Abstract
The FOXO3a and FOXM1 forkhead transcription factors are key players in cancer initiation, progression, and drug resistance. Recent research shows that FOXM1 is a direct transcriptional target of FOXO3a, a vital downstream effector of the PI3K-AKT-FOXO signaling cascade. In addition, FOXM1 and FOXO3a also antagonize each other's activity by competitively binding to the same target genes, which are involved in chemotherapeutic drug sensitivity and resistance. Understanding the role and regulation of the FOXO-FOXM1 axis will provide insight into chemotherapeutic drug action and resistance in patients, and help to identify novel therapeutic approaches as well as diagnostic and predictive biomarkers.
Keywords: FOXO3a, FOXM1, transcription factor, cancer, drug resistance, tumorigenesis
Cancer is a widespread disease and a major cause of death worldwide. In 2008, 12.7 million new cases and 7.6 million deaths were reported[1]. Although novel therapeutics have improved patient survival rates, resistance to conventional anticancer drugs remains the principal reason for treatment failure, relapse, and cancer mortality. Patients for whom hormone or molecular-targeted therapies are not an option usually receive cytotoxic chemotherapeutic agents, including taxanes (e.g., paclitaxel and docetaxel) and anthracyclines (e.g., epirubicin and doxorubicin)[2]. For example, anthracyclines and taxanes are usually used as first- and/or second-line option(s) in chemotherapeutic regimens for patients with advanced or metastatic breast cancer or those with EGFR/HER2-negative and estrogen receptor (ER)-negative tumors. In most cases, these patients eventually become insensitive to taxane- and anthracycline-based chemotherapy and develop recurrent cancer, despite an initial response to these cytotoxic and genotoxic therapies.
Anthracyclines block RNA and DNA synthesis, triggering DNA damage through interfering with topoisomerase II and inducing the formation of reactive oxygen species. Taxanes, however, act specifically during the G2/M phase of the cell cycle, blocking cell cycle progression by impairing centrosome formation, inducing abnormal spindles, and suppressing microtubule dynamics during spindle development. Although these drugs' mode of action is known, the molecular mechanisms by which drug-resistant cancer clones evade their cytotoxic or genotoxic effects remain largely undefined[2],[3].
FOXO-FOXM1 Axis in Tumorigenesis and Drug Resistance
Forkhead box (FOX) proteins belong to a superfamily of transcription factors defined by their highly evolutionarily conserved “winged-helix” DNA- binding domain (DBD). FOX proteins are responsible for the spatio-temporal regulation of a wide range of transcriptional programs required for normal homeostasis and development[4]. These FOX-regulated biological processes include cell proliferation, cell cycle progression, cell differentiation, tissue homeostasis, angiogenesis, and apoptosis. As a result, it is not surprising that deregulation of FOX transcription factors can lead to severe pathologic conditions, including cancer. The forkhead box class O (FOXO) subfamily of transcription factors (i.e., FOXO1, FOXO3a, FOXO4, and FOXO6) act downstream of the phosphoinositol-3-kinase (PI3K)-AKT oncogenic signaling cascade (Figure 1), and are required for the execution of diverse cellular functions, including cell cycle arrest, apoptosis, development, differentiation, invasion, metabolism, migration, and resistance to oxidative stress and DNA damage[4]–[8].
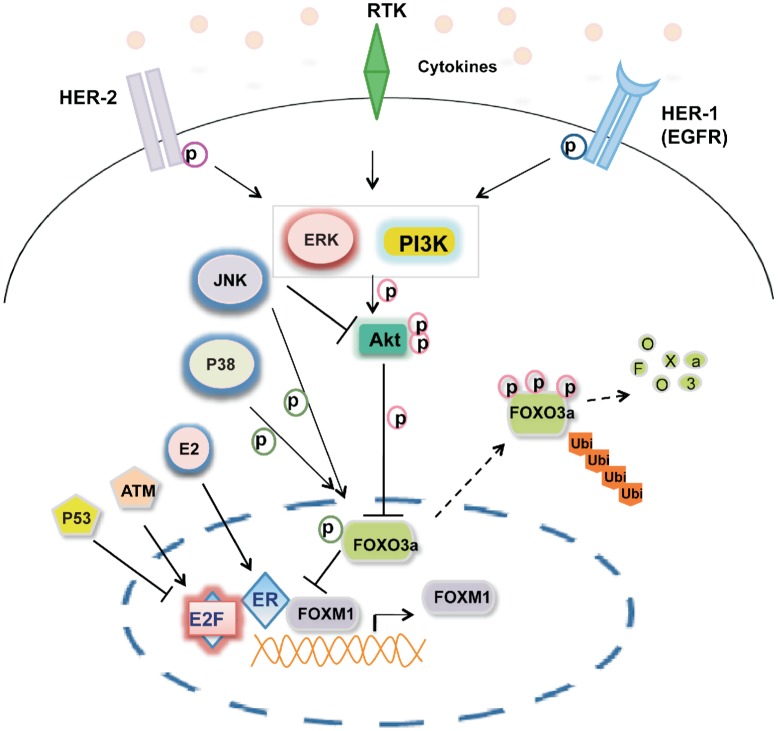
Figure 1. Integration of intracellular and extracellular signals with the forkhead box class O3a (FOXO3a) and forkhead box protein M1 (FOXM1) axis.
Intracellular and extracellular signals converge on the PI3K-AKT-FOXO3a-FOXM1 signaling cascade. FOXO3a antagonizes the transcription output of FOXM1, which controls cancer-related processes including proliferation, survival, drug resistance, angiogenesis, migration, and DNA damage repair. EGFR, human epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; P38, P38 mitogen-activated protein kinase; P53, tumor suppressor protein 53; E2F, E2F transcription factor; ER, estrogen receptor; E2, estradiol; ATM, ataxia telangiectasia mutated protein; Ub, ubiquitination; P, phosphorylation.
The PI3K-AKT cascade is a commonly deregulated signaling pathway in cancer. During tumorigenesis and the evolution toward cancer drug resistance, this oncogenic pathway is frequently hyperactivated, leading to FOXO inactivation. Overexpression of receptor tyrosine kinases, loss of function mutations within phosphatase and tensin homolog (PTEN), and activating mutations within PIK3CA (p110α) and AKT all result in increased AKT activity, which promotes tumor survival and cancer progression. Moreover, enhanced AKT activity also confers resistance to endocrine and molecular-targeted therapeutics as well as conventional cytotoxic and genotoxic drugs, further highlighting the importance of the PI3K-AKT signaling pathway and FOXOs in determining the outcome of drug sensitivity. Although it is well established that AKT-mediated phosphorylation of FOXO proteins induces their cytoplasmic translocation and degradation, FOXOs—in particular FOXO3a—are also regulated by the extracellular signal-related kinase (ERK)/mitogen-activated protein kinase (MAPK) and IκB kinase (IKK) pathways[4]. Crosstalk of these frequently deregulated signaling cascades culminates in the inactivation of the tumor suppressive function of FOXO3a. Emerging evidence has revealed that the cytostatic and cytotoxic effects of many anticancer therapeutics, including paclitaxel[9],[10], doxorubicin[11],[12], lapatinib[13], gefitinib[14],[15], imatinib[16]–[18], cisplatin[19], and tamoxifen[4], are mediated through FOXO activation, primarily through inhibition of the PI3K-AKT signaling axis. In addition, in response to cytotoxic anticancer agent treatment, FOXO3a is targeted by the stress-activated MAPKs, including JNK and P38. For instance, JNK has been shown to induce FOXO3a activity and nuclear localization by repressing AKT phosphorylation and activity following paclitaxel treatment[9],[10]. Moreover, P38 phosphorylates FOXO3a on Ser7, promoting FOXO3a nuclear relocalization and activation in response to doxorubicin[20]. Together these findings suggest that chemotherapeutic drugs integrate their signals with FOXO3a to mediate their effects primarily through the PI3K-AKT, ERK, JNK, and P38-MAPK signaling pathways.
For all “good” FOX proteins, there is always a “bad” cousin. While the FOXOs are bona fide tumor suppressors, another closely related forkhead subfamily member, forkhead box protein M1 (FOXM1), behaves like a classic oncogene. The FOXM1 transcription factor has a central function in cell cycle progression and cell proliferation. As such, Foxm1-deficient mice die in utero due to a failure to enter mitosis[4],[21]. Consistent with its role in proliferation, FOXM1 overexpression has been identified in many types of cancers, including liver, prostate, breast, lung, and colon cancers[4],[22]. This was further confirmed by independent gene expression profiling studies of cancers, which identified FOXM1 as a commonly up-regulated gene in human solid tumors[22]–[24]. These findings suggest a key role for FOXM1 in tumorigenesis. In addition to cell proliferation, FOXM1 controls and influences other biologic processes, including cell differentiation, apoptosis, angiogenesis, senescence, tissue homeostasis, and DNA damage repair[4],[21]. Not surprisingly, there is a remarkable correlation between increasing levels of FOXM1 and progressive stages of human cancers.
Consistent with a role in tumor initiation and progression, FOXM1 has also been shown to induce the expansion of stem cell compartments, resulting in hyperplasia during tumorigenesis. FOXM1 plays a part in cancer progression by inducing the expression of downstream targets that promote the stem cell and epithelial-to-mesenchymal transition (EMT) phenotypes, including the stem and mesenchymal cell markers ZEB1, ZEB2, Snail2, E-cadherin, and vimentin. Ultimately, FOXM1-mediated expression of these genes culminates in increased cell proliferation, self-renewal capacity, long-term viability, cell migration, angiogenesis, and drug resistance[25]. In addition, FOXM1-deficient cells also display polyploidy, aneuploidy, chromosome missegregation, and an increase in the number of DNA breaks, all of which highlight the importance of FOXM1 in safeguarding mitosis and genomic integrity[26],[27]. The role of FOXM1 in the clinical context of drug responsiveness and resistance has also been studied[4],[13],[21],[28]. Increased FOXM1 expression has been found to confer resistance to chemotherapeutic drugs, such as cisplatin and epirubicin, equip cancer cells with protective mechanisms against DNA damage-induced cell death, and disrupt mitosis/cytokinesis control[13],[29],[30]. Furthermore, these chemotherapy-resistant cancer cells also expressed higher levels of FOXM1 compared with drug-sensitive counterparts[13],[29],[30]. The ability of FOXM1 to confer resistance to the genotoxic and cytotoxic drugs has been linked to its ability to activate the expression of target genes important for DNA damage response, including breast cancer type 2 susceptibility protein (BRCA2), X-ray repair complementing defective repair in Chinese hamster cells (XRCC1), exonuclease 1 (EXO1), polo-like kinase 4 (PLK4), polymerase (DNA directed), epsilon 2, accessory subunit (POLE2), and replication factor C4 (RFC4)[31]–[33]. How these cytotoxic and genotoxic agents regulate FOXM1 expression remains unclear. Nevertheless, recent research demonstrates that the p38 MAPK-MK2 signaling pathway is involved in the control of drug sensitivity, as well as E2F1 and FOXM1 expression in response to epirubicin[3]. In addition, upon treatment with genotoxic agents, the checkpoint kinases Chk1 and Chk2 also phosphorylate and activate FOXM1. FOXM1 is also involved in sensitivity and resistance of endocrine-related cancers to hormone therapy. Because ERα and FOXM1 regulate each other's expression, it is not surprising that FOXM1 also contributes to the development of resistance to endocrine therapy in breast cancer[34],[35]. This reciprocal regulation culminates in a positive feedback mechanism that, when deregulated, can contribute to tumorigenesis and hormone insensitivity in endocrine-related cancers. Furthermore, recent evidence suggests that the anti-proliferative role of ERβ1 in the development of breast cancer is mediated through competing with ERα in the regulation of FOXM1 expression[36].
Interestingly, FOXO3a and FOXM1 compete for binding to similar DNA sequences and share a number of downstream target genes[37]. As a result, FOXO3a antagonizes the transcriptional output of FOXM1[37],[38], such that genes activated by FOXM1 are often repressed by FOXO3a[13],[14],[39] (Figure 2). For instance, FOXM1 activates whereas FOXO3a represses VEGF expression to control angiogenesis and migration in breast cancer cells[37]. Moreover, the antagonistic action between FOXO3a and FOXM1 is also extended to the FOXM1 gene, which is negatively regulated by FOXO3a but positively regulated by FOXM1 at the transcriptional level[13],[14],[39]. On the other hand, FOXO3a and FOXM1 may cooperate to regulate ERα gene transcription in breast cancer cells[35], and this may have implications for the action of estrogens and anti-estrogens in cancer development and treatment.
Figure 2. Integration of signals with the FOXO3a-FOXM1 axis in chemotherapeutic drug response.
Chemotherapeutic drugs have various modes of action but ultimately integrate signals with the FOXO3a-FOXM1 signaling axis. FOXO3a then antagonizes the expression of FOXM1 target genes, which control cancer-related processes, including cell cycle progression, cell proliferation, anti-oxidative stress, cell self-renewal, drug resistance, DNA damage repair, senescence suppression, angiogenesis, and migration. RTK, receptor tyrosine kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositol-3-kinase.
Summary and Future Perspectives
In summary, the FOXO3a-FOXM1 axis, which functions downstream of the PI3K-AKT oncogenic signaling pathway, is the target of many conventional genotoxic and cytotoxic chemotherapeutic drugs. The FOXO3a and FOXM1 transcription factors are also cellular targets of endocrine and molecular targeting anticancer agents. Genes activated by FOXM1 are commonly repressed by FOXO3a. The FOXO3a-FOXM1 axis can modulate cancer initiation, progression, and drug resistance by regulating the expression of genes essential for cell proliferation, survival, self-renewal, migration, angiogenesis, cell cycle/checkpoint transition, and DNA damage repair. Given the key roles of FOXO and FOXM1 proteins in cancer, a better understanding of the mechanisms by which FOXO and FOXM1 are regulated, as well as a greater appreciation of their roles and downstream targets in cancer, will help to develop these proteins into reliable diagnostic markers and therapeutic targets for cancer.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wong ST, Goodin S. Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy. 2009;29:954–965. doi: 10.1592/phco.29.8.954. [DOI] [PubMed] [Google Scholar]
- 3.de Olano N, Koo CY, Monteiro LJ, et al. The p38 MAPK-MK2 axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Mol Cancer Res. 2012;10:189–202. doi: 10.1158/1541-7786.MCR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 5.Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FOXO. Oncogene. 2008;27:2300–2311. doi: 10.1038/onc.2008.23. [DOI] [PubMed] [Google Scholar]
- 6.Lam EW, Francis RE, Petkovic M. FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans. 2006;34:722–726. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- 7.Gomes AR, Brosens JJ, Lam EW. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–3136. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 8.Brosens JJ, Parker MG, McIndoe A, et al. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200(615):e1–e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Sunters A, Fernandez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 10.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 11.Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–678. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 12.Hui RC, Gomes AR, Constantinidou D, et al. The forkhead transcription factor FOXO3a increases phosphoinositide-3 kinase/Akt activity in drug-resistant leukemic cells through induction of PIK3CA expression. Mol Cell Biol. 2008;28:5886–5898. doi: 10.1128/MCB.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis RE, Myatt SS, Krol J, et al. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. Int J Oncol. 2009;35:57–68. doi: 10.3892/ijo_00000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern UB, Francis RE, Peck B, et al. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582–591. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
- 15.Krol J, Francis RE, Albergaria A, et al. The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther. 2007;6:3169–3179. doi: 10.1158/1535-7163.MCT-07-0507. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez de Mattos S, Essafi A, Soeiro I, et al. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol. 2004;24:10058–10071. doi: 10.1128/MCB.24.22.10058-10071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkenkamp KU, Essafi A, van der Vos KE, et al. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–2220. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 18.Essafi A, Fernandez de Mattos S, Hassen YA, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez de Mattos S, Villalonga P, Clardy J, et al. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther. 2008;7:3237–3246. doi: 10.1158/1535-7163.MCT-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho KK, McGuire VA, Koo CY, et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem. 2012;287:1545–1555. doi: 10.1074/jbc.M111.284224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myatt SS, Lam EW. Targeting FOXM1. Nat Rev Cancer. 2008;8:242. doi: 10.1038/nrc2223-c2. [DOI] [PubMed] [Google Scholar]
- 22.Pilarsky C, Wenzig M, Specht T, et al. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddin S, Ahmed M, Hussain A, et al. Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. Am J Pathol. 2011;178:537–547. doi: 10.1016/j.ajpath.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okabe H, Satoh S, Kato T, et al. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cdna microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–2137. [PubMed] [Google Scholar]
- 25.Bao B, Wang Z, Ali S, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Laoukili J, Kooistra MR, Bras A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Gomes AR, Monteiro LJ, et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One. 2010;5:e12293. doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok JM, Peck B, Monteiro LJ, et al. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res. 2010;8:24–34. doi: 10.1158/1541-7786.MCR-09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millour J, de Olano N, Horimoto Y, et al. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol Cancer Ther. 2011;10:1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol. 2007;27:1007–1016. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YY, Jung SY, Jennings NB, et al. FOXM1 mediates Dox resistance in breast cancer by enhancing DNA repair. Carcinogenesis. 2012;33:1843–1853. doi: 10.1093/carcin/bgs167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro LJ, Khongkow P, Kongsema M, et al. The Forkhead Box M1 protein regulates BRIP1 expression and DNA damage repair in epirubicin treatment. Oncogene. 2012 Oct 29; doi: 10.1038/onc.2012.491. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millour J, Constantinidou D, Stavropoulou AV, et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madureira PA, Varshochi R, Constantinidou D, et al. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. J Biol Chem. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 36.Horimoto Y, Hartman J, Millour J, et al. ERbeta1 represses FOXM1 expression through targeting ERalpha to control cell proliferation in breast cancer. Am J Pathol. 2011;179:1148–1156. doi: 10.1016/j.ajpath.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karadedou CT, Gomes AR, Chen J, et al. FOXO3a represses VEGF expression through FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2012;31:1845–1858. doi: 10.1038/onc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo CY, Muir KW, Lam EW. FOXM1: from cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MS, Brosens JJ, Schwenen HD, et al. FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets. 2011;12:1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]