Abstract
Both the incidence and mortality of nasopharyngeal carcinoma (NPC) have decreased in Hong Kong and Taiwan but not in mainland China. The goal of this study was to analyze trends in NPC patient survival between 1976 and 2005 in Sihui, an area of mainland China with a population at high risk for NPC. A total of 1,761 patients diagnosed with NPC between 1976 and 2005 according to the records of Sihui Cancer Registry were followed to the end of 2006. We determined their observed and relative survival rates and used Cox proportional hazards regression analysis to predict prognosis. Our results showed that the 5-year and 10-year observed survival rates of NPC patients in Sihui were 50.5% and 36.9%, respectively, and the median survival time was 5.1 years. The 5-year observed survival rate of NPC patients diagnosed after 2000 was 69.8%, significantly higher than that of patients diagnosed between 1976 and 1985 (42.5%; P < 0.001, relative risk = 0.28). Similarly, the 5-year relative survival rate was 84.8% between 2000 and 2005 but 51.8% between 1976 and 1985. Besides date of diagnosis, other prognostic factors included patient sex and age and NPC clinical stage and histologic type. The relative risks of death from NPC were 0.76 [95% confidence interval (CI): 0.65–0.90] for female comparing to male and 1.28 (95% CI: 1.00–1.64) for WHO type I comparing to WHO types II and III. For the eldest age group and the latest clinical stage group, the relative risks were 2.22 (95% CI: 1.73–2.84) and 3.41 (95% CI: 2.34–4.49), respectively. Our results indicate that the survival of NPC patients in Sihui has significantly increased in recent years and this increase is not influenced by patient's sex, age, histologic type, and clinical stage. A reduction in mortality rate is expected in coming years.
Keywords: Nasopharyngeal carcinoma, survival, relative survival, time trend
The incidence and mortality of nasopharyngeal carcinoma (NPC) have declined in Chinese populations of Hong Kong, Taiwan, Singapore, and Los Angeles[1]–[3], but a similar trend has not been observed among the high-risk population of mainland China[4],[5]. The city of Sihui has the highest incidence of NPC in China. Between 1998 and 2002, the age-standardized incidence of NPC was 30.94 per 100,000 person-years for males and 13.0 per 100,000 person-years for females. At the same time, the age-standardized mortality was 21.95 per 100,000 person-years for males and 7.73 per 100,000 person-years for females[4]. In Sihui, NPC incidence has remained stable over the last 30 years, and mortality has trended downward in recent years but have not reached statistical significance.
Since 1986, several large-scale screening programs for NPC have been conducted in the high-risk population of Sihui using antibodies to test for Epstein-Barr virus. These programs have significantly impacted early diagnosis of NPC, but whether overall survival for NPC patients has changed for this high-risk population remains unknown. Here, we investigated trends in the survival of NPC patients in Sihui over a 30-year period (1976–2005).
Materials and Methods
Materials
In 1973, a cancer registry system was established mainly covering NPC and other common cancer incidences and all causes of death in Sihui, China. In 1978, this cancer registry system was extended to all cancer, and a follow-up system for patients with NPC was established. Besides the local reporting system for cancer, the case records in all hospitals with diagnostic and treatment capability for NPC in major cities near Sihui were checked annually, and the information of patients with cancer from Sihui were registered. Therefore, most cases have been doubly verified, and the completeness and validity of data were ensured.
Because the records in the register were deemed to be incomplete during the initial period of 1973–1975, we limited our analysis to start from 1976. Anonymous records of incident cases of NPC (ICD-9 147; ICD-10 C11) for the period of diagnosis 1976–2005 were extracted from the Sihui Cancer Registry. At the time of data extraction, follow-up (by linkage to death records) was available to the end of 2006. In total, 1,761 cases with NPC were identified, among which 1,239 cases (70.4%) had pathologic diagnosis. Follow-up ended on December 31, 2006, and the longest follow-up was 30.4 years.
Based on the data from the death registry system in Sihui, age-specific death rates and life tables of Sihui general population were estimated.
Statistical analyses
The Kaplan-Meier estimate of survival was used to observe the overall survival outcome with respect to each covariate. The statistical significance of covariates compared over strata was determined using the log-rank test. Cox proportional hazards regression modeling[6] was used to analyze censored data over time and to estimate the impact of prognostic factors on survival. The following variables were analyzed in univariate and multivariate analysis: sex, age at diagnosis, period of diagnosis, clinical stage of NPC, pathohistologic types of NPC, and hospital where diagnosed. These variables were not regarded as time-dependent.
Relative survival probabilities for NPC were assessed by matching sex, age group, and period of diagnosis with the general population. The method bases on the hypothesis that patients had the same age, sex-specific mortality in each period (background mortality) as the general population and that the ratio of the observed (absolute) survival and the relative survival of cancer patients would be stable in different sex and age groups[7]. Relative survival analysis was performed by stratification with sex, age at diagnosis, and clinical and pathological stage of NPC[8].
Observed and relative survival rates were analyzed in four calendar periods according to clinical stage and histologic type of NPC. The four calendar periods were 1976–1985, 1986–1992, 1993–1999, and 2000–2005. The general survival analysis was performed with SAS 9.2. The software SURV3[9] was used for estimation of relative survival rate.
Results
Patient characteristics
The distributions of sex, age, hospital where diagnosed, and clinical stage among the 1,761 patients with NPC are listed in Table 1. The ratio of males to females among NPC patients was 2.08. The median age of patients was 46, and NPC was diagnosed most commonly between the age 40 and 60. More than 60% of patients were diagnosed at Sun Yat-sen University Cancer Center. Other medical facilities where NPC was diagnosed include Sihui Cancer Institute (n = 311, 17.7%), Zhaoqing Hospital (n = 127, 7.3%), and other hospitals (n = 247, 13.9%). Among patients for whom clinical stage was known, 81.7% were diagnosed with stage II or III NPC. In cases wherein NPC was pathologically diagnosed, 12.8% had well-differentiated keratinizing type (WHO type I) and 87.1% had moderately-differentiated nonkeratinizing type (WHO type II) or undifferentiated carcinoma or lymphoepitheliomas (WHO type III). NPC diagnosis gradually increased in calendar periods from 1976 to 1999 and decreased in the period from 2000 to 2005.
Table 1. The demographic and clinical characteristics of patients with nasopharyngeal carcinoma (NPC) in Sihui, China, 1976–2005.
| Characteristic | Cases | Percentage (%) |
| Sex | ||
| Male | 1,189 | 67.5 |
| Female | 572 | 32.5 |
| Age at diagnosis | ||
| <40 | 498 | 28.2 |
| 40∼ | 545 | 30.9 |
| 50∼ | 414 | 23.5 |
| ≥60 | 304 | 17.3 |
| Clinical stage | ||
| I | 107 | 6.1 |
| II | 546 | 31.0 |
| III | 467 | 26.5 |
| IV | 119 | 6.8 |
| Unknown or missing | 522 | 29.6 |
| Pathologic diagnosis | ||
| WHO I | 159 | 9.0 |
| WHO II or III | 1,080 | 61.3 |
| Unknown or missing | 522 | 29.6 |
| Period of diagnosis | ||
| 1976–1985 | 409 | 23.2 |
| 1986–1992 | 472 | 26.8 |
| 1993–1999 | 534 | 30.3 |
| 2000–2005 | 346 | 19.6 |
| Hospital where diagnosed | ||
| SYSUCC | 1,076 | 61.1 |
| Other medical facilities | 685 | 38.9 |
WHO I, well-differentiated keratinizing type; WHO II, moderately-differentiated nonkeratinizing type; WHO III, undifferentiated carcinoma or lymphoepitheliomas; SYSUCC, Sun Yat-sen University Cancer Center.
Observed and relative survival rates in relation to demographic and clinic characteristics
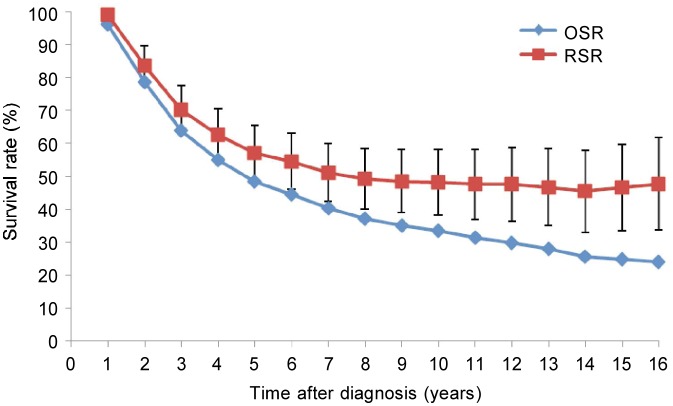
The overall 5-year observed and relative survival rates were 50.5% and 57.9%, respectively (Figure 1). Median survival time was 5.1 years [95% confidence interval (CI): 4.5–5.8 years]. Clinical stage was the critical prognostic factor for NPC patients. Patients with stage I NPC had a 10-year overall survival rate of 60.9% and a median survival time of 14.4 years. In contrast, in patients with stage IV NPC, these figures were 18.1% and 2.3 years, respectively. Similarly, the 10-year relative survival rate was 83.4% (95% CI: 71.0%– 95.9%) for patients with stage I NPC and 28.1% (95% CI: 16.7%–39.4%) for patients with stage IV NPC.
Figure 1. The observed andrelative survival (95% confidence interval) of patients with nasopharyngeal cancer, Sihui, China, 1976–2005. OSR, cumulative observed survival rate; RSR, cumulative relative survival rate.
The observed and relative survival of patients diagnosed after 1990 (1993–1999, 2000–2005) generally increased (Table 2). Note that relative survival takes account of any changes in background mortality over time and is therefore focused on survival from NPC, irrespective of competing causes of death. Survival was lower in the period 1976–1992 but improved since then (log-rank χ2 = 80.30, d.f. = 3, P = 0.002). The 5-year observed survival increased from 42.5% in 1976–1985 to 69.8% in 2000–2005, and relative survival increased from 51.8% in 1976–1985 to 84.8% in 2000–2005.
Table 2. Correlation of 5-year survival with demographic and clinical characteristics of patients with NPC in Sihui, China, 1976–2005.
| Characteristic | MTS | Observed survival (%, 95% CI) | Relative survival (%, 95% CI) |
| All cases | 5.1 | 50.5 (48.1–52.9) | 60.3 (57.5–63.1) |
| Sexa | |||
| Male | 4.5 | 48.4 (45.5–51.3) | 59.3 (55.8–62.8) |
| Female | 6.2 | 54.5 (50.4–58.7) | 62.2 (57.6–66.8) |
| Age at diagnosisa | |||
| < 40 | 8.7 | 56.4 (51.9–60.9) | 59.4 (54.7–64.1) |
| 40∼ | 5.9 | 53.9 (49.6–58.1) | 60.1 (55.4–64.8) |
| 50∼ | 4.8 | 48.7 (43.8–53.6) | 62.5 (56.3–68.6) |
| ≥60 | 2.8 | 36.4 (30.8–41.9) | 59.1 (50.5–67.6) |
| Clinical Stagea | |||
| I | 14.4 | 73.7 (65.3–82.1) | 85.2 (75.8–94.5) |
| II | 6.3 | 57.4 (53.1–61.7) | 69.4 (64.5–74.3) |
| III | 2.8 | 38.3 (33.8–42.8) | 44.7 (39.5–50.0) |
| IV | 2.3 | 31.5 (22.8–40.2) | 37.9 (27.6–48.2) |
| Unknown or missing | 6.1 | 52.8 (48.4–57.2) | 64.4 (59.2–69.6) |
| Pathologic stagea | |||
| WHO I | 5.1 | 49.8 (41.1–58.6) | 56.9 (52.3–61.5) |
| WHO II or III | 5.5 | 52.1 (49.1–55.1) | 62.3 (58.8–65.8) |
| Unknown or missing | 4.2 | 46.4 (42.0–50.9) | 58.8 (53.5–64.1) |
| Period of diagnosisa | |||
| 1976–1985 | 3.8 | 42.5 (37.8–47.3) | 51.8 (46.2–57.4) |
| 1986–1992 | 4.0 | 43.4 (39.0–47.9) | 51.1 (45.9–56.3) |
| 1993–1999 | 5.5 | 52.1 (47.8–56.3) | 62.5 (57.5–67.6) |
| 2000–2005 | NA | 69.8 (64.1–75.4) | 84.8 (79.1–90.6) |
| Hospital where diagnosed | |||
| SYSUCC | 5.5 | 52.3 (49.3–55.3) | 62.3 (58.8–65.8) |
| Others | 4.3 | 47.4 (43.5–51.2) | 56.9 (52.3–61.5) |
alog-rank test, P < 0.05.
MTS, median time of survival (year); CI, confidence interval; NA, not available due to the small size of cases; SYSUCC, Sun Yat-sen University Cancer Center.
In addition to clinical stage and calendar period, sex, age at diagnosis, and histologic type were significantly related to the prognosis of NPC patients. However, the difference of survival between patients diagnosed at Sun Yat-sen University Cancer Center and those diagnosed at other hospitals had no statistically significance.
Stage/histology-specific survival of NPCs in different diagnosis periods
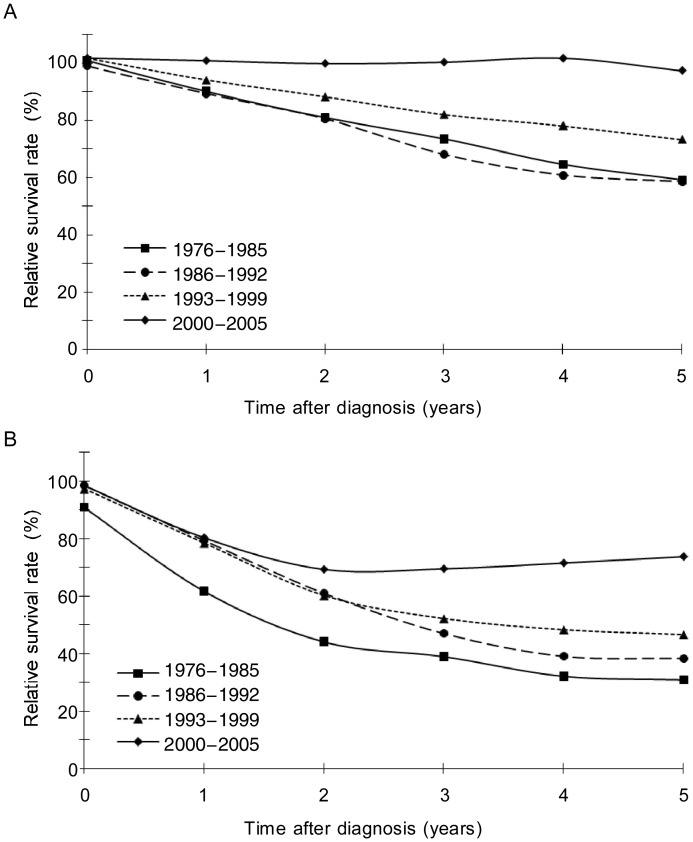
Among patients with different clinical stages of NPC, both the observed and the relative survival increased during the recent decade compared with all previous decades studied (Table 3, Figure 2). The 5-year relative survival of patients with stage I NPC was 83.8% during 1976–1985 and increased to 91.3% during 1993–1999. The 5-year relative survival of patients with stage II NPC was 60.5% during 1976–1985, then increased to 74.3% during 1993–1999. The 5-year relative survival of stage III NPC was 35.0% during 1976–1985, then increased to 49.7% during 1993–1999. For stage IV patients, 5-year relative survival increased from 14.9% in 1976–1985 to 59.6% in 2000–2005. The increasing survival was also found in the patients whose stage information was not available.
Table 3. Stage and histology-specific relative survival and observed survival of patients with NPC stratified by calendar period of diagnosis, Sihui, China, 1976–2005.
| Variate | Period of diagnosis | Number |
Observed survival (%, 95% CI) |
Relative survival (%, 95% CI) |
|||
| Cases | Death | 3-year | 5-year | 3-year | 5-year | ||
| Clinical stage | |||||||
| I | 1976–1985 | 35 | 29 | 82.8 (70.3–95.3) | 68.6 (53.2–83.9) | 91.7 (76.9–100) | 83.8 (67.1–100) |
| 1986–1992 | 31 | 15 | 71.0 (54.9–86.9) | 61.3 (44.1–78.4) | 87.2 (71.9–100) | 74.1 (54.4–93.9) | |
| 1993–1999 | 34 | 11 | 88.3 (77.4–99.1) | 79.4 (65.8–93.0) | 99.8 (91.2–100) | 91.3 (76.9–100) | |
| 2000–2005 | 7 | 0 | NA | NA | NA | NA | |
| II | |||||||
| 1976–1985 | 172 | 146 | 64.5 (57.4–71.7) | 49.4 (41.9–56.9) | 78.6 (71.3–85.8) | 60.5 (51.9–69.2) | |
| 1986–1992 | 142 | 102 | 66.9 (59.1–74.6) | 47.9 (39.7–56.1) | 79.1 (71.0–87.1) | 57.9 (48.4–67.5) | |
| 1993–1999 | 129 | 64 | 70.5 (62.7–78.4) | 60.5 (52.0–68.9) | 84.9 (76.9–92.9) | 74.3 (64.4–84.2) | |
| 2000–2005 | 103 | 15 | 87.3 (80.4–94.2) | 80.4 (70.6–90.2) | 99.3 (93.1–100) | NA | |
| III | |||||||
| 1976–1985 | 134 | 122 | 38.1 (29.8–46.3) | 29.1 (21.4–36.8) | 47.6 (38.3–56.8) | 35.0 (25.9–44.1) | |
| 1986–1992 | 151 | 128 | 46.4 (38.4–54.3) | 33.8 (26.2–41.3) | 60.9 (52.2–69.7) | 39.3 (30.5–48.2) | |
| 1993–1999 | 109 | 72 | 45.9 (36.5–55.2) | 41.3 (32.1–50.5) | 60.8 (50.5–71.2) | 49.7 (38.8–60.6) | |
| 2000–2005 | 73 | 24 | 66.5 (55.3–77.7) | NA | 71.5 (59.4–83.6) | NA | |
| IV | |||||||
| 1976–1985 | 23 | 23 | 13.0 (0.0–26.8) | 8.7 (0.0–20.2) | 23.4 (4.9–42.0) | 14.9 (0.0–30.9) | |
| 1986–1992 | 34 | 30 | 47.1 (30.3–63.8) | 29.4 (14.1–44.7) | 60.8 (42.3–79.3) | 37.4 (18.9–56.0) | |
| 1993–1999 | 38 | 28 | 50.0 (34.1–65.9) | 34.2 (19.1–49.3) | 57.7 (39.9–75.4) | 43.6 (25.1–62.1) | |
| 2000–2005 | 24 | 11 | 53.5 (32.8–74.1) | NA | 62.7 (40.7–84.7) | 59.6 (35.5–83.7) | |
| Unknown or missing | |||||||
| 1976–1985 | 45 | 37 | 57.8 (43.3–72.2) | 44.4 (29.9–58.9) | 69.5 (53.3–85.7) | 62.2 (44.0–80.3) | |
| 1986–1992 | 114 | 79 | 63.2 (54.3–72.0) | 46.5 (37.3–55.6) | 78.4 (69.4–87.4) | 55.9 (45.3–66.6) | |
| 1993–1999 | 224 | 129 | 58.9 (52.5–65.4) | 49.6 (43.0–56.1) | 71.2 (64.1–78.3) | 60.6 (52.6–68.5) | |
| 2000–2005 | 139 | 42 | 72.8 (65.3–80.3) | 66.4 (57.4–75.4) | 81.4 (73.3–89.5) | 81.1 (71.7–90.6) | |
| Histologic type | |||||||
| WHO I | |||||||
| 1976–1985 | 33 | 30 | 54.6 (37.6–71.5) | 42.4 (25.6–59.3) | 77.5 (60.9–94.1) | 54.2 (34.8–73.7) | |
| 1986–1992 | 34 | 27 | 52.9 (36.2–69.7) | 35.3 (19.2–51.4) | 68.7 (51.3–86.1) | 45.8 (27.0–64.5) | |
| 1993–1999 | 32 | 21 | 53.1 (35.8–70.4) | 40.6 (23.6–57.6) | 78.6 (59.9–97.4) | 58.1 (36.2–79.9) | |
| 2000–2005 | 60 | 12 | 77.4 (63.9–90.9) | NA | 93.3 (83.1–100) | NA | |
| WHO II or III | |||||||
| 1976–1985 | 280 | 243 | 56.4 (50.6–62.2) | 43.9 (38.1–49.7) | 66.4 (60.1–72.6) | 52.6 (45.9–59.4) | |
| 1986–1992 | 310 | 232 | 60.3 (54.9–65.8) | 44.5 (38.9–50.0) | 73.5 (67.8–79.2) | 51.7 (45.3–58.1) | |
| 1993–1999 | 332 | 178 | 66.5 (61.5–71.6) | 57.8 (52.5–63.1) | 78.8 (73.5–84.1) | 66.9 (60.7–73.1) | |
| 2000–2005 | 158 | 44 | 73.0 (66.0–80.0) | 70.4 (62.7–78.0) | 81.2 (73.8–88.6) | 82.7 (74.5–90.9) | |
CI, confidence interval; NA, not available due to the small size of cases.
Figure 2. Relative survival of patients with nasopharyngeal cancer, stages I and II (A) and stages III and IV (B), diagnosed in Sihui, China, during four calendar periods: 1976–1985, 1986–1992, 1993–1999, and 2000–2005.
Both the observed and the relative survival increased during the recent decade in patients with different pathologic stages and histologic types of NPC (Table 3). In patients with WHO type I NPC, the 3-year observed survival remained stable at 53% in the first three calendar periods but increased to 77.4% in the most recent. However, this trend may be due to small sample size.
Multivariate Cox proportional hazards regression
Multivariate Cox regression analysis revealed age, sex, clinical stage, histologic type, and period of diag- nosis to be significant, suggesting that these variables are independent prognostic factors for NPC (Table 4).
Table 4. The independent prognostic factors related to death among patients with NPC in Sihui, China, 1976–2005 by multivariate proportional hazard regression analysisa.
| Characteristic | Total No. | Death No. | RR | 95% CI | P |
| Sex | <0.001 | ||||
| Male | 1189 | 767 | 1.00 | Ref. | |
| Female | 572 | 340 | 0.76 | 0.65–0.90 | |
| Age (years) | <0.001 | ||||
| < 40 | 498 | 261 | 1.00 | Ref. | |
| 40∼ | 545 | 334 | 1.21 | 0.99–1.49 | |
| 50∼ | 414 | 281 | 1.51 | 1.22–1.88 | |
| ≥60 | 304 | 231 | 2.22 | 1.73–2.84 | |
| Clinical stage | <0.001 | ||||
| I | 107 | 55 | 1.00 | Ref. | |
| II | 546 | 327 | 1.61 | 1.17–2.22 | |
| III | 467 | 346 | 2.61 | 1.89–3.60 | |
| IV | 119 | 92 | 3.41 | 2.34–4.99 | |
| Histologic type | 0.048 | ||||
| WHO II or III | 1080 | 697 | 1.00 | Ref. | |
| WHO I | 159 | 90 | 1.28 | 1.00–1.64 | |
| Period of diagnosis | <0.001 | ||||
| 1976–1985 | 409 | 357 | 1.00 | Ref. | |
| 1986–1992 | 472 | 354 | 0.76 | 0.63–0.91 | |
| 1993–1999 | 534 | 304 | 0.55 | 0.45–0.68 | |
| 2000–2005 | 346 | 92 | 0.28 | 0.20–0.40 |
aThe multiple regression models included all variables listed in the Table. RR, relative risk; CI, confidence interval; Ref., reference group.
Within years after diagnosis, 1,107 of the 1,761 patients (62.9%) had died. In the Cox proportional hazards regression model for all patients, the adjusted relative risks (RRs) for death from NPC according to sex (lower risk for women than men), age (increasing risk with rising age), and clinical stage (increasing risk with later clinical stage) were statistically significant. Multiple Cox proportional hazards regression analysis showed that the adjusted RR for patients diagnosed in the period 2000– 2005 compared with the period 1976–1985 was 0.28 (95% CI, 0.20–0.40, P < 0.001). There was a 72% reduction in the RR of death from NPC among those diagnosed after 2000 compared with those diagnosed in the mid-1970s to mid-1980s after controlling for sex and age at diagnosis. RR of death from NPC among patients diagnosed in 1986–1992 and in 1993–1999 was 0.76 (95% CI: 0.63–0.91) and 0.55 (95% CI: 0.45–0.68), respectively, compared with those diagnosed in 1976–1985 after controlling for sex and age at diagnosis. Patients with WHO I histologic type NPC were in a slightly more hazard compared with patients with WHO type II or III NPC (RR = 1.28, 95% CI: 1.00–1.64).
Discussion
Our analyses show an overall improvement in longterm survival among NPC patients diagnosed between 1976 and 2005 in Sihui, China. The 5-year observed survival and relative survival of NPC patients increased during last 3 decades regardless of clinical stage. In addition, the improvement of survival was more remarkable in the patients with advanced-stage NPC.
Since the highest incidence and mortality, specific concern was given on NPC in Sihui Cancer Registry and its death report system there, resulting in more accurate diagnosis and higher percentage of cases with pathologic confirmation. The follow-up system of reported cancer incidence cases is established only for NPC. Because of the incompleteness of case report and the unsatisfied accuracy of diagnosis in the first three years of the cancer registry, we excluded these data and limited our study from the year 1976 to 2005. Also, to avoid the influence of other cancers on survival rates, we only included patients without any previous cancer diagnoses recorded in the cancer registry.
In this study, we report both the observed survival and the relative survival of NPC patients. The observed survival is of interest in clinical practice, but for comparing time periods, relative rates are more accurate because they take into account the general mortality change in the Sihui population.
The staging system for NPC has been changed several times over the decades[10] and the proportion of stage was incomparable between calendar period. However, we found no significant change in the proportion of stages among NPC patients in Sihui, despite large-scale screening being conducted in the study area and marked improvement in diagnostic skills. Stage migration is often the result of technological advancement that enables early detection of a metastatic tumor. In the earlier years, patients would have been clinically classified as having localized cancer or cancer with regional spread. With improved technology, patients are now diagnosed as having metastatic disease. The technological advancement and metabolic standard of staging may explain the stable proportion of stages during the study period.
The results from our study are inconsistent with findings from a population-based study published in 1998, which showed no significant change in survival in nine European countries over the much shorter period of 1978–1989[11]. One potential explanation is that the observed time is not long enough to allow calendar factors to emerge. In a Scotland-based study[12], survival was higher in the most affluent quintile of patients than in the poorest quintile of patients. Socioeconomic factors may have more or less impact to the improvements of survival. Furthermore, in that study, the 5-year survival in the age group of 15–44 years old in a recent period was almost double that in the period in 1980s. The 5-year survival rate is 84% in the period 1995–2001 whereas 46% in the period 1975–1979. This observation is strikingly similar to what we observed—that the 5-year relative survival in 0- to 40-year old group was 84.5% in 2000–2005 whereas 46.3% in 1976–1985 (data not shown). This strong resemblance between two different populations in relative survival indicated that there may be a potential predictor of NPC prognosis that changed over the past three decades regardless of the area and background mortality. Interestingly, the age-specific incidence of NPC remained relatively stable in Sihui during two recent decades. A study in a high-risk population in southern China (Zhongshan city) showed that the 5-year observed survival was 33.7% in 1975–1979 and 58.0% after the 1990s[13]. Likewise, we determined the 5-year overall survival to be 42.5% in 1976–1985 and 52.1% in 1993–1999 in our study. Similar survival trends have also been reported in other high-risk areas in China like Changle and Qidong[14],[15], where there are complete cancer registry systems.
Previous studies showed that early discovery of NPC in high-risk families is an important predictor of long-term disease-free survival[16],[17]. Furthermore, largescale screening programs for Epstein-Barr virus have been in place in Sihui since the mid-1980s. Although the distribution of stages was not an apparent variable over the time course of this study, it remains to be evaluated whether screening programs and early detection of NPC have an impact on survival among the general high-risk population.
In conclusion, our study in an NPC high-risk population during a period of nearly three decades revealed that the prognosis of NPC is alterative. Although incidence remained stable during these recent decades, the prognosis of NPC patients has significantly improved for different stages and pathologies. Although the factors responsible for the improved survival and stable incidence are unknown, there are several possibilities. Improvements to radiotherapy during the mid-1990s, including use of a linear accelerator, application of a three-dimensional conformal technique, and development of intensity-modulated radiotherapy, may have contributed to the survival trends seen in NPC patients. Furthermore, the alarm over NPC in Sihui, fueled by large-scale propaganda and the repeated screening program, may have prompted individuals with symptoms and signs of NPC to visit the doctor earlier. Although there was no apparent change in the proportion of stages in our study, early diagnosis has a definite impact on the prognosis of NPC.
Further population-based studies of survival in NPC including detailed data of treatment, socioeconomic factors, and screening program information may provide more evidence of the factors that contribute to improved NPC prognosis. Thus, these studies can identify and evaluate therapeutic strategies and the effectiveness of screening programs, and may find different features of NPC survival in various high-risk area populations.
Acknowledgments
This study was supported by a grant from the 11th National Science and Technology Support Program of China (No. 2006BA102A11).
References
- 1.Lee AW, Foo W, Mang O, et al. et al. Changing epidemiology of nasopharyngeal carcinoma in Hong Kong over a 20-year period (1980-99): an encouraging reduction in both incidence and mortality. Int J Cancer. 2003;103:680–685. doi: 10.1002/ijc.10894. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Chia KC, Chia SE, et al. et al. Secular trends of nasopharyngeal carcinoma incidence in Singapore, Hong Kong and Los Angeles Chinese population, 1973–1997. Eur J Epidemiol. 2007;22:513–521. doi: 10.1007/s10654-007-9148-8. [DOI] [PubMed] [Google Scholar]
- 3.Hsu C, Shen YC, Cheng CC, et al. et al. Difference in the trend of nasopharyngeal and oropharyngeal carcinoma in Taiwan implication from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:856–861. doi: 10.1158/1055-9965.EPI-05-0821. [DOI] [PubMed] [Google Scholar]
- 4.Jia WH, Huang QH, Liao J, et al. et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20–25 year period (1978/1983–2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei KR, Yu YL, Yang YY, et al. et al. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev. 2010;11:29–32. [PubMed] [Google Scholar]
- 6.Cox DR. Regression models and life tables (with discussion) J Royal Statist Society-B. 1972;34:187–220. [Google Scholar]
- 7.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 8.Hakulinen T. On long-term relative survival rates. J Chronic Dis. 1977;30:431–443. doi: 10.1016/0021-9681(77)90036-4. [DOI] [PubMed] [Google Scholar]
- 9.Voutilainen ET, Dickman PW, Hakulinen T. SURV3: relative survival analysis program, version 3.01. In Helsinki: Finish Cancer Registry. :2002. [Google Scholar]
- 10.Mould RF, Tai TH. Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. Br J Radiol. 2002;75:307–339. doi: 10.1259/bjr.75.892.750307. [DOI] [PubMed] [Google Scholar]
- 11.Jiong L, Berrino F, Coebergh JW. Variation in survival for adults with nasopharyngeal cancer in Europe, 1978–1989. Eur J Cancer. 1998;34:2162–2166. doi: 10.1016/s0959-8049(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 12.Anandan C, Elton R, Hitchings A, et al. et al. Nasopharyngeal cancer incidence and survival in Scotland, 1975–2001. Clin Otolaryngol. 2008;33:12–17. doi: 10.1111/j.1749-4486.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 13.Wei K, Liu X, Liang Z. Survival rate of nasopharyngeal carcinoma (NPC) in all population of Zhongshan in 1970–1994. Zhong Liu Fang Zhi Yan Jiu. 2003;30:157–159. [in Chinese] [Google Scholar]
- 14.Chen J, Zhu J, Zhang Y. An analysis of survival in major malignancies during 1972–2000 in Qidong, China. Zhongguo Zhong Liu. 2006;15:575–578. [in Chinese] [Google Scholar]
- 15.Xiao JR, Chen JS, Zhou Y, et al. Survival analysis of 10409 cases with malignant cancers during 1989 to 1998 in Changle City. Zhongguo Man Xing Bing Yu Fang Yu Kong Zhi. 2005;13:225–227. [in Chinese] [Google Scholar]
- 16.Ng WT, Choi CW, Lee MC, et al. et al. Familial nasopharyngeal carcinoma in Hong Kong: epidemiology and implication in screening. Fam Cancer. 2009;8:103–108. doi: 10.1007/s10689-008-9213-9. [DOI] [PubMed] [Google Scholar]
- 17.Ng WT, Choi CW, Lee MC, et al. et al. Outcomes of nasopharyngeal carcinoma screening for high risk family members in Hong Kong. Fam Cancer. 2010;9:221–228. doi: 10.1007/s10689-009-9296-y. [DOI] [PubMed] [Google Scholar]