Abstract
Pancreatic neuroendocrine tumors (PNETs), a group of endocrine tumors arising in the pancreas, are among the most common neuroendocrine tumors. The genetic causes of familial and sporadic PNETs are somewhat understood, but their molecular pathogenesis remains unknown. Most PNETs are indolent but have malignant potential. The biological behavior of an individual PNET is unpredictable; higher tumor grade, lymph node and liver metastasis, and larger tumor size generally indicate a less favorable prognosis. Endocrine testing, imaging, and histological evidence are necessary to accurately diagnose PNETs. A 4-pronged aggressive treatment approach consisting of surgery, locoregional therapy, systemic therapy, and complication control has become popular in academic centers around the world. The optimal application of the multiple systemic therapeutic modalities is under development; efficacy, safety, availability, and cost should be considered when treating a specific patient. The clinical presentation, diagnosis, and treatment of specific types of PNETs and familial PNET syndromes, including the novel Mahvash disease, are summarized.
Keywords: Pancreatic neuroendocrine tumors, insulinoma, gastrinoma, VIPoma, glucagonoma, non-functioning, multiple endocrine neoplasia syndrome type 1, Mahvash disease
Neuroendocrine tumors are neoplasms that exhibit neuroendocrine phenotypes such as the production of neuropeptides, large dense-core secretory vesicles, and a lack of neural structures[1]–[3]. Pancreatic neuroendocrine tumors (PNETs), a group of endocrine tumors arising in the pancreas, are among the most common neuroendocrine tumors (NETs)[4]. Functioning PNETs include insulinoma, gastrinoma, VIPoma, glucagonoma, and others that produce specific hormonal hypersecretion syndromes. Non-functioning PNETs comprise the largest group of PNETs and do not produce syndromes of hormonal excess; rather, they cause morbidity and mortality by invading normal tissue and metastasizing[1]–[3]. There are no clear differences in the epidemiology of PNETs based on race, sex, geographic area, or socioeconomic status[4]. A conservative estimate of the prevalence of PNETs is approximately 25–30 per 100,000 population in the United States, and the incidence of PNETs is increasing due to improvements in diagnosis and case finding. The behaviors of these tumors are highly variable and range from nearly benign to extremely aggressive, but the majority of PNETs are moderately malignant[1]–[3]. In this review, we summarize the commonalities of PNET biology, diagnosis, and treatment and briefly discuss specific PNETs and PNET-associated syndromes.
Pathogenesis
The pathogenesis of PNETs is largely unknown but is growing as a research topic[5],[6]. Approximately 10% of all PNETs are components of familial endocrine tumor syndromes such as multiple endocrine neoplasia syndrome type 1 (MEN1), von Hippel-Lindau disease (VHL), neurofibromatosis type 1 (NF-1), and tuberous sclerosis (TSC)[5],[6]. The etiology of PNETs within the context of these familial syndromes is the inherited germline loss of the respective tumor suppressor gene. For example, MEN1 is caused by inactivating mutations of the MEN1 gene. For a more detailed discussion, please refer to the section “Endocrine Tumor Syndromes” of this paper. The exact pathogenetic mechanism that leads to PNET tumorigenesis in these syndromes, however, is unclear. Several studies have been performed on the pathogenesis of sporadic PNETs, which comprise 90% of all PNETs. Loss of chromosome 1, 3p, 6q, 11q, 17p, or 22q and gains of chromosome 4 or 9q have been observed in PNETs[7]–[11]. It is generally assumed that the loss of a tumor suppressor gene or the gain of an Oncogene is the mechanism by which chromosomal alterations cause PNETs, but stochastic chromosomal number changes are also possible. A few genes that regulate cell proliferation have also been studied. Inhibitors of cell proliferation, including the tumor suppressor genes pRB and p53, and the cyclin-dependent kinase inhibitor (CKI) p16INK4a are usually intact in well-differentiated PNETs, but p53 abnormalities are common in poorly differentiated PNETs [12]–[17]. The Oncogene CCND1 (cyclin D1) is often up-regulated in PNETs, but the ras family oncogenes are not usually detected[14],[18]. Recently, an exome study of apparently sporadic PNETs from 68 patients demonstrated that 44% of those tumors harbored mutations in the MEN1 gene (the same gene, if inactivated, causes MEN1), 43% harbored mutations in two subunits of a transcription/chromatin remodeling complex [death domain-associated protein (DAXX) and thalassemia/mental retardation syndrome X-linked (ATRX)], and 14% harbored mutations in the mammalian target of rapamycin (mTOR) pathway[19].
Just as the cellular origin of PNETs is unknown[20],[21], it is unclear whether PNETs arise from precursor lesions[22],[23]. Diffuse endocrine cell hyperplasia, dysplasia, and microadenoma are present in the pancreas of patients with MEN1 and VHL and are considered to be precursor lesions[24],[25]. The hyperplastic pancreatic endocrine cells in patients with MEN1 and in heterozygous MEN1 mutant mice are polyclonal and retain the normal menin allele, indicating that the deletion of one copy of MEN1 results in pancreatic endocrine cell proliferation without tumorigenesis[24],[25]. Loss of heterozygosity (LOH) at the MEN1 locus is present in adenomas as small as 0.3 mm in diameter, demonstrating that these microadenomas are true tumors according to Knudson's two-hit hypothesis of tumor development[26]. Interestingly, the exact pattern of LOH differs between microadenomas, suggesting that these microadenomas arise independently from the hyperplastic background[21],[27]–[29]. Because few clinical adenomas develop but numerous microadenomas are observed, additional mutations must accrue to form larger and clinically significant PNETs. Similar precursor lesions in sporadic PNETs have not been reported. Endocrine hyperplasia, dysplasia, and microadenoma, however, are relatively common incidental pathologic findings in the pancreas; if carefully screened during autopsy, up to 10% of adults harbor these lesions[30]. It is not known whether these lesions are monoclonal. Although most of these lesions are not likely clinically significant, they could represent precursor lesions that give rise to sporadic PNETs because all clinical PNETs have to pass through a microadenoma stage during their growth[29]. It is therefore plausible that PNETs develop from precursor (pre-malignant) lesions, such as hyperplasia and microadenoma, in familial PNET syndromes and in some sporadic cases[29].
Natural History
Most PNETs are indolent but have malignant potential[1]–[3]. The true natural history of PNETs has not been studied systemically. PNETs in MEN1 grow slowly and remain stable for years, at least when they are small [31],[32]. Whether the natural history of PNETs in MEN1 is representative of that of sporadic PNETs is unknown. If untreated, most PNETs grow and eventually metastasize to the liver; extensive liver metastasis is the most common cause of death for patients with PNETs[33],[34]. The biological behavior of an individual PNET is unpredictable; a higher tumor grade, lymph node and liver metastasis, and a larger primary tumor generally portend a less favorable prognosis[35],[36]. Molecular markers that predict PNET behavior are necessary to improve clinical decision-making. Most patients survive many years after diagnosis.
PNETs are classified as functioning or non-functioning depending on whether they cause hormonal hypersecretion syndrome[1]–[3]. Functioning PNETs result in hormonal hypersecretion syndromes, as elaborated below (Table 1). Non-functioning PNETs cause nonspecific symptoms, such as vague abdominal pain, and can be an incidental finding. The distinction between functioning and non-functioning PNETs is based on clinical presentation, and there is no absolute difference in hormone expression between functioning and non-functioning PNETs. For example, the tumor cells in a small PNET may express glucagon, resulting in a borderline elevation of glucagon levels; clinically, however, this PNET is classified as non-functioning because the slight elevation of glucagon is not sufficient to cause glucagonoma syndrome. Through immunochemical examination, most PNETs express numerous hormones. Some hormones are expressed in normal islets (e.g., insulin, glucagon, somatostatin, and pancreatic polypeptide), and others [e.g., gastrin, vasoactive intestinal peptide (VIP), serotonin, adrenocorticotropin (ACTH), corticotropin-releasing hormon (CRH), parathyroid hormone related peptide (PTHrp), parathyroid hormone (PTH), growth hormone-releasing hormone (GHRH), growth hormone (GH), calcitonin, ghrelin, human choriogonadotropin (hCG), and renin] that are not normally expressed in the islets are expressed by PNETs[37]–[49]. The expressed hormones may or may not be secreted or biologically active, and the hormone expression profile of each tumor can change over time. It is common that an initially non-functioning PNET later triggers hormonal hypersecretion syndrome[38],[40],[48].
Table 1. Functioning pancreatic neuroendocrine tumor (PNET) syndromes.
| Tumor | Symptom(s) |
| Insulinoma | Hypoglycemia |
| Gastrinoma | Severe peptic ulceration |
| VIPoma | Watery diarrhea, hypokalemia, achlorhydria (WDHA syndrome) |
| Glucagonoma | Glucose intolerance, necrolytic migratory erythema, stomatitis/glossitis, hypoaminoacidemia |
| Somatostatinoma | Hyperglycemia, cholelithiasis, steatorrhea, achlorhydria |
Diagnostic Approach
Because the recognition of hormonal hypersecretion syndrome requires considerable clinical experience and the symptoms of non-functioning PNETs are nonspecific, the diagnosis of PNET is often delayed[1]–[3]. Endocrine testing, imaging, and histological evidence are all required to accurately diagnose PNETs. A complete diagnosis should establish the PNET nature, assess the tumor grade, identify the primary and metastatic loci, and determine whether the tumor is functioning. If hormonal hypersecretion syndrome is suspected, appropriate biochemical testing is performed to determine hormonal hypersecretion and followed by imaging, endoscopy, and biopsy. If pancreatic or liver masses are incidentally identified by imaging, they are usually biopsied to confirm the presence of a NET. Biochemical testing should ideally be performed even if hormonal hypersecretion syndrome is not evident because it could be at the subclinical stage, and the hypersecreted hormones can be used as tumor markers during follow-up evaluations. Chromogranin A (CGA), neuron-specific enolase (NSE), and pancreastatin are the most useful PNET markers[50]–[52]. Fasting levels of pancreatic polypeptide (PP), gastrin, proinsulin, insulin, glucagon, and vasoactive intestinal peptide (VIP) are worth measuring because they are the hormones most frequently produced by functioning PNETs (Table 1). False positive results are common, especially for CGA, because it is often elevated in patients taking anti-acids or in those with atrophic gastritis[53],[54]. Anatomical computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and pelvis is important to evaluate the pancreatic, liver, lymph node, and peritoneal metastases[55],[56]. Nuclear imaging with octreotide should be performed at least once to determine if the tumors have a high affinity for somatostatin and if there are occult tumors not detected by anatomical imaging[57],[58]. FDG-PET is not usually indicated because most PNETs are negative; however, FDG-PET can ascertain the overall tumor burden in high-grade PNETs[59],[60]. Recently, PET with gallium 68–labeled octreotide has been demonstrated to be extremely sensitive at detecting small and extra-hepatic PNET metastases but is not widely available[61],[62]. Liver masses are typically biopsied transcutaneously with ultrasound or CT guidance, and pancreatic masses are biopsied with endoscopic ultrasound guidance [63],[64]. Tumor biopsy is critical for PNET diagnosis, not only to demonstrate the neuroendocrine nature of the tumor but also to preliminarily grade the tumor and to perform immunocytochemical staining for hormones and islet markers, which is useful for determining the pancreatic origin of liver metastases[65],[66]. Currently, the best predictor of PNET behavior is tumor grade; therefore, the cytologic examination of the biopsied tumor sample should classify the tumor as a well-differentiated endocrine tumor (low grade of malignancy), a well-differentiated endocrine carcinoma (intermediate grade), or a poorly differentiated endocrine carcinoma (high grade)[67]–[69].
Treatment Strategy
Because most PNETs are indolent, a “wait-and-see” approach has historically predominated. The treatment strategy for PNETs has undergone a paradigm shift in the last 10–20 years[70]. An “aggressive” approach has become popular in academic centers throughout the world[71]. The aggressive approach is based on the reasonable assumptions that patients benefit from reducing the tumor burden and that interventions are increasingly safer in academic centers. Therefore, the aggressive approach advocates removing as much of the primary and metastatic tumors as possible. Although no prospective randomized trials have been performed to study the efficacy and safety of the aggressive approach, most experts on PNETs agree that it is theoretically superior to the “wait-and-see” method, and experience is confirming this[70],[72],[73]. The aggressive approach is only possible when a local expert team is available to design and implement a comprehensive treatment plan[74],[75]. The leaders of the team can be from any specialty, but the team has to include members from endocrinology, oncology, gastroenterology, diagnostic radiology, nuclear medicine, pathology, surgery, and Interventional radiology. For this reason, it is in the best interest the patients with PNETs to be referred to academic centers to undergo treatment from such expert teams.
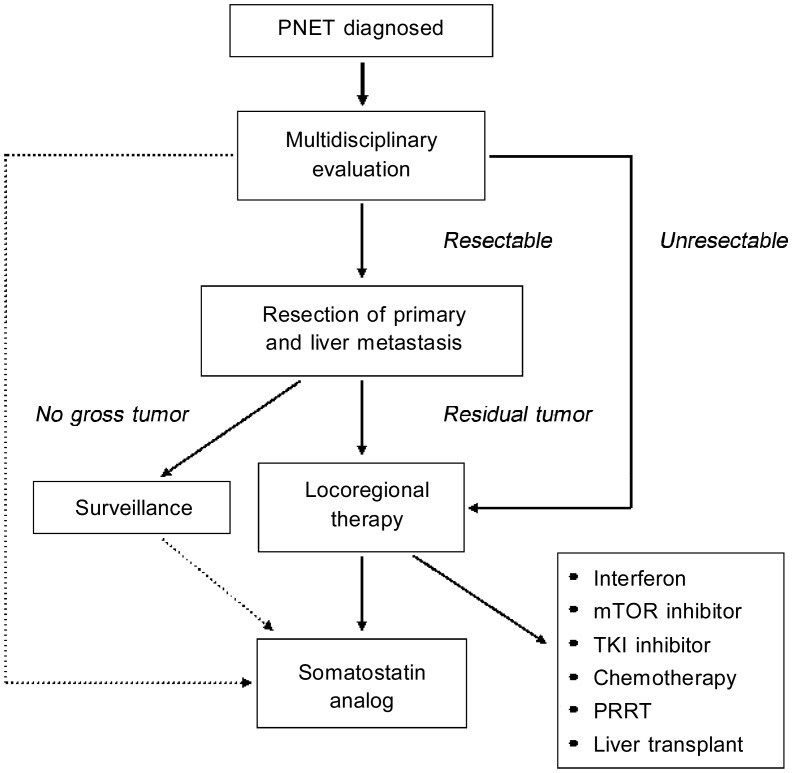
The aggressive approach has 4 components: surgery, locoregional therapy, systemic therapy, and complication control[71]. Surgical removal of primary, non-metastatic PNET is the only clinical cure, and surgical debulking of liver PNET metastases reduces the hormone secretion from functioning PNETs and the tumor mass effects of all PNETs[76],[77]. Surgical removal of PNETs requires considerable experience and should be performed in academic centers with an expert team for PNETs. Because surgery has intrinsic operative and anesthetic risks, patient selection is key to achieving the best outcomes. Locoregional therapy of liver metastases is indicated for most patients with liver metastases [78] Radioactive polymer microspheres, chemoembolization, bland embolization, radiofrequency ablation (RFA), transcutaneous alcohol ablation, and microwave ablation have all been successfully used to treat PNET liver metastasis[79]–[84]. Systemic therapy is required for patients with residual disease after surgery and locoregional therapy. Somatostatin analogs are effective against functioning PNETs, especially VIPoma and glucago-noma[85],[86]. There is no clear evidence that somatostatin analogs treat non-functioning PNETs, but they most likely restrain tumor growth[87],[88]. Two somatostatin analogs, octreotide and lanreotide, and their long-acting release forms are currently available, and a new analog, pasireotide, is in clinical trials with encouraging preliminary results[89]. Bile sludge and hyperglycemia are the predominant adverse events, so cholecystectomy and blood glucose monitoring are important for patients taking somatostatin analogs[90]. Because somatostatin analogs have a high benefit/risk ratio, we recommend somatostatin analogs for all PNET patients with a tumor burden. Systemic interferon alpha can be used to treat PNETs that progress on somatostatin analogs[91]. In recent randomized clinical trials, the mTOR inhibitor everolimus (RAD001) and the tyrosine kinase inhibitor sunitinib prolonged progression-free survival (PFS) by approximately 10 months in patients with well-differentiated PNETs, but the best indications for these drugs remain unknown[92],[93]. Chemotherapy is reserved for intermediate- and high-grade PNETs. Temozolomide and capecitabine are oral agents that may be used in patients with rapidly progressing PNETs[94]. Cisplatin and etoposide or 5-fluorouracil and streptozocin are appropriate treatment combinations for patients with high-grade PNETs[95],[96]. Numerous experimental drugs for treatment-resistant PNETs are being tested in clinical trials[97],[98]. Peptide receptor radionuclide therapy (PRRT) is efficient against well-differentiated PNETs but is not widely available, so it is best for patients with large disease burdens that are resistant to other systemic therapies[71],[99],[100]. Liver transplantation is highly controversial for patients with liver-dominant metastatic PNETs[101],[102]. The main concerns are post-transplantation immunosuppression, which often allows for the rapid growth of occult extra-hepatic metastases, and the availability of multiple other systemic treatments. Common PNET complications and treatment side effects include hyperglycemia, malabsorption, vitamin deficiency, and metastasis to the bone, brain, or spinal cord, which can be treated with insulin, diet and pancreatic enzyme replacement, vitamin supplementation, and external beam radiation, respectively [103]–[105]. We provide an algorithm that summarizes the treatment strategies for PNETs (Figure 1).
Figure 1. Algorithm for pancreatic neuroendocrine tumor (PNET) treatment.
Please refer to the text for details. Solid lines indicate a strong recommendation, and dotted lines indicate a weak recommendation.
Specific Types of Pancreatic Neuroendocrine Tumors
Functioning pancreatic neuroendocrine tumors
Insulinoma
Insulinomas are the most common functioning pancreatic endocrine tumors. They are rare (approximately 4 cases per million per year) but are the most common cause of hyperinsulinemic hypoglycemia in adults[106]. It has a slight female predominance, and the median age at diagnosis is in the fifth decade of life[107]. Insulinomas are typically solitary pancreatic lesions that are small (90% are less than 2 cm), well-circumscribed, and equally distributed throughout the pancreas (head, body, and tail). Rarely, insulinomas occur as multiple lesions (8% of the total insulinoma cases); this is usually associated with MEN1. On occasion, beta cell hyperplasia clinically mimics insulinoma[108].
To begin the work-up of a hyperinsulinemic hypoglycemia patient, Whipple's triad must first be established[106]. Patients should have symptoms of hypoglycemia with concomitant low blood glucose levels (< 50 mg/dL). In addition, symptoms should resolve with glucose intake or correction of the low blood glucose levels. Once Whipple's triad is confirmed, insulin levels should be checked. Inappropriately normal or elevated insulin levels in the presence of hypoglycemia are typically diagnostic of insulinoma after other factors such as exogenous insulin or hypoglycemic drugs have been ruled out. If it is difficult to obtain such results in an outpatient setting, a 72-hour fast should be conducted in a hospital setting[109]. Once hypoglycemia symptoms due to a suspected insulinoma are confirmed, the tumor should be localized prior to attempts at surgical resection. Localization of the tumor prior to surgery assists in planning the operation and avoiding extensive exploration. The best imaging modality is based on local experience and availability. CT and MRI identify 30%–66% of insulinomas but are limited in their ability to identify small tumors[106],[107]. Angiography along with calcium stimulation and venous sampling was once considered the gold standard for localizing insulinomas[110]. Recently, endoscopic ultrasound, with a sensitivity of 93% and a specificity of 95%, has become a more common method for preoperatively localizing insulinomas[111]. Intraoperative ultrasounds are also useful in identifying small tumors and may be better than endoscopic ultrasonography (EUS) because there is minimal to no interference of overlying organs or bowel gas[112]. Imaging methods with glucagon-like peptide-1 (GLP-1) receptor targeting have recently been introduced[113]. GLP-1 receptor is highly expressed in insulinomas. GLP-1-like ligands labeled with radioisotope lndium-111 (111ln-DOTA-exendin-4) are infused into the patient, and total body planar images and single photon emission computed tomography (SPECT) in combination with CT scans are performed. In this study, GLP-1 scintigraphy correctly detected the insulinoma in all 6 patients. Surgical removal of insulinoma is the best treatment because it is curative in 85%–95% of patients[106],[107]. Medical management is only appropriate for patients with unresectable or metastatic insulinomas. Traditional medications, including diazoxide and calcium channel blockers such as verapamil, have limited values [114]. Somatostatin analogs and especially mTOR inhibitors have demonstrated dramatic effects in controlling hypoglycemia and reducing tumor burden[115],[116]. Genetic markers such as microsatellite instability and MLH1 gene inactivation may be helpful in predicting insulinoma behavior[117].
Gastrinoma
Gastrinoma is a NET of the pancreas or duodenum that secretes gastrin and gastrin precursors (progastrins) that mimic the action of gastrin secreted by the G cells of the gastric antrum[118]. Gastrinoma syndrome, also known as Zollinger-Ellison syndrome (ZES), is a rare disease (1–3 cases per million per year). It has a slight male predominance and is generally diagnosed in the fifth decade of life[119],[120]. Peptic ulcer is the most common presentation of ZES. Interestingly, diarrhea occurs in up to 75% of patients and is occasionally the chief complaint. Most gastrinomas are sporadic, but in up to 20%–30% of the cases, it is associated with MEN1. Approximately 50%–60% of gastrinomas are in the pancreas, and 40%–50% are in the duodenum[121]. Approximately 60%–90% of gastrinomas are malignant, and up to 50% of patients will have distant liver metastases at the time of diagnosis. The 5-year survival rate for all gastrinoma patients ranges between 62% and 75%.
Gastrinoma is suspected in patients with resistant or multiple peptic ulcers. Biochemical diagnosis of gastrinoma is challenging because gastrin levels increase for many reasons, such as the use of proton pump inhibitors, H. pylori infection, renal failure, and gastric outlet obstruction[122]. Fasting gastrin level detection should be the first laboratory screening test. Normal gastrin levels rule out gastrinoma, and fasting gastrin levels > 500 pg/mL or greater than 5-fold the upper limit of normal suggest gastrinoma. In addition, fasting gastrin levels >1,000 pg/mL are highly suggestive of gastrinoma, especially if the patient has an acidic gastric pH (< 2). Because many other conditions increase gastrin levels, a provocative test must be performed to confirm the diagnosis. The most common provocative tests use secretin or calcium gluconate infusion. Secretin does not stimulate gastrin release from G cells of the stomach but does stimulate gastrin release from gastrinomas. An increase in fasting gastrin level higher than 200 pg/mL after secretin infusion is considered diagnostic[120]. Gastrinoma localization may be challenging. CT/MRI, octreotide scan, and endoscopic ultrasound may all be required to localize the gastrinoma[123],[124].
The treatment of sporadic gastrinomas entails complete surgical resection of the tumor and dissection of the regional lymph nodes[125]–[127]. If the tumor is in the pancreas, enucleation is appropriate, if it is feasible. If not, duodenopancreatectomy with or without preservation of the pylorus is necessary to completely remove the tumor. Even if complete tumor excision cannot be achieved, removing as much tumor as possible can improve the efficacy of postoperative medical therapy. Medical management is warranted for patients who cannot undergo surgical resection, have an incomplete resection, or whose gastrinoma is a component of MEN1, which is often multifocal[128]. Proton pump inhibitors are currently the gold standard therapy[129]. For aggressive tumors and advanced gastrinomas, chemotherapy and other systemic modalities are required (please refer to the section “Treatment Strategy”).
VIPoma
VIPoma is a vasoactive intestinal polypeptide (VIP)–secreting tumor that commonly arises from the gastrointestinal tract [130],[131]. VIPoma syndrome is also known as WDHA syndrome and includes watery diarrhea, hypokalemia, and achlorhydria (Table 1). VIPomas are rare and occur at a rate of 1 per 10 million per year. The median age at diagnosis is in the fifth decade of life, and there is a slight female predominance. Approximately 90% of these tumors are located in the pancreas, mostly in the body or tail. VIPomas are usually solitary (70%–80%) and have a diameter of 1–7 cm. Many tumors are larger than 2 cm at the time of diagnosis, and symptoms typically appear after the tumor reaches a certain size [132]. More often than not, these tumors are metastatic at the time of diagnosis.
The diagnosis of VIPomas requires recognition of the VIPoma syndrome and exclusion of more common causes of chronic diarrhea, such as chronic gastrointestinal infection, inflammatory bowel disease, microscopic colitis, malabsorption syndrome, and laxative abuse[133]. Fasting VIP levels greater than 200 pg/mL are required to confirm the diagnosis. Most patients with VIPomas have much higher VIP levels, sometimes as high as 7,000 pg/mL. One institutional study reported that in their 29-patient cohort, the median VIP level was 632 pg/mL with a mean of 1,209 pg/mL[134]. Because most VIPomas are bulky, localization by CT/MRI, octreotide scan, and endoscopic ultrasound is generally straightforward[132],[134].
The initial treatment of VIPoma entails replacing large fluid losses, correcting electrolyte imbalances, and reversing acidosis[131]. Severe cases may require hospitalization and intravenous fluid and potassium replacement. Somatostatin analogs are particularly useful because they lower VIP levels and directly inhibit diarrhea[85]. Once stabilized, the patient can be considered for surgical management, which is the most definitive treatment for VIPoma[132],[134]. For aggressive tumors and advanced VIPomas, chemotherapy and other systemic modalities are required (please refer to the section “Treatment Strategy”).
Glucagonoma
Glucagonoma is a rare type of functioning PNET, with an estimated incidence of 1 per 20 million per year[135],[136]. Glucagonoma syndrome is characterized by a skin rash known as necrolytic migratory erythema, diabetes mellitus, weight loss, anemia, stomatitis, thromboembolism, gastrointestinal disturbances, and neuropsychiatric symptoms. Glucagonomas vary in size from 2 to 25 cm and predominantly occur in the tail of the pancreas. Most glucagonomas have already metastasized to the liver at the time of diagnosis.
The diagnosis of glucagonoma requires a high index of suspicion. Non-specific elevations in glucagon levels are common under physiologic stress or in carcinoid syndrome, but glucagon levels are usually less than 500 pg/mL (upper limit of normal < 100 pg/mL) [137]. Glucagonoma is associated with a markedly elevated serum glucagon level (> 500 pg/mL, mean ∼1,400 pg/mL), and glucagon levels above 1,000 pg/mL are diagnostic of glucagonoma if the patient has glucagonoma syndrome. In asymptomatic patients with very high glucagon levels, familial hyperglucagonemia and Mahvash disease (see below) are worth considering [138]–[140]. Because most glucagonomas are bulky, localization by CT/MRI, octreotide scan, and endoscopic ultrasound is typically straightforward[135],[136].
Multidisciplinary treatments are particularly important for glucagonomas[135],[136]. Surgical debulking with preoperative nutrition support and routine deep venous thrombosis prophylaxis reduce the tumor burden and glucagon levels in approximately 30% of the patients. Somatostatin analogs are highly effective in inhibiting hormone secretion and improving glucagonoma syndrome [86],[141]. Please refer to the section “Treatment Strategy” for information on the treatment of aggressive and advanced glucagonomas.
Non-functioning pancreatic neuroendocrine tumors
Non-functioning PNETs are clinically defined as PNETs that are not associated with a clear hormonal hypersecretion syndrome. Non-functioning PNETs produce and secrete hormones, but the quantity and the biological activity of these hormones do not produce a distinct syndrome[142]–[144]. Non-functioning PNETs result in non-specific symptoms resulting from tumor mass effects. Sometimes, non-functioning PNETs are discovered incidentally during abdominal imaging for other purposes. In most academic centers in North America and Europe, non-functioning PNETs are more common than functioning PNETs[142]–[144]. Non-functioning PNETs are usually diagnosed in the forth or fifth decades of life and have often already metastasized to the liver at the time of diagnosis.
The diagnosis of non-functioning PNETs ultimately requires a biopsy of the primary pancreatic tumor or the liver metastasis[142],[143]. The measurement of NET markers such as CGA, NSE, and pancreastatin is recommended to confirm the diagnosis and can be used during the follow-up evaluations (please refer to the section “Diagnostic Approach”). Although measuring common hormones secreted by PNETs is not routinely required, it is beneficial in academic centers because apparent non-functioning PNETs can actually be functioning at a subclinical level, and the elevated hormones can be used as tumor markers. CT/MRI, octreotide scan, and endoscopic ultrasound are usually sufficient to estimate the overall tumor burden.
The treatment of non-functioning PNETs follows the general principles for the treatment of PNETs (Figure 1).
Endocrine Tumor Syndromes
Multiple endocrine neoplasia syndrome type 1 (MEN1)
MEN1 is an autosomal dominant genetic disease caused by an inactivating mutation of the tumor suppressor gene MEN1 on chromosome 11q13[145],[146]. The function of the encoded protein, menin, has been extensively studied, but the role of menin in tumor suppression is not entirely clear. Regulating chromatin remodeling may be a key function of menin in preventing tumorigenesis. No genotype-phenotype correlation exists, and patients within the same family often have different presentations of MEN1 lesions. Hyperparathyroidism due to parathyroid hyperplasia, pituitary tumors, and PNETs are components of MEN1[147]. Although only approximately 50% of MEN1 patients harbor gross PNETs, endocrine cell hyperplasia and dysplasia and micro-PNETs are present in all MEN1 patients[22],[24]. Detailed morphologic analyses and tissue microdissection demonstrated that lacking one normal MEN1 allele results in endocrine cell hyperplasia, whereas the loss of the normal MEN1 allele results in endocrine cell dysplasia and micro-PNETs[21]. Additional genetic changes are required for the formation of gross PNETs.
An index MEN1 case is suspected in patients with 2 of the 3 MEN1 lesions[148]. If a patient has a family history of MEN1, the emergence of any MEN1 lesion indicates that the patient is a MEN1 mutation carrier. Patients with known MEN1 mutations should be classified as having MEN1, regardless of whether MEN1 lesions are present. Most PNETs in MEN1 are benign, but metastasis can occur if the tumors are larger than 2–3 cm[147]. Functioning PNETs, such as gastrinoma and insulinoma, and non-functioning PNETs are most common. Patients with a clinical or genetic diagnosis of MEN1 need to undergo life-long biochemical and imaging surveillance to detect MEN1 lesions, including gross PNETs, early[148]. Once detected, the management program for the PNETs should be individualized, balancing the benefits of tumor removal and the risks of pancreatic endocrine and exocrine deficiency[149],[150]. Functioning PNETs as well as PNETs that are larger than 2–3 cm should be surgically removed to prevent significant hormonal hypersecretion symptoms, local tumor mass effects, and metastasis; non-functioning PNETs that are smaller than 2–3 cm can simply be monitored.
Von Hippel-Lindau disease (VHL)
VHL is an autosomal dominant inherited disorder characterized by the development of multiple benign and malignant tumors and cysts[151]. These tumors are located in the central nervous system and visceral organs and commonly present as hemangioblastomas in the central nervous system and retina, pheochromocytoma, renal cell carcinoma or renal cysts, and PNETs or pancreatic cysts. The VHL tumor suppressor gene on chromosome 3p25–26 encodes the VHL protein; inherited inactivating mutations of VHL cause this disease[152],[153]. The loss of function of the VHL protein results in an elevation of non-degraded hypoxia-inducible factor (HIF), which results in increased transcription of vascular epidermal growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-α (TGF-α). This promotes cell growth and the development of microvascular vessels, accelerating the growth of hemangioblastomas, renal cell carcinoma, and other VHL-related tumors.
The most common pancreatic lesions in VHL are single or multiple cysts and serous cystadenomas[25],[154],[155]. They are present in 35%–75% of patients with VHL. Most cystic lesions are benign and asymptomatic. Solid lesions of the pancreas are less common and are usually NETs or microscopic adenomas. The incidence of gross NETs in VHL patients is approximately 17%, and most of those are non-functioning PNETs. Although neuroendocrine substances are secreted, hormonally functioning tumors are rare in VHL. Unlike sporadic non-functioning NETs, which have a high malignant potential (60%–100%), PNETs associated with VHL have a much lower rate of metastatic spread (11%–20%). This is thought to be because sporadic non-functioning NETs do not produce hormonal symptoms and are therefore larger and have already metastasized when they are discovered. In the case of VHL-related PNETs, diagnosis occurs earlier because of the early screening for abdominal manifestations of this disease. PNETs in VHL disease grow slowly; consequently, patients have a relatively good prognosis. However, given the malignant potential of these tumors, partial resection of the pancreas is recommended, especially if the tumor diameter is greater than 1 cm.
Mahvash disease
Mahvash disease is a newly discovered disease caused by homozygous inactivating mutations in the glucagon receptor gene[139],[156]. It is likely an autosomal-recessive inherited disease. Characteristics of Mahvash disease include pancreatic alpha cell hyperplasia, PNETs, and extremely elevated glucagon levels. The glucagonoma syndrome, however, is not present because the defective glucagon receptor (P86S) has a lower affinity to glucagon and produces less cyclic adenosine monophosphate (cAMP) and intracellular calcium[156],[157]. The glucagon receptor-knockout mice exhibit a phenotype that is identical to the patients with Mahvash disease[158]. Long-term observation of the glucagon receptor-knockout mice, a model of Mahvash disease, revealed that these mice continuously develop PNETs and have a shortened life span compared with their wild-type counterparts[159]. Mahvash disease is particularly rare but should be suspected in patients with PNETs and extremely high glucagon levels without glucagonoma syndrome. Patients with Mahvash disease should undergo surgery to remove the gross tumors and perhaps treated with somatostatin analogs to suppress glucagon production[139]. Long-term imaging surveillance is necessary to postoperatively screen for gross PNETs. Molecular studies have demonstrated that the primary mechanism for the defective signaling by the P86S mutant glucagon receptor is abnormal receptor trafficking, which can be rescued by pharmacologic chaperones, providing a targeted therapeutic solution[160].
Summary and Future Perspectives
Pancreatic neuroendocrine tumors (PNETs) are becoming increasingly important both clinically and from a research standpoint. Numerous advances, including the discovery of novel diseases, have been made in recent decades through clinical, basic, and translational research. The knowledge of and experience with PNETs are sufficient so that most patients with PNETs today should expect a satisfactory diagnosis and treatment at academic centers with a multidisciplinary team experienced in PNETs. To increase the understanding of PNETs and to develop more diagnostic and treatment modalities, we advocate that patients should be encouraged to seek opinions from academic centers with a multidisciplinary team experienced in PNETs and to be enrolled in clinical studies. We also suggest that in countries or regions where such centers are lacking, interested physicians should form their own multidisciplinary teams. Clinically, the natural history of sporadic PNETs needs to be addressed directly, and comparative efficacy studies need to be performed to elucidate the best indications for the ever-expanding list of systemic therapeutic modalities. We believe that basic research using animal models and PNET cell lines will yield novel insights into human PNETs.
References
- 1.Rindi G, Bordi C. Highlights of the biology of endocrine tumours of the gut and pancreas. Endocr Relat Cancer. 2003;10:427–436. doi: 10.1677/erc.0.0100427. [DOI] [PubMed] [Google Scholar]
- 2.Massironi S, Sciola V, Peracchi M, et al. et al. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008;14:5377–5384. doi: 10.3748/wjg.14.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehehalt F, Saeger HD, Schmidt CM, et al. et al. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14:456–467. doi: 10.1634/theoncologist.2008-0259. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, et al. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 5.Oberg K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors) Curr Opin Endocrinol Diabetes Obes. 2009;16:72–78. doi: 10.1097/med.0b013e328320d845. [DOI] [PubMed] [Google Scholar]
- 6.Antonello D, Gobbo S, Corbo V, et al. et al. Update on the molecular pathogenesis of pancreatic tumors other than common ductal adenocarcinoma. Pancreatology. 2009;9:25–33. doi: 10.1159/000178872. [DOI] [PubMed] [Google Scholar]
- 7.Speel EJ, Scheidweiler AF, Zhao J, et al. et al. Genetic evidence for early divergence of small functioning and nonfunctioning endocrine pancreatic tumors: gain of 9Q34 is an early event in insulinomas. Cancer Res. 2009;61:5186–5192. [PubMed] [Google Scholar]
- 8.Zhao J, Moch H, Scheidweiler AF, et al. et al. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer. 2001;32:364–372. doi: 10.1002/gcc.1201. [DOI] [PubMed] [Google Scholar]
- 9.Chung DC, Smith AP, Louis DN, et al. et al. A novel pancreatic endocrine tumor suppressor gene locus on chromosome 3p with clinical prognostic implications. J Clin Invest. 1997;100:404–410. doi: 10.1172/JCI119547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wild A, Langer P, Celik I, et al. et al. Chromosome 22q in pancreatic endocrine tumors: identification of a homozygous deletion and potential prognostic associations of allelic deletions. Eur J Endocrinol. 2002;147:507–513. doi: 10.1530/eje.0.1470507. [DOI] [PubMed] [Google Scholar]
- 11.Beghelli S, Pelosi G, Zamboni G, et al. et al. Pancreatic endocrine tumours: evidence for a tumour suppressor pathogenesis and for a tumour suppressor gene on chromosome 17p. J Pathol. 1998;186:41–50. doi: 10.1002/(SICI)1096-9896(199809)186:1<41::AID-PATH172>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Chung DC, Smith AP, Louis DN, et al. et al. Analysis of the retinoblastoma tumour suppressor gene in pancreatic endocrine tumours. Clin Endocrinol. 1997;47:523–528. doi: 10.1046/j.1365-2265.1997.2861110.x. [DOI] [PubMed] [Google Scholar]
- 13.Lohmann DR, Funk A, Niedermeyer HP, et al. et al. Identification of p53 gene mutations in gastrointestinal and pancreatic carcinoids by nonradioisotopic SSCA. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:293–296. doi: 10.1007/BF02915125. [DOI] [PubMed] [Google Scholar]
- 14.Pellegata NS, Sessa F, Renault B, et al. et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–1560. [PubMed] [Google Scholar]
- 15.Lam KY, Lo CY. Role of p53 tumor suppressor gene in pancreatic endocrine tumors of Chinese patients. Am J Gastroenterol. 1998;93:1232–1235. doi: 10.1111/j.1572-0241.1998.401_w.x. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa S, Sessa F, Capella C, et al. et al. Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Archiv. 1996;429:323–333. doi: 10.1007/BF00198436. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch DK, Kersting M, Wild A, et al. et al. Low frequency of p16 (INK4a) alterations in insulinomas. Digestion. 2000;62:171–177. doi: 10.1159/000007810. [DOI] [PubMed] [Google Scholar]
- 18.Chung DC, Brown SB, Graeme-Cook F, et al. et al. Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab. 2000;85:4373–4378. doi: 10.1210/jcem.85.11.6937. [DOI] [PubMed] [Google Scholar]
- 19.Jiao Y, Shi C, Edil BH, et al. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vortmeyer AO, Huang S, Lubensky I, et al. et al. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab. 2004;89:1934–1938. doi: 10.1210/jc.2003-031575. [DOI] [PubMed] [Google Scholar]
- 21.Perren A, Anlauf M, Henopp T, et al. et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab. 2007;92:1118–1128. doi: 10.1210/jc.2006-1944. [DOI] [PubMed] [Google Scholar]
- 22.Anlauf M, Perren A, Kloppel G. Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: criteria, molecular concepts and clinical significance. Pathobiology. 2007;74:279–284. doi: 10.1159/000105810. [DOI] [PubMed] [Google Scholar]
- 23.Kloppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol. 2007;18:150–155. doi: 10.1007/s12022-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 24.Thompson NW, Lloyd RV, Nishiyama RH, et al. et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg. 1984;8:561–574. doi: 10.1007/BF01654938. [DOI] [PubMed] [Google Scholar]
- 25.Lubensky IA, Pack S, Ault D, et al. et al. Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease patients: histopathological and molecular genetic analysis. Am J Pathol. 1998;153:223–231. doi: 10.1016/S0002-9440(10)65563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anlauf M, Perren A, Henopp T, et al. et al. Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions. Gut. 2007;56:637–644. doi: 10.1136/gut.2006.108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabtree JS, Scacheri PC, Ward JM, et al. et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci USA. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol. 2011;8:54–64. doi: 10.1038/nrendo.2011.120. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard DM. Pathogenesis of gastrinomas associated with multiple endocrine neoplasia type 1. Gut. 2007;56:606–607. doi: 10.1136/gut.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933–942. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai A, Katai M, Yamashita K, et al. et al. Long-term follow-up of patients with multiple endocrine neoplasia type 1. Endocr J. 2007;54:295–302. doi: 10.1507/endocrj.k06-147. [DOI] [PubMed] [Google Scholar]
- 32.Davì MV, Boninsegna L, Dalle Carbonare L, et al. et al. Presentation and outcome of pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1 syndrome. Neuroendocrinology. 2011;94:58–65. doi: 10.1159/000326164. [DOI] [PubMed] [Google Scholar]
- 33.Broder LE, Carter SK. Pancreatic islet cell carcinoma. I. Clinical features of 52 patients. Ann Intern Med. 1973;79:101–107. doi: 10.7326/0003-4819-79-1-101. [DOI] [PubMed] [Google Scholar]
- 34.Cubilla AL, Hajdu SI. Islet cell carcinoma of the pancreas. Arch Pathol. 1975;99:204–207. [PubMed] [Google Scholar]
- 35.La Rosa S, Klersy C, Uccella S, et al. et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40:30–40. doi: 10.1016/j.humpath.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt AM, Anlauf M, Rousson V, et al. et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol. 2007;31:1677–1682. doi: 10.1097/PAS.0b013e31805f675d. [DOI] [PubMed] [Google Scholar]
- 37.Mao C, el Attar A, Domenico DR, et al. et al. Carcinoid tumors of the pancreas. Status report based on two cases and review of the world s literature. Int J Pancreatol. 1998;23:153–164. doi: 10.1385/IJGC:23:2:153. [DOI] [PubMed] [Google Scholar]
- 38.Zhu L, Domenico DR, Howard JM. Metastatic pancreatic neuroendocrine carcinoma causing Cushing's syndrome. ACTH secretion by metastases 3 years after resection of nonfunctioning primary cancer. Int J Pancreatol. 1996;19:205–208. doi: 10.1007/BF02787369. [DOI] [PubMed] [Google Scholar]
- 39.Saeger W, Reincke M, Scholz GH, et al. et al. Ectopic ACTH- or CRH-secreting tumors in Cushing's syndrome. Zentralbl Pathol. 1993;139:157–163. [PubMed] [Google Scholar]
- 40.Milanesi A, Yu R, Geller SA, et al. et al. Concurrent primary hyperparathyroidism and humoral hypercalcemia of malignancy in a patient with multiple endocrine neoplasia type 1. Pancreas. 2011;40:634–637. doi: 10.1097/MPA.0b013e318214f65e. [DOI] [PubMed] [Google Scholar]
- 41.Vacher-Coponat H, Opris A, Denizot A, et al. et al. Hypercalcaemia induced by excessive parathyroid hormone secretion in a patient with a neuroendocrine tumour. Nephrol Dial Transplant. 2005;20:2832–2835. doi: 10.1093/ndt/gfi065. [DOI] [PubMed] [Google Scholar]
- 42.Doga M, Bonadonna S, Burattin A, et al. et al. Ectopic secretion of growth hormone-releasing hormone (GHRH) in neuroendocrine tumors: relevant clinical aspects. Ann Oncol. 2001;2:S89–S94. doi: 10.1093/annonc/12.suppl_2.s89. [DOI] [PubMed] [Google Scholar]
- 43.Melmed S, Ezrin C, Kovacs K, et al. et al. Acromegaly due to secretion of growth hormone by an ectopic pancreatic islet-cell tumor. N Engl J Med. 1985;312:9–17. doi: 10.1056/NEJM198501033120103. [DOI] [PubMed] [Google Scholar]
- 44.Machens A, Haedecke J, Hinze R, et al. et al. Hypercalcitoninemia in a sporadic asymptomatic neuroendocrine tumor of the pancreatic tail. Dig Surg. 2000;17:522–524. doi: 10.1159/000051953. [DOI] [PubMed] [Google Scholar]
- 45.Corbetta S, Peracchi M, Cappiello V, et al. et al. Circulating ghrelin levels in patients with pancreatic and gastrointestinal neuroendocrine tumors: identification of one pancreatic ghrelinoma. J Clin Endocrinol Metab. 2003;88:3117–3120. doi: 10.1210/jc.2002-021842. [DOI] [PubMed] [Google Scholar]
- 46.Bidart JM, Baudin E, Troalen F, et al. et al. Eutopic and ectopic production of glycoprotein hormones alpha and beta subunits. Ann Endocrinol (Paris) 1997;58:125–128. [PubMed] [Google Scholar]
- 47.Langer P, Bartsch D, Gerdes B, et al. et al. Renin producing neuroendocrine pancreatic carcinoma—a case report and review of the literature. Exp Clin Endocrinol Diabetes. 2002;110:43–49. doi: 10.1055/s-2002-19994. [DOI] [PubMed] [Google Scholar]
- 48.Miehle K, Tannapfel A, Lamesch P, et al. et al. Pancreatic neuroendocrine tumor with ectopic adrenocorticotropin production upon second recurrence. J Clin Endocrinol Metab. 2004;89:3731–3736. doi: 10.1210/jc.2003-032164. [DOI] [PubMed] [Google Scholar]
- 49.Kaltsas G, Androulakis II, de Herder WW, et al. et al. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr Relat Cancer. 2010;17:R173–R193. doi: 10.1677/ERC-10-0024. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence B, Gustafsson BI, Kidd M, et al. et al. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–134. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62:33–38. doi: 10.1159/000051853. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 52.ODorisio TM, Krutzik SR, Woltering EA, et al. et al. Development of a highly sensitive and specific carboxy-terminal human pancreastatin assay to monitor neuroendocrine tumor behavior. Pancreas. 2010;39:611–616. doi: 10.1097/MPA.0b013e3181c68d7a. [DOI] [PubMed] [Google Scholar]
- 53.Sanduleanu S, De Bruïe A, Stridsberg M, et al. et al. Serum chromogranin A as a screening test for gastric enterochromaffin-like cell hyperplasia during acid-suppressive therapy. Eur J Clin Invest. 2001;31:802–811. doi: 10.1046/j.1365-2362.2001.00890.x. [DOI] [PubMed] [Google Scholar]
- 54.Peracchi M, Gebbia C, Basilisco G, et al. et al. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur J Endocrinol. 2005;152:443–448. doi: 10.1530/eje.1.01862. [DOI] [PubMed] [Google Scholar]
- 55.Raman SP, Hruban RH, Cameron JL, et al. et al. Pancreatic imaging mimics: part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am J Roentgenol. 2012;199:309–318. doi: 10.2214/AJR.12.8627. [DOI] [PubMed] [Google Scholar]
- 56.Debray MP, Geoffroy O, Laissy JP, et al. et al. Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74:1065–1070. doi: 10.1259/bjr.74.887.741065. [DOI] [PubMed] [Google Scholar]
- 57.Krausz Y, Bar-Ziv J, de Jong RB, et al. et al. Somatostatin-receptor scintigraphy in the management of gastroenteropancreatic tumors. Am J Gastroenterol. 1998;93:66–70. doi: 10.1111/j.1572-0241.1998.066_c.x. [DOI] [PubMed] [Google Scholar]
- 58.Shi W, Johnston CF, Buchanan KD, et al. et al. Localization of neuroendocrine tumours with [111In] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM. 1998;91:295–301. doi: 10.1093/qjmed/91.4.295. [DOI] [PubMed] [Google Scholar]
- 59.Adams S, Baum R, Rink T, et al. et al. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 60.Ambrosini V, Tomassetti P, Franchi R, et al. et al. Imaging of NETs with PET radiopharmaceuticals. Q J Nucl Med Mol Imaging. 2010;54:16–23. [PubMed] [Google Scholar]
- 61.Krausz Y, Freedman N, Rubinstein R, et al. et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan) Mol Imaging Biol. 2011;13:583–593. doi: 10.1007/s11307-010-0374-1. [DOI] [PubMed] [Google Scholar]
- 62.Hofman MS, Kong G, Neels OC, et al. et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Naran S, Lallu S, et al. et al. Fine needle aspiration diagnosis of neuroendocrine tumors in the liver. Pathology. 2000;32:16–20. doi: 10.1080/003130200104501. [DOI] [PubMed] [Google Scholar]
- 64.Atiq M, Bhutani MS, Bektas M, et al. et al. EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience. Dig Dis Sci. 2012;57:791–800. doi: 10.1007/s10620-011-1912-7. [DOI] [PubMed] [Google Scholar]
- 65.Collins BT, Cramer HM. Fine-needle aspiration cytology of islet cell tumors. Diagn Cytopathol. 1996;15:37–45. doi: 10.1002/(SICI)1097-0339(199607)15:1<37::AID-DC8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 66.Haynes CM, Sangoi AR, Pai RK. PAX8 is expressed in pancreatic well-differentiated neuroendocrine tumors and in extrapancreatic poorly differentiated neuroendocrine carcinomas in fine-needle aspiration biopsy specimens. Cancer Cytopathol. 2011;119:193–201. doi: 10.1002/cncy.20136. [DOI] [PubMed] [Google Scholar]
- 67.Verbeke CS. Endocrine tumours of the pancreas. Histopathology. 2010;56:669–682. doi: 10.1111/j.1365-2559.2010.03490.x. [DOI] [PubMed] [Google Scholar]
- 68.Klöpel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18:S1–S16. doi: 10.1530/ERC-11-0013. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 69.Kaklamanos M, Karoumpalis I, Salla C, et al. et al. Diagnostic accuracy and clinical significance of the fine needle aspiration Ki-67 labelling index in pancreatic endocrine tumours. Endocr Relat Cancer. 2011;18:L1–L3. doi: 10.1530/ERC-10-0191. [DOI] [PubMed] [Google Scholar]
- 70.Kvols LK, Revisiting CG. Moertel's land of small tumors. J Clin Oncol. 2008;26:5005–5007. doi: 10.1200/JCO.2008.19.2161. [DOI] [PubMed] [Google Scholar]
- 71.Yu R. Radiotherapy: radioactive somatostatin analog therapy against carcinoids. Nat Rev Endocrinol. 2010;6:428–430. doi: 10.1038/nrendo.2010.94. [DOI] [PubMed] [Google Scholar]
- 72.Ong SL, Garcea G, Pollard CA, et al. et al. A fuller understanding of pancreatic neuroendocrine tumours combined with aggressive management improves outcome. Pancreatology. 2009;9:583–600. doi: 10.1159/000212085. [DOI] [PubMed] [Google Scholar]
- 73.Konstantinidis IT, Dursun A, Zheng H, et al. et al. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749–753. doi: 10.1016/j.jamcollsurg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Herder WW, Mazzaferro V, Tavecchio L, et al. et al. Multidisciplinary approach for the treatment of neuroendocrine tumors. Tumori. 2010;96:833–846. doi: 10.1177/030089161009600537. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchikawa T, Hirano S, Tanaka E, et al. et al. Multidisciplinary treatment strategy for advanced pancreatic neuroendocrine tumors—a single center experience. Hepatogastroenterology. 2012;59:2623–2626. doi: 10.5754/hge12116. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen SQ, Angel LP, Divino CM, et al. et al. Surgery in malignant pancreatic neuroendocrine tumors. J Surg Oncol. 2007;96:397–403. doi: 10.1002/jso.20824. [DOI] [PubMed] [Google Scholar]
- 77.Fendrich V, Waldmann J, Bartsch DK, et al. et al. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol. 2009;6:419–428. doi: 10.1038/nrclinonc.2009.82. [DOI] [PubMed] [Google Scholar]
- 78.Proye C. Natural history of liver metastasis of gastroentero-pancreatic neuroendocrine tumors: place for chemoembolization. World J Surg. 2001;25:685–688. doi: 10.1007/s00268-001-0013-8. [DOI] [PubMed] [Google Scholar]
- 79.Kennedy AS, Dezarn WA, McNeillie P, et al. et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 80.Gupta S, Johnson MM, Murthy R, et al. et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 81.Strosberg JR, Choi J, Cantor AB, et al. et al. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 82.Mazzaglia PJ, Berber E, Milas M, et al. et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 83.Atwell TD, Charboneau JW, Que FG, et al. et al. Treatment of neuroendocrine cancer metastatic to the liver: the role of ablative techniques. Cardiovasc Intervent Radiol. 2005;28:409–221. doi: 10.1007/s00270-004-4082-6. [DOI] [PubMed] [Google Scholar]
- 84.Frilling A, Sotiropoulos GC, Li J, et al. et al. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361–379. doi: 10.1111/j.1477-2574.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ODorisio TM, Gaginella TS, Mekhjian HS, et al. et al. Somatostatin and analogues in the treatment of VIPoma. Ann N Y Acad Sci. 1988;527:528–535. doi: 10.1111/j.1749-6632.1988.tb27006.x. [DOI] [PubMed] [Google Scholar]
- 86.Maton PN, Gardner JD, Jensen RT. Use of long-acting somatostatin analog SMS 201–995 in patients with pancreatic islet cell tumors. Dig Dis Sci. 1989;34:28S–39S. doi: 10.1007/BF01536043. [DOI] [PubMed] [Google Scholar]
- 87.Lamberts SW. Somatostatin analogs in the management of gastrointestinal tumors. Horm Res. 1988;29:118–120. doi: 10.1159/000180985. [DOI] [PubMed] [Google Scholar]
- 88.Sidéris L, Dubé P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist. 2012;17:747–755. doi: 10.1634/theoncologist.2011-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kvols L, Oberg KE, ODorisio T, et al. et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a Phase II study. Endocr Relat Cancer. 2012;19:657–666. doi: 10.1530/ERC-11-0367. [DOI] [PubMed] [Google Scholar]
- 90.Eriksson B, Oberg K. Summing up 15 years of somatostatin analog therapy in neuroendocrine tumors: future outlook. Ann Oncol. 1999;10:S31–S38. doi: 10.1093/annonc/10.suppl_2.s31. Suppl. 2. [DOI] [PubMed] [Google Scholar]
- 91.Fazio N, de Braud F, Delle Fave G, et al. et al. Interferon-alpha and somatostatin analog in patients with gastroenteropancreatic neuroendocrine carcinoma: single agent or combination? Ann Oncol. 2007;18:13–19. doi: 10.1093/annonc/mdl144. [DOI] [PubMed] [Google Scholar]
- 92.Yao JC, Shah MH, Ito T, et al. et al. RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raymond E, Dahan L, Raoul JL, et al. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 94.Strosberg JR, Fine RL, Choi J, et al. et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwasa S, Morizane C, Okusaka T, et al. et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40:313–318. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- 96.Turner NC, Strauss SJ, Sarker D, et al. et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106–1112. doi: 10.1038/sj.bjc.6605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pavel ME, Wiedenmann B. Novel therapeutic agents for the treatment of gastroenteropancreatic neuroendocrine tumors. Horm Metab Res. 2011;43:844–853. doi: 10.1055/s-0031-1291368. [DOI] [PubMed] [Google Scholar]
- 98.Oberstein PE, Saif MW. Update on novel therapies for pancreatic neuroendocrine tumors. JOP. 2012;13:372–375. doi: 10.6092/1590-8577/964. [DOI] [PubMed] [Google Scholar]
- 99.Kwekkeboom DJ, de Herder WW, Kam BL, et al. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 100.Bushnell DL, Jr, ODorisio TM, ODorisio MS, et al. et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation. 1998;66:1307–1312. doi: 10.1097/00007890-199811270-00007. [DOI] [PubMed] [Google Scholar]
- 102.Gulati AP, Saif MW. Is there a role for liver transplantation in metastatic pancreatic neuroendocrine tumors (PNET)? JOP. 2012;13:320–321. [PubMed] [Google Scholar]
- 103.Lillemoe KD, Kaushal S, Cameron JL, et al. et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–700. doi: 10.1097/00000658-199905000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crucitti F, Doglietto G, Bellantone R, et al. et al. Digestive and nutritional consequences of pancreatic resections. The classical vs the pylorus-sparing procedure. Int J Pancreatol. 1995;17:37–45. doi: 10.1007/BF02788357. [DOI] [PubMed] [Google Scholar]
- 105.Kim SJ, Kim JW, Han SW, et al. et al. Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: tumor grade and metastatic site are important for treatment strategy. BMC Cancer. 2010;10:448. doi: 10.1186/1471-2407-10-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–798. doi: 10.1016/j.bpg.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 107.Zhao YP, Zhan HX, Zhang TP, et al. et al. Surgical management of patients with insulinomas: result of 292 cases in a single institution. J Surg Oncol. 2011;103:169–174. doi: 10.1002/jso.21773. [DOI] [PubMed] [Google Scholar]
- 108.Service FJ, Natt N, Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84:1582–1589. doi: 10.1210/jcem.84.5.5645. [DOI] [PubMed] [Google Scholar]
- 109.Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab. 2000;85:3973–3974. doi: 10.1210/jcem.85.11.6934. [DOI] [PubMed] [Google Scholar]
- 110.Guettier JM, Kam A, Chang R, et al. et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94:1074–1080. doi: 10.1210/jc.2008-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sotoudehmanesh R, Hedayat A, Shirazian N, et al. et al. Endoscopic ultrasonography (EUS) in the localization of insulinoma. Endocrine. 2007;31:238–241. doi: 10.1007/s12020-007-0045-4. [DOI] [PubMed] [Google Scholar]
- 112.Wong M, Isa SH, Zahiah M, et al. et al. Intraoperative ultrasound with palpation is still superior to intra-arterial calcium stimulation test in localising insulinoma. World J Surg. 2007;31:586–592. doi: 10.1007/s00268-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 113.Christ E, Wild D, Forrer F, et al. et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–4405. doi: 10.1210/jc.2009-1082. [DOI] [PubMed] [Google Scholar]
- 114.Hirshberg B, Cochran C, Skarulis MC, et al. et al. Malignant insulinoma: spectrum of unusual clinical features. Cancer. 2005;104:264–272. doi: 10.1002/cncr.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bourcier ME, Sherrod A, DiGuardo M, et al. et al. Successful control of intractable hypoglycemia using rapamycin in an 86-year-old man with a pancreatic insulin-secreting islet cell tumor and metastases. J Clin Endocrinol Metab. 2009;94:3157–3162. doi: 10.1210/jc.2009-0788. [DOI] [PubMed] [Google Scholar]
- 116.Ong GS, Henley DE, Hurley D, et al. et al. Therapies for the medical management of persistent hypoglycaemia in two cases of inoperable malignant insulinoma. Eur J Endocrinol. 2010;162:1001–1008. doi: 10.1530/EJE-09-1010. [DOI] [PubMed] [Google Scholar]
- 117.Mei M, Deng D, Liu TH, et al. et al. Clinical implications of microsatellite instability and MLH1 gene inactivation in sporadic insulinomas. J Clin Endocrinol Metab. 2009;94:3448–3457. doi: 10.1210/jc.2009-0173. [DOI] [PubMed] [Google Scholar]
- 118.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6:454–463. doi: 10.1007/s11894-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 119.Cameron AJ, Hoffman HN., 2nd Zollinger-Ellison syndrome. Clinical features and long-term follow-up. Mayo Clin Proc. 1974;49:44–51. [PubMed] [Google Scholar]
- 120.Berna MJ, Hoffmann KM, Long SH, et al. et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stabile BE, Morrow DJ, Passaro E., Jr The gastrinoma triangle: operative implications. Am J Surg. 1984;147:25–31. doi: 10.1016/0002-9610(84)90029-1. [DOI] [PubMed] [Google Scholar]
- 122.Berna MJ, Hoffmann KM, Serrano J, et al. et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prinz RA. Localization of gastrinomas. Int J Pancreatol. 1996;19:79–91. doi: 10.1007/BF02805221. [DOI] [PubMed] [Google Scholar]
- 124.Pfannenberg AC, Burkart C, Kröer SM, et al. et al. Dual-phase multidetector thin-section CT in detecting duodenal gastrinoma. Abdom Imaging. 2005;30:543–547. doi: 10.1007/s00261-004-0299-8. [DOI] [PubMed] [Google Scholar]
- 125.Norton JA, Fraker DL, Alexander HR, et al. et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Campana D, Piscitelli L, Mazzotta E, et al. et al. Zollinger-Ellison syndrome. Diagnosis and therapy. Minerva Med. 2005;96:187–206. [PubMed] [Google Scholar]
- 127.Cisco RM, Norton JA. Surgery for gastrinoma. Adv Surg. 2007;41:165–176. doi: 10.1016/j.yasu.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1) Surg Oncol. 2003;12:145–151. doi: 10.1016/s0960-7404(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 129.Lew EA, Pisegna JR, Starr JA, et al. et al. Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 2000;118:696–704. doi: 10.1016/s0016-5085(00)70139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mekhjian HS, ODorisio TM. VIPoma syndrome. Semin Oncol. 1987;14:282–291. [PubMed] [Google Scholar]
- 131.Park SK, ODorisio MS, ODorisio TM. Vasoactive intestinal polypeptide-secreting tumours: biology and therapy. Baillieres Clin Gastroenterol. 1996;10:673–696. doi: 10.1016/s0950-3528(96)90018-4. [DOI] [PubMed] [Google Scholar]
- 132.Peng SY, Li JT, Liu YB, et al. et al. Diagnosis and treatment of VIPoma in China: (case report and 31 cases review) diagnosis and treatment of VIPoma. Pancreas. 2004;28:93–97. doi: 10.1097/00006676-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 133.Koch TR, Michener SR, Go VL. Plasma vasoactive intestinal polypeptide concentration determination in patients with diarrhea. Gastroenterology. 1991;100:99–106. doi: 10.1016/0016-5085(91)90588-c. [DOI] [PubMed] [Google Scholar]
- 134.Ghaferi AA, Chojnacki KA, Long WD, et al. et al. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg. 2008;12:382–393. doi: 10.1007/s11605-007-0177-0. [DOI] [PubMed] [Google Scholar]
- 135.Wermers RA, Fatourechi V, Wynne AG, et al. et al. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine (Baltimore) 1996;75:53–63. doi: 10.1097/00005792-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 136.Kindmark H, Sundin A, Granberg D, et al. et al. Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years. Med Oncol. 2007;24:330–337. doi: 10.1007/s12032-007-0011-2. [DOI] [PubMed] [Google Scholar]
- 137.Chastain MA. The glucagonoma syndrome: a review of its features and discussion of new perspectives. Am J Med Sci. 2001;321:306–320. doi: 10.1097/00000441-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 138.Boden G, Owen OE. Familial hyperglucagonemia—an autosomal dominant disorder. N Engl J Med. 1977;296:534–538. doi: 10.1056/NEJM197703102961003. [DOI] [PubMed] [Google Scholar]
- 139.Yu R, Nissen NN, Dhall D, et al. et al. Nesidioblastosis and hyperplasia of alpha cells, microglucagonoma, and nonfunctioning islet cell tumor of the pancreas. Pancreas. 2008;36:428–431. doi: 10.1097/MPA.0b013e31815ceb23. [DOI] [PubMed] [Google Scholar]
- 140.Ouyang D, Dhall D, Yu R. Pathologic pancreatic endocrine cell hyperplasia. World J Gastroenterol. 2011;17:137–143. doi: 10.3748/wjg.v17.i2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boden G, Ryan IG, Eisenschmid BL, et al. et al. Treatment of inoperable glucagonoma with the long-acting somatostatin analogue SMS 201–995. N Engl J Med. 1986;314:1686–1689. doi: 10.1056/NEJM198606263142606. [DOI] [PubMed] [Google Scholar]
- 142.Solorzano CC, Lee JE, Pisters PW, et al. et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 143.Kouvaraki MA, Solorzano CC, Shapiro SE, et al. et al. Surgical treatment of non-functioning pancreatic islet cell tumors. J Surg Oncol. 2005;89:170–185. doi: 10.1002/jso.20178. [DOI] [PubMed] [Google Scholar]
- 144.Kuo SC, Gananadha S, Scarlett CJ, et al. et al. Sporadic pancreatic polypeptide secreting tumors (PPomas) of the pancreas. World J Surg. 2008;32:1815–1822. doi: 10.1007/s00268-008-9499-7. [DOI] [PubMed] [Google Scholar]
- 145.Marx SJ, Agarwal SK, Kester MB, et al. et al. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent Prog Horm Res. 1999;54:397–439. [PubMed] [Google Scholar]
- 146.Agarwal SK, Kennedy PA, Scacheri PC, et al. et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res. 2005;37:369–374. doi: 10.1055/s-2005-870139. [DOI] [PubMed] [Google Scholar]
- 147.Agarwal SK, Lee Burns A, Sukhodolets KE, et al. et al. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–198. doi: 10.1196/annals.1294.020. [DOI] [PubMed] [Google Scholar]
- 148.Brandi ML, Gagel RF, Angeli A, et al. et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 149.Tonelli F, Giudici F, Fratini G, et al. et al. Pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: review of literature. Endocr Pract. 2011;17:33–40. doi: 10.4158/EP10376.RA. Suppl. 3. [DOI] [PubMed] [Google Scholar]
- 150.Thakker RV, Newey PJ, Walls GV, et al. et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 151.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–623. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 153.Shuin T, Yamazaki I, Tamura K, et al. et al. Recent advances in ideas on the molecular pathology and clinical aspects of Von Hippel-Lindau disease. Int J Clin Oncol. 2004;9:283–287. doi: 10.1007/s10147-004-0415-3. [DOI] [PubMed] [Google Scholar]
- 154.Libutti SK, Choyke PL, Bartlett DL, et al. et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124:1153–1159. doi: 10.1067/msy.1998.91823. [DOI] [PubMed] [Google Scholar]
- 155.Tamura K, Nishimori I, Ito T, et al. et al. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol. 2010;16:4515–4518. doi: 10.3748/wjg.v16.i36.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou C, Dhall D, Nissen NN, et al. et al. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas. 2009;38:941–946. doi: 10.1097/MPA.0b013e3181b2bb03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu R, Wawrowsky K, Zhou C. A natural inactivating mutant of human glucagon receptor exhibits multiple abnormalities in processing and signaling. Endocrinol Nutr. 2011;58:258–266. doi: 10.1016/j.endonu.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 158.Yu R, Dhall D, Nissen NN, et al. et al. Pancreatic neuroendocrine tumors in glucagon receptor-deficient mice. PLoS One. 2011;6:e23397. doi: 10.1371/journal.pone.0023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu R, Ren SG, Miroha J. Glucagon receptor is required for long-term survival: a natural history study of the Mahvash disease in a murine model. Endocrinol Nutr. 2012;59:523–530. doi: 10.1016/j.endonu.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 160.Yu R, Chen CR, Liu X, et al. et al. Rescue of a pathogenic mutant human glucagon receptor by pharmacological chaperones. J Mol Endocrinol. 2012;49:69–78. doi: 10.1530/JME-12-0051. [DOI] [PubMed] [Google Scholar]