Abstract
For patients with unresectable pancreatic cancer, current chemotherapies have negligible survival benefits. Thus, developing effective minimally invasive therapies is currently underway. This study was conducted to evaluate the efficacy of transarterial chemoembolization plus radiofrequency ablation and/or 125I radioactive seed implantation on unresectable pancreatic cancer. We analyzed the outcome of 71 patients with unresectable pancreatic carcinoma who underwent chemoembolization plus radiofrequency ablation and/or radioactive seed implantation. Of the 71 patients, the median survival was 11 months, and the 1-, 2-, and 3-year overall survival rates were 32.4%, 9.9%, and 6.6% respectively. Patients who had no metastasis, who had oligonodular liver metastases (≤3 lesions), and who had multinodular liver metastases (>3 lesions) had median survival of 12, 18, and 8 months, respectively, and 1-year overall survival rates of 50.0%, 68.8%, and 5.7%, respectively. Although the survival of patients without liver metastases was worse than that of patients with oligonodular liver metastasis, the result was not significant (P = 0.239). In contrast, the metastasis-negative patients had significantly better survival than did patients with multinodular liver metastases (P < 0.001). Patients with oligonodular liver lesions had a significanthg longer median survival than did patients with multinodular lesions (P < 0.001). In conclusion, combined minimally invasive therapies had good efficacy on unresectable pancreatic cancer and resulted in a good control of liver metastases. In addition, the number of liver metastases was a significant factor in predicting prognosis and response to treatment.
Keywords: Pancreatic cancer, minimally invasive therapies, transarterial chemoembolization, radiofrequency ablation, median survival
Pancreatic cancer is an aggressive gastroin-testinal cancer and is responsible for more than 250,000 deaths per year worldwide[1]. Patient survival is overwhelmingly short[2]. Almost 50% of pancreatic cancer patients have distant metastases at diagnosis, and many have subclinical liver metastases. In those with unresectable disease, current chemotherapies have negligible survival benefits[3]. Indeed, pancreatic cancer persists as a major therapeutic challenge largely characterized by chemotherapy-refractory disease and poor response to currently available treatments. Thus, minimally invasive therapies have come under development.
Gemcitabine, a drug often used to treat liver metastasis, has been approved as an chemotherapeutic drug for advanced pancreatic cancer in the past two decades[4]; however, its efficacy is limited. For patients with liver metastases from pancreatic tumors, transarterial chemoembolization (TACE) has been proven efficient for the control of both symptoms and tumor masses[5]. TACE is a locoregional procedure that provides a highly concentrated dose of chemotherapeutic drug(s) to tumor cells, prolonging drug-cell contact time and minimizing systemic toxicity. We hypothesized that TACE would result in good locoregional disease control and improve overall survival (OS). Percutaneous radiofrequency ablation (RFA) and radioactive seed implantation are minimally invasive local therapies capable of tumor destruction or complete tumor eradication. RFA has been established for treating liver metastases[6]–[8]. Our previous studies indicated that computed tomography (CT)–guided 125I radioactive seed implantation is an effective treatment for pancreatic cancer with minimal damage and few complications[8],[9]. The aim of this study was to evaluate the results of TACE plus RFA for liver metastasis and/or 125I radioactive seed implantation for pancreatic cancer. Our goal was to determine if combined minimally invasive therapies could improve the control of tumor growth and the outcomes of patients with unresectable pancreatic cancer.
Patients and Methods
Patient selection
Seventy-one patients (22 women and 49 men; median age, 60 years; interquartile range, 55–64 years) with advanced pancreatic cancer who underwent TACE and RFA and/or radioactive seed implantation between February 1, 1998 and October 31, 2011 at Sun Yat-sen University Cancer Center were considered for the present study. For inclusion, the following criteria met:(1) patients had histopathologically documented pancreatic cancer; (2) patients underwent minimally invasive therapies; (3) patients were not surgical candidates or underwent no systemic chemotherapy before minimally invasive therapies; (4) patients underwent routine CT or magnetic resonance imaging (MRI) every month; and (5) follow-up documents were available. Patients who underwent previous surgery, chemotherapy, or radiotherapy, had severe and/or uncontrolled medical conditions, were pregnant or breastfeeding, or had evidence of bleeding diathesis were excluded from the study. Patients who had metastases to other organs (except the liver) during minimally invasive treatments and switched to undergo systemic therapy were also excluded; however, these patients were included for survival analysis. The clinical characteristics of the 71 patients with unresectable pancreatic cancer are listed in Table 1.
Table 1. Clinical data of 71 patients with pancreatic cancer.
| Parameter | Metastases-negative group | Oligonodular metastases groupa | Multinodular metastases groupb | Pc |
| Age (years, range) | 61 (49–82) | 57 (42–77) | 62 (20–74) | 0.532d |
| Sex | 0.771 | |||
| Male | 15 | 9 | 25 | |
| Female | 5 | 4 | 13 | |
| Tumor location | 0.704 | |||
| Pancreatic head | 15 | 8 | 27 | |
| Others | 5 | 5 | 11 | |
| History | 0.746 | |||
| Elevated CA-199 | 17 | 9 | 30 | |
| Elevated CEA | 6 | 4 | 10 | |
| With Jaundice | 9 | 6 | 20 | |
| With DM | 4 | 3 | 8 | |
| ECOG | 0.642 | |||
| 0 | 6 | 6 | 14 | |
| 1 | 14 | 7 | 24 | |
| Local treatments | 0.227 | |||
| RFA | 2 | 2 | 12 | |
| 125I | 10 | 5 | 9 | |
| RFA + 125I | 8 | 6 | 17 | |
| Chemotherapy | 0.110 | |||
| No | 12 | 6 | 29 | |
| Yes | 8 | 7 | 9 |
CA-199, carbohydrate antigen 199; CEA, cancer embryo antigen; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; RFA, radiofrequency ablation; 125I, 125I radioactive seed implantation. a Oligonodular metastases were defined as ≤3 metastatic lesions. b Multinodular metastases were defined as >3 metastatic lesions. c Except what was specially marked, P values were obtained with the Pearson χ2 test. dObtained with one-way ANOVA.
Treatment schedule
TACE with gemcitabine (1,000 mg/m2) plus cisplatin (30–60 mg) was performed for all patients. The Seldinger technique was used under local anesthesia to access the femoral artery, and the embolization was performed with 5–30 mL of iodized oil (lipiodol). When necessary, doses were held for grade 3 or worse adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE) and resumed when adverse events were grade 2 or better. Tumor responses were assessed by CT or MRI one month after every procedure. Objective tumor responses were evaluated based on Response Evaluation Criteria in Solid Tumors (RECIST)[10].
Residual lesions at the primary site, liver metastases and malignant lymph nodes were treated with 125I radioactive seed implantation and RFA, both guided by CT (Figure 1). When patients with inoperable hepatic metastases showed disease progression, systemic chemotherapy of cisplatin (7.5 mg/m2) plus gemcitabine (1,000 mg/m2) was administered via the peripheral vein once per month. If the patients manifested grade 3 or higher adverse events, treatment was interrupted until recovery.
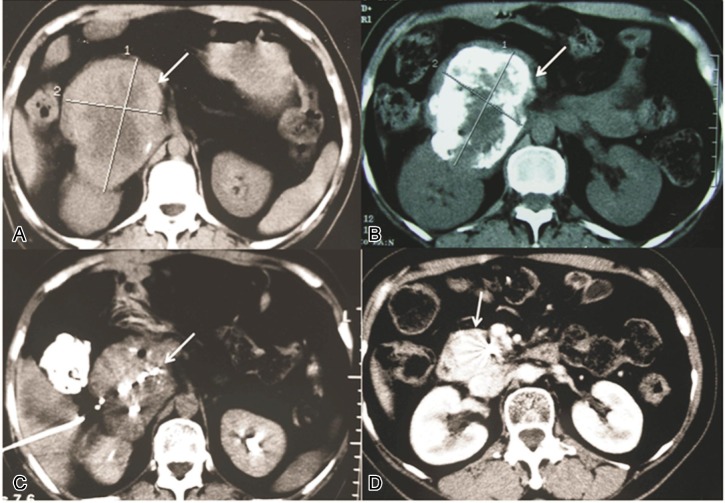
Figure 1. Representative computed tomography (CT) images showing the effect of minimally invasive therapies on an unresectable pancreatic tumor.
This 53-year-old patient was treated with 6 courses of TACE, followed by radioactive seed implantation to treat residual disease in the pancreas. A, typical CT imaging findings of pancreatic head tumor (arrow) before administration of any treatment. B, lipiodol deposit (arrow) in pancreatic carcinoma after 6 TACE procedures. C, the pancreatic tumor dimension (arrow) reduced after TACE, and CT-guided 125I radioactive seed implantation was carried out to treat the residual lesion. D, the pancreatic carcinoma shrank significantly after 125I radioactive seed implantation was performed.
Follow-up and response assessment
All patients underwent contrast-enhanced helical CT or MRI every month after treatment or when signs of progression were evident. Using RECIST guidelines, complete response (CR) was defined as the disappearance of all lesions; partial response (PR) was defined as ≥30% reduction in the tumor load, which was estimated as the sum of the longest diameters of all measurable lesions, using the sum of the longest diameters at baseline as a reference; progressive disease (PD) was defined as ≥20% increase in the tumor load, using the smallest sum of the longest diameters recorded since treatment initiation or development of new lesions in a previously uninvolved site as a reference; stable disease (SD) was defined as disease that showed neither sufficient shrinkage nor increase that would qualify as PR or PD.
OS, the primary index determined in this study, was measured from the time of diagnosis to the end of follow-up or the date of death. Sex, age, presence of diabetes, tumor localization, and tumor number were also observed and analyzed for all patients.
Statistical analysis
Tumor size (according to RECIST) and survival index were assessed overall as well as by the number of liver metastases and patient sex. Categorical measurements were summarized with count and percentage. All statistical tests were 2-sided, and P < 0.05 was considered significant (with 95% confidence interval). Comparisons between the subgroups were performed using one-way ANOVA for continuous data and Chi-square test for categorical data. Patient survival was analyzed using the Kaplan-Meier method and compared with the log-rank test. All analyses were performed using Statistical Package for the Social Science (SPSS) version 16.0 for Windows.
Results
All patients underwent TACE, with a mean of 3 sessions per patient (range: 2–6), in 4-week or 8-week intervals. Residual lesions in the primary tumor site and liver as well as metastatic lymph nodes were treated with RFA and/or by radioactive seed implantation. The numbers of patients who underwent RFA and/or by radioactive seed implantation for primary tumor site, liver metastases, and metastatic lymph nodes are shown in Table 1.
Overall tumor response
Seventy-one patients underwent TACE with gemcitabine plus cisplatin, RFA, and/or radioactive seed implantation for treatment of primary tumor, residual lesions, liver metastases, and lymph node metastases. The overall response rate was 45%, 30.8%, and 26.3% in the metastasis-negative group, oligonodular metastases group, and multinodular metastases group, respectively. Nine patients with PD group showed metastasis to the lung or spleen during treatment. No patients experienced CR. Treatment response and OS rates for all patients are summarized in Table 2. In our study, there were 20 patients (28.2%) without liver metastases, 13 (18.3%) with ≤3 (oligonodular) liver metastases, and 38 (53.5%) with >3 (multinodular) liver metastases.
Table 2. Treatment responses and overall survival rates of pancreatic cancer patients.
| Item | Metastases-negative group | Oligonodular metastases group | Multinodular metastases group | P |
| MST (months) | 12 | 18 | 8 | <0.001a |
| Treatment response (cases) | 0.409 | |||
| PR | 9 | 4 | 10 | |
| SD | 8 | 4 | 19 | |
| PD | 3 | 5 | 9 | |
| ORR (%) | 45.0 | 30.8 | 26.3 | |
| Overall survival rate (%) | <0.001a | |||
| 1-year | 50.0 | 68.8 | 5.7 | |
| 2-year | 10.0 | 31.2 | 0.0 | |
| 3-year | 5.0 | 15.6 | 0.0 |
MST, median survival time; PR, partial remission; SD, stable disease; PD, progressive disease; ORR, objective response rate. No patients experienced CR in this study. aObtained with the log-rank test.
Overall survival
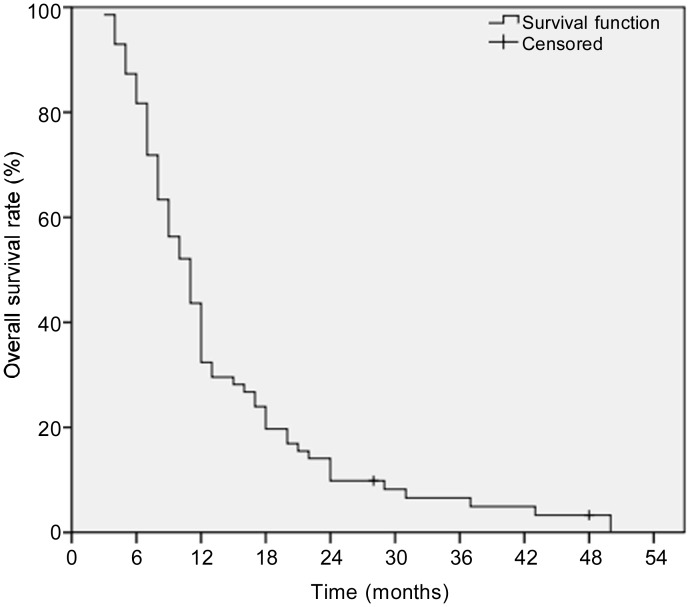
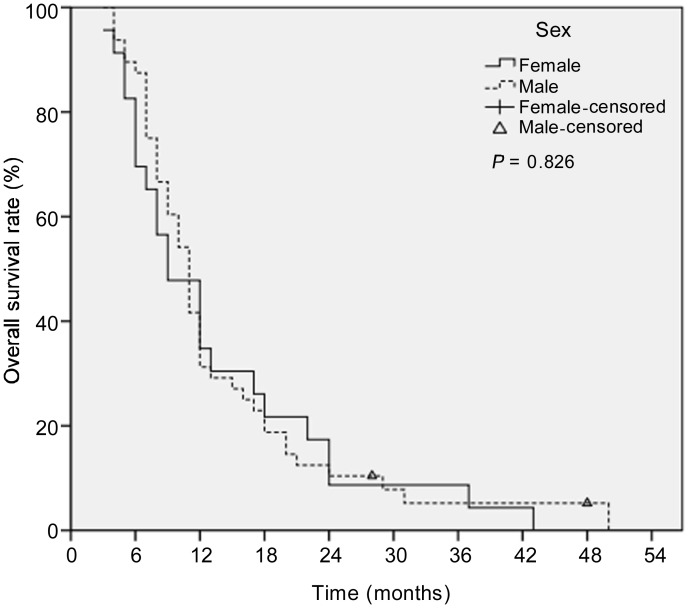
Of the 71 patients, the median OS was 11 months. The 1-, 2-, and 3-year OS rates were 32.4%, 9.9%, and 6.6%, respectively (Figure 2). The 1-, 2-, and 3-year OS rates were 31.2%, 10.4%, and 5.2% for male patients, respectively, and 34.8%, 8.7%, and 4.3% for female patients, respectively (Figure 3). The median OS was not significantly different between male and female patients (9 months vs. 11 months, P = 0.826).
Figure 2. Kaplan-Meier curve showing the overall survival (OS) of patients with unresectable pancreatic cancer who were treated with minimally invasive therapies.
The 1-, 2-, and 3-year OS rates were 32.4%, 9.9%, and 6.6%, respectively.
Figure 3. Kaplan-Meier survival curves for male and female patients.
The median OS was not significantly different between male and female patients (9 months vs. 11 months, P = 0.826).
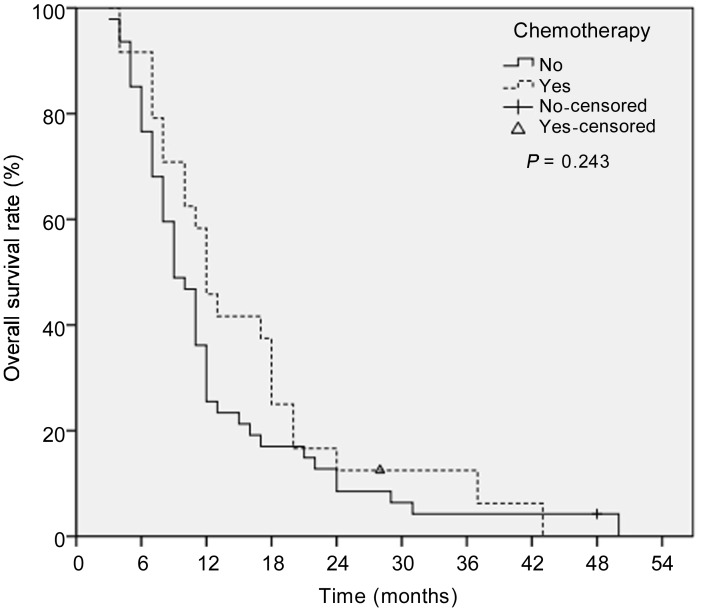
Systemic chemotherapy was administered when patients with inoperable hepatic metastases showed disease progression. The median OS was not significantly different between patients who did and did not undergo chemotherapy (P = 0.243). The 1-, 2-, and 3-year OS rates were 45.8%, 12.5%, and 6.2%, respectively, in the group that underwent chemotherapy and 25.5%, 8.5%, and 4.3%, respectively, in the group that did not undergo chemotherapy (Figure 4).
Figure 4. Kaplan-Meier survival curves for patients who did or did not undergo chemotherapy.
The median OS was not significantly different between the two groups (12 months vs. 9 months, P = 0.243).
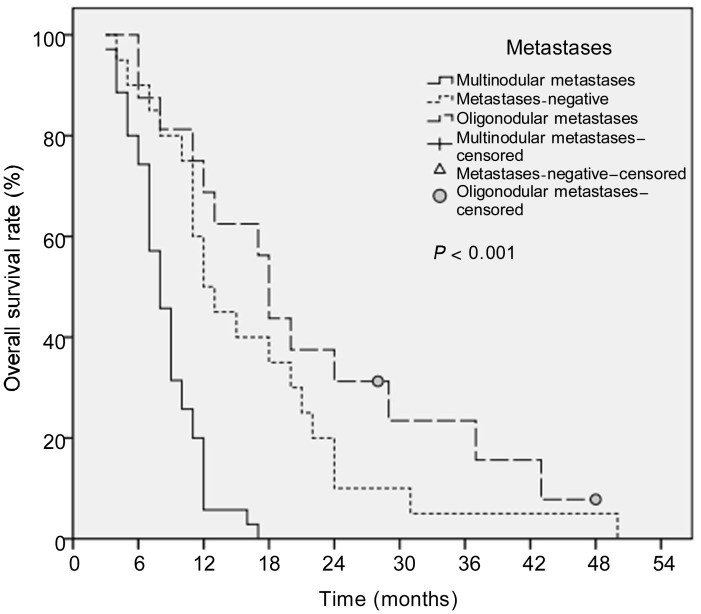
This study indicated that patients with oligonodular liver lesions had better survival than did those with multinodular liver metastases. The median OS of patients without liver metastases, with oligonodular liver metastases, and with multinodular liver metastases were 12, 18, and 8 months, respectively. The 1-, 2-, and 3-year OS rates were 50%, 10%, and 5%, respectively, for patients without metastases, 68.8%, 31.2%, and 15.6%, respectively, for patients with oligonodular metastases, and 5.7%, 0.0%, and 0.0%, respectively, for patients with multinodular metastases. Interestingly, patients with stage III pancreatic cancer who had no liver metastases had worse survival than did patients with stage IV disease who had oligonodular liver metastases (12 months vs. 18 months), but the difference was not statistically significant (P = 0.239). Furthermore, patients without liver metastases had significantly better survival than did those with multinodular liver metastases (12 months vs. 8 months, P < 0.001). Also, patients with oligonodular liver metastases had a significantly better median survival time than did patients with multinodular liver metastases (18 months vs. 8 months, P < 0.001) (Figure 5, Table 2).
Figure 5. Kaplan-Meier survival curves for patients without metastases, with oligonodular metastases (≤3 liver lesions), and with multinodular metastases (> 3 liver lesions).
The median OS of the three groups were 12, 18, and 9 months, respectively. Survival was not significantly different between the metastases-negative group and the oligonodular metastases group (P = 0.239), but the metastases-negative group had better survival than did the multinodular metastases group (P < 0.001). Furthermore, the oligonodular metastases group had a significantly better survival than did the multinodular metastases group (P < 0.001).
Discussion
For patients with unresectable pancreatic cancer, current chemotherapies have negligible survival benefits. Thus, developing effective minimally invasive therapies is currently underway. This study was conducted to evaluate the efficacy of TACE plus RFA and/or 125I radioactive seed implantation on unresectable pancreatic cancer. In the present study, there was no significant difference between the group without liver metastases and the group with oligonodular liver metastases in survival rate(P = 0.239). However, the group without liver metastases had better survival than did patients with multinodular liver metastases (P < 0.001). Also, patients with oligonodular liver metastases had better survival than did patients with multinodular liver metastases (P < 0.001). Combined minimally invasive therapies resulted in a good tumor response for the control of liver metastases. In addition, the number of liver metastases was a significant factor in predicting prognosis and treatment response. These results are similar to our previous study of nasopharyngeal carcinoma[11].
Data from the Surveillance, Epidemiology and End Results (SEER) [12] registry between 2000 and 2007 indicate that the majority of pancreatic cancer is advanced (50.5% metastatic and 25.9% regional spread) at diagnosis. Liver metastasis is common in patients with pancreatic cancer and is difficult to treat. Therefore, the presence and extension of liver metastases are considered important prognostic factors[13]. Gemcitabine was the first clinical drug to clearly improve patient's quality of life and prolong survival. Single-agent gemcitabine has evolved as a standard treatment of locally advanced and metastatic pancreatic cancer, producing median OS ranging from 5 months to 8 months and 1-year OS rates ranging from 17% to 25%[14]. Many combination regimens have been compared with gemcitabine alone for metastatic pancreatic cancer, but only erlotinib in combination with gemcitabine has been found to significantly boost survival (median OS: 6 months) compared with gemcitabine alone[15],[16]. To attain locoregional disease control for patients with advanced pancreatic cancer, selecting effective treatments for liver metastases has become more crucial. Multimodal treatment protocols, including TACE, RFA, and 125I radioactive seed implantation, have been established for the treatment of metastatic tumors. Because liver metastases are little supplied by the hepatic artery, to date, TACE was developed as a palliative treatment for liver metastasis[17]. The goal of this study was to evaluate the efficacy of combining TACE with RFA and/or radioactive seed implantation on disease control and survival. Although the results were not significant, we found that patients with oligonodular liver metastases, who were treated with RFA and/or radioactive seed implantation, had longer median survival than did patients without liver metastases and those who did not undergo these treatments. This suggests that RFA and radioactive seed implantation are effective options for obtaining locoregional disease control and survival benefits.
Most pancreatic cancer patients are diagnosed with advanced disease and have a median survival of approximately 6 months[18]. Late-stage clinical trials have generally failed to demonstrate improvement in outcome or to predict better survival in patients with metastatic pancreatic cancer[19]. In the current study, the median OS was 11 months. The 1-, 2-, and 3-year OS rates were 32.4%, 9.9%, and 6.6%, respectively. For all stages of pancreatic cancer, the 1-year relative survival rate is 20%[19]. In our cohort, the 1-year survival rate was 32.4% for all patients and 25.5% for patients with liver metastases. Since patients with oligonodular liver metastases had longer survival than did patients with multinodular liver metastases in this study, we observed that the number of liver metastases had a negative effect on local tumor response. In retrospective studies by Kelley et al.[19] and Moureau-Zabotto et al.[20], the reported median survival was 8–14 months and the 1-year OS was 32%–62% for patients with locoregionally advanced disease. For metastatic pancreatic cancer patients who underwent chemotherapy, the median OS was 6–7.1 months[15], whereas it was 9 months in our study. This result further confirms that RFA and radioactive seed implantation can be used to gain locoregional disease control and better survival benefits than that obtained with chemotherapy only for pancreatic cancer patients with liver metastases.
Our results confirm that oligonodular liver metastases predict longer survival in pancreatic cancer patients with hepatic metastases. However, this finding is inconsistent with results from other published studies[21]. There are several possible reasons for the disparate results. First, all patients in our study had stage III or stage IV disease and were subjected to different treatment strategies compared with patients enrolled in other studies. Second, we defined 3 as the critical number of liver lesions, whereas other studies set 5 as the critical level[21]. Third, most of our patients had good performance status at diagnosis [Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1], which predicts better survival[22]. Finally, in this study, the survival of all patients was calculated from the time of diagnosis other than from the first time of treatment. This criterion may be different from other studies, which may make the median survival of patients with metastases in our study longer than that reported by others.
The clinical predictors of survival in patients with metastatic pancreatic cancer that we identified here should be helpful in improving the design of clinical trials involving pancreatic cancer patients with liver metastases. These predictors can be easily assessed when liver metastasis is diagnosed, and they can be used to more accurately stratify patients into groups with fairly consistent outcomes and thus help to standardize reporting results of any therapeutic interventions with less samples and expense.
However, the retrospective nature and limited sample size of the study perhaps reduce the generalizability of our results. Furthermore, the results are based on a single institution specializing in cancer treatment and may not be generalized well to other institutions, given the different nature of health care delivery.
Conclusions
In conclusion, our results show that this approach may be a suitable choice in the management of unresectable pancreatic cancer and that this approach may offer a promising contribution to the control of liver metastases. Patients with oligonodular liver metastases had better survival than did those with multinodular liver metastases. In addition, the number of liver metastases is a significant factor in predicting prognosis and treatment response.
The lack of stage-specific completed randomized trials does not allow us to draw evidence-based guidelines for clinical practice. However, there is a growing trend towards recommending upfront combination minimally invasive therapies. Accordingly, the roles of minimally invasive therapies deserve to be confirmed by prospective trials.
References
- 1.Pantano F, Baldi A, Santini D, et al. et al. MUC2 but not MUC5 expression correlates with prognosis in radically resected pancreatic cancer patients. Cancer Biol Ther. 2009;8:996–999. doi: 10.4161/cbt.8.11.8537. [DOI] [PubMed] [Google Scholar]
- 2.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–282. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Tempero M, Arnoletti JP, Ben-Josef E, et al. et al. Pancreatic adenocarcinoma. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2007;5:998–1033. doi: 10.6004/jnccn.2007.0085. [DOI] [PubMed] [Google Scholar]
- 4.Fryer RA, Galustian C, Dalgleish AG. Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol. 2009;4:102–112. doi: 10.2174/157488409788185007. [DOI] [PubMed] [Google Scholar]
- 5.Vogl TJ, Zangos S, Heller M, et al. et al. Transarterial chemoperfusion with gemcitabine and mitomycin c in pancreatic carcinoma: results in locally recurrent tumors and advanced tumor stages. Rofo. 2007;179:1181–1188. doi: 10.1055/s-2007-963568. [DOI] [PubMed] [Google Scholar]
- 6.Khajanchee YS, Hammill CW, Cassera MA, et al. et al. Hepatic resection vs minimally invasive radiofrequency ablation for the treatment of colorectal liver metastases: a Markov analysis. Arch Surg. 2011;146:1416–1423. doi: 10.1001/archsurg.2011.212. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Cai YC, Cao Y, et al. et al. Radiofrequency ablation combined with systemic chemotherapy in nasopharyngeal carcinoma liver metastases improves response to treatment and survival outcomes. J Surg Oncol. 2012;106:322–326. doi: 10.1002/jso.23034. [DOI] [PubMed] [Google Scholar]
- 8.Wu PH, Pan CC, Huang ZL, et al. et al. Percutaneous radiofrequency ablation approach through the spleen: initial case report for pancreatic tail gastrinoma. Chin J Cancer. 2010;29:836–841. doi: 10.5732/cjc.009.10755. [DOI] [PubMed] [Google Scholar]
- 9.Zhang FJ, Wu PH, Zhao M, et al. et al. CT guided radioactive seed 125I implantation in treatment of pancreatic cancer. Zhonghua Yi Xue Za Zhi. 2006;86:223–227. [in Chinese] [PubMed] [Google Scholar]
- 10.Park JO, Lee SI, Song SY, et al. et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–537. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 11.Pan C, He N, Zhao M, et al. et al. Subdividing the M1 stage of liver metastasis for nasopharyngeal carcinoma to better predict metastatic survival. Med Oncol. 2011;28:1349–1355. doi: 10.1007/s12032-010-9643-8. [DOI] [PubMed] [Google Scholar]
- 12.Statistics USC Surveillance epidemiology and end results (SEER). 1999–2007 incidence and mortality report. Accessed January 31, 2011. Available at: http://www.seer.cancer.gov/publications/uses.html.
- 13.Vogl TJ, Gruber T, Naguib NN, et al. et al. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol. 2009;193:941–947. doi: 10.2214/AJR.08.1879. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann V, Boeck S, Hinke A, et al. et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saif MW. New developments in the treatment of pancreatic cancer. Highlights from the “44th ASCO Annual Meeting”. Chicago, IL, USA. May 30–June 3, 2008. JOP. 2008;9:391–397. [PubMed] [Google Scholar]
- 16.Campen CJ, Dragovich T, Baker AF. Management strategies in pancreatic cancer. Am J Health Syst Pharm. 2011;68:573–584. doi: 10.2146/ajhp100254. [DOI] [PubMed] [Google Scholar]
- 17.Wang LQ, Persson BG, Bergqvist L, et al. et al. Influence of dearterialization on distribution of absolute tumor blood flow between hepatic artery and portal vein. Cancer. 1994;74:2454–2459. doi: 10.1002/1097-0142(19941101)74:9<2454::aid-cncr2820740911>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Kelley RK, Ko AH. Erlotinib in the treatment of advanced pancreatic cancer. Biologics. 2008;2:83–95. doi: 10.2147/btt.s1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moureau-Zabotto L, Phelip JM, Afchain P, et al. et al. Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II study. J Clin Oncol. 2008;26:1080–1085. doi: 10.1200/JCO.2007.12.8223. [DOI] [PubMed] [Google Scholar]
- 21.Azizi A, Naguib NN, Mbalisike E, et al. et al. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas. 2011;40:1271–1275. doi: 10.1097/MPA.0b013e318220e5b9. [DOI] [PubMed] [Google Scholar]
- 22.Ong YK, Heng DM, Chung B, et al. et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–1541. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]