Abstract
Recurrent viral pressure has acted on host-encoded antiviral genes during primate and mammalian evolution. This selective pressure has resulted in dramatic episodes of adaptation in host antiviral genes, often detected via positive selection. These evolutionary signatures of adaptation have the potential to highlight previously unrecognized antiviral genes (also called restriction factors). Although the TRIM multigene family is recognized for encoding several bona fide restriction factors (e.g., TRIM5alpha), most members of this expansive gene family remain uncharacterized. Here, we investigated the TRIM multigene family for signatures of positive selection to identify novel candidate antiviral genes. Our analysis reveals previously undocumented signatures of positive selection in 17 TRIM genes, 10 of which represent novel candidate restriction factors. These include the unusual TRIM52 gene, which has evolved under strong positive selection despite its encoded protein lacking a putative viral recognition (B30.2) domain. We show that TRIM52 arose via gene duplication from the TRIM41 gene. Both TRIM52 and TRIM41 have dramatically expanded RING domains compared with the rest of the TRIM multigene family, yet this domain has evolved under positive selection only in primate TRIM52, suggesting that it represents a novel host–virus interaction interface. Our evolutionary-based screen not only documents positive selection in known TRIM restriction factors but also highlights candidate novel restriction factors, providing insight into the interfaces of host–pathogen interactions mediated by the TRIM multigene family.
Keywords: TRIM5, TRIM52, positive selection, dN/dS, restriction factors
Introduction
Host-encoded restriction factors confer an intrinsic line of defense that inhibits viruses at various stages of the viral life cycle (Goff 2004; Duggal and Emerman 2012; Yan and Chen 2012). One example of this type of antiviral defense gene is TRIM5, which was identified as the block to HIV-1 infection in rhesus macaques (Stremlau et al. 2004). The potent restriction by TRIM5 is conserved in other mammals, including primates (Yap et al. 2004; Song, Javanbakht et al. 2005; Zhang et al. 2006; Kratovac et al. 2008; Yap et al. 2008; Rahm et al. 2011) and closely related paralogs belonging to glires (Schaller et al. 2007; Tareen et al. 2009; Fletcher et al. 2010) and cows (Si et al. 2006; Ylinen et al. 2006). Restriction activity is attributed to the assembly of a TRIM5 lattice directly to the surface of the retroviral core (Ganser-Pornillos et al. 2011) that is thought to mediate premature capsid disassembly (Stremlau et al. 2006). Antiviral activity of TRIM5 has also been attributed to the induction of an inflammatory response (Pertel et al. 2011; Tareen and Emerman 2011). Retroviral specificity of TRIM5 dramatically differs among primate orthologs due to ancient and ongoing selective pressures reflected by variation in the Coiled-Coil and B30.2 domains, which influence the interaction with viral proteins (Sawyer et al. 2005; Sebastian and Luban 2005; Kirmaier et al. 2010; Maillard et al. 2010).
TRIM5 is a member of the TRIM multigene family, which encodes as many as 100 genes in humans and is similarly expansive throughout primates (Han et al. 2011). Proteins encoded by the TRIM multigene family are characterized by a tripartite motif consisting of a RING domain, one or two B-boxes, and a Coiled-Coil motif, the order and spacing of which are generally conserved (Reymond et al. 2001; Meroni and Diez-Roux 2005; Nisole et al. 2005). Like TRIM5, several other TRIM genes have been implicated in innate immunity and antiviral defense (reviewed in Nisole et al. 2005; Ozato et al. 2008; Johnson and Sawyer 2009; Kawai and Akira 2011; McNab et al. 2011). However, the majority of TRIM genes remain largely uncharacterized, along with their potential for encoding antiviral activities.
Previous studies have used functional characterizations to identify TRIM gene family members that encode antiviral activity. For example, a screen of a subset of human and mouse TRIM genes highlighted members not previously known to positively or negatively impact retroviral fitness (Uchil et al. 2008). Other functional characterizations have focused on hallmarks of restriction factors, including induction on interferon treatment (Carthagena et al. 2009; Uchil et al. 2013). Although candidate restriction factors were identified from each of these approaches, functional identification of novel restriction factors in the TRIM gene family is complicated due to a number of reasons. First, multiple alternatively spliced transcripts are produced from each TRIM gene. PML, for instance, is only one of the 11 TRIM19 protein isoforms. TRIM5alpha is the longest of at least nine reported transcripts of the TRIM5 gene (Reymond et al. 2001; Brennan et al. 2007; Battivelli et al. 2011) but the only protein isoform with antiviral activity. Homodimerization of TRIM5alpha with other TRIM5 isoforms (gamma, delta, and iota) causes dominant negative suppression of the antiviral activity of TRIM5alpha (Stremlau et al. 2004; Passerini et al. 2006; Battivelli et al. 2011), so antiviral activity requires that the correct isoform or combination of isoforms be appropriately expressed in the cells being assayed. Second, viral restriction specificity may further impede identification of antiviral function especially for those restriction factors that act directly at the host–virus interface (like TRIM5alpha) compared with those that may indirectly affect the immune response (like PML); for the former case, detection of antiviral activity would depend on the right combination of TRIM genes and viruses. For instance, although rhesus macaque TRIM5 has potent antiviral activity against HIV-1, the human ortholog only has relatively modest effects (Stremlau et al. 2004).
In order to bypass these difficulties associated with a functional screening approach, we have taken a complementary, evolutionary approach to identify candidate antiviral restriction factors in this family. This approach exploits a common feature of restriction genes: the unique selective pressures they are subjected to by virtue of their antagonistic relationship with viral pathogens (Meyerson and Sawyer 2011; Daugherty and Malik 2012). Any mutation that improves the ability of an antiviral gene to recognize the virus is advantageous to the host genome. In contrast, the virus selectively favors mutations that weaken or destroy this interaction. Repeated rounds of mutation in which one party increases affinity while the other party decreases affinity can lead to rapid evolution at the protein–protein binding interface. Specifically, such interactions will result in the rapid accumulation of changes at nonsynonymous (amino acid altering) positions in coding DNA compared with the relatively benign mutations at synonymous sites, a selective regime referred to as positive selection. Such positive selection analysis was successfully used to precisely identify the region of TRIM5alpha that determines its specificity for different retroviral capsids (Sawyer et al. 2005). Importantly, positive selection has also been detected in nearly all other known restriction factors (Duggal and Emerman 2012). Indeed, signals of adaptive evolution are often a hallmark among restriction factors with roles at the direct interface of host–pathogen interactions.
Here, we analyzed members from the TRIM gene family for positive selection in primates. Via our evolutionary screen, we recovered two TRIM genes previously identified to be under positive selection due to their antiviral role (i.e. TRIM5 and TRIM22 [Sawyer et al. 2005, 2007]), five antiviral genes whose evolutionary signatures were previously unknown (i.e., TRIM15 [Uchil et al. 2008; Uchil et al. 2013], TRIM21 [Mallery et al. 2010], TRIM25 [Gack et al. 2007], TRIM31 [Uchil et al. 2008], and TRIM38 [Uchil et al. 2008; Xue et al. 2012; Zhao, Wang, Zhang, Yuan et al. 2012; Zhao, Wang, Zhang, Wang et al. 2012]), and ten novel TRIM gene antiviral factor candidates. We also present a more detailed analysis of the most intriguing restriction factor candidate revealed by our screen, TRIM52. TRIM52 lacks a C-terminal B30.2 domain but encodes a massively expanded RING domain that we find has been subject to intense positive selection. Our analysis of TRIM52 evolution reveals its age and birth via a partial duplication of the TRIM41 gene, followed by independent loss or pseudogenization of TRIM52 in multiple mammalian and primate lineages. Based both on the strong signatures of adaptive evolution and the recurrent losses, we propose that TRIM52 represents a novel, noncanonical antiviral TRIM gene in primate genomes with unique specificity determined by the rapidly evolving RING domain. Our evolutionary screen to identify novel restriction factors reveals several intriguing candidates that warrant further study to fully elucidate the role played by TRIM genes either directly or indirectly in mediating antiviral defense.
Materials and Methods
Collecting TRIM Orthologs
Human (Homo sapiens) TRIM gene sequences were obtained from Ensembl (Flicek et al. 2012) and GenBank. Chimpanzee (Pan troglodytes), bonobo (Pan paniscus), gorilla (Gorilla gorilla), orangutan (Pongo abelii), white-cheeked gibbon (Nomascus leucogenys), rhesus macaque (Macaca mulatta), baboon (Papio anubis), squirrel monkey (Saimiri boliviensis), marmoset (Callithrix jacchus), tarsier (Tarsius syrichta), mouse lemur (Microcebus murinus), and bushbaby (Otolemur garnettii) orthologs were obtained when reported from NCBI by BLAST (Altschul et al. 1990) searches of the “RefSeq RNA” databases with the human TRIM sequence as the query and from Ensembl gene orthology/paralogy predictions (Vilella et al. 2009). Additional primate orthologs were collected when available (e.g., African green monkey [Chlorocebus aethiops]). Subsequent collection of TRIM sequences, specifically TRIM52 and TRIM41, via publically available databases were carried out utilizing Ensembl’s genome databases to recover annotated sequences from available animals, including Reptilia, Avian, and Mammalian species.
Sequencing TRIM52
To expand our collection of primate TRIM52 sequences to improve the power of downstream evolutionary analysis, we amplified TRIM52 using genomic DNA from the following primates: human, chimpanzee, bonobo, gorilla, orangutan, rhesus macaque, African green monkey, talapoin monkey (Miopithecus talapoin), colobus monkey (Colobus guereza), Francois’ leaf monkey (Trachypithecus francoisi), purple-faced langur (Trachypithecus vetulus), and silvery langur (Trachypithecus cristatus). Exon 1 was amplified and sequenced using the following primer pair: Forward: CCACCGATCCCAGAGAGAGG and Reverse: CCTCTGGGGAAGCCAATCTGC. We amplified exon2 by nested PCR with the following primer pairs: Initial primer pair: Forward: GTYGCATGATTTAGAAYTTTACTGACCAA and Reverse: GACAATCCAGGCATCCAGTTATGC. Second, nested primer pair: Forward: ATWATGGTTTATTTAATAYARTATACATTATC and Reverse: GAACTCTAACTCATGGGATGGACAAA. The second, nested primer pair was used to sequence exon2. We used PCR Supermix (Invitrogen, Inc.) for amplification reactions. Reactions used 1 µl of each 10 µM forward primer and 10 µM reverse primer and had a final volume of 12.5 µl. Cycling parameters were 94 °C for 3 min; 40 cycles of 94 °C for 15 s, 55 °C for 15 s, 72 °C for 1 min; 72 °C for 10 min; 10 °C thereafter. Sequencing reactions were carried out using BigDye. TRIM52 sequences have been deposited in the GenBank database (accession numbers JX896135.1–JX896146.1).
Phylogenetic Analysis
Nonprimate TRIM52 and TRIM41 sequences were obtained by Blast (Altschul et al. 1990) analysis with the human TRIM52 protein as query and psi-blast (Altschul, Madden et al. 1997) analysis with the human TRIM52 RING expansion as query. Psi-blast of the RING expansion recovers only TRIM52 and TRIM41 orthologs, suggesting that these are the only TRIMs with this expansion. We found no evidence of a protein domain downstream of the B-Box2 domain, with homology to TRIM41, in any of the TRIM52 orthologs. For instance, there is no identifiable Coiled-Coil domain or B30.2 domain downstream of the human TRIM52 gene in the human genome assembly. All of the TRIM41 sequences are predicted to encode a Coiled-Coil and B30.2 domain. Nonprimate and primate TRIM sequences (TRIM52 and TRIM41) that we recovered from Blast (Altschul et al. 1990) and Ensembl (Flicek et al. 2012) were aligned using ClustalX (Larkin et al. 2007). We only included the RING (omitting the region containing the RING expansion) and B-Box domain. Using this alignment, we constructed a tree using maximum likelihood methodology (Guindon et al. 2010) and used the program Dendroscope (Huson, Richter et al. 2007) to present a phylogram.
Delineation of TRIM Protein Domain Boundaries and Secondary Structure
RING, B-box1, and B-box2 domains were identified based on the consensus sequences (Meroni and Diez-Roux 2005). Coiled-Coil domain boundaries were identified by predicting secondary protein structure with PSIPRED (McGuffin, Bryson et al. 2000) and identifying the long alpha helix that is associated with this motif (Lupas 1996). B30.2 or other C-terminal domains were identified by using the CDD (Marchler-Bauer, Anderson et al. 2005) and SMART (Schultz, Copley et al. 2000) domain databases, and the N-terminal boundary of B30.2 domains was aided by secondary structure prediction, as the B30.2 domain consists entirely of sequential tandem beta-strands (Seto, Liu et al. 1999; Masters, Yao et al. 2006).
Computational Analysis of Positive Selection
Detection of recurrent positive selection by multiple alignment comparisons was carried out using the CODEML program from the PAML package (Yang 1997). Constrained model M7 was tested against unconstrained model M8 under the following parameters: f61 (codon frequencies of 61 nonstop codons are calculated), starting omega: 0.4 and 1.5. All simulations converged and results are consistent between both codon models (2lnλ; P values were calculated assuming two degrees of freedom). We present the percentage of sites estimated to evolve under positive selection and the average dN/dS for those sites. Posterior probabilities were calculated according to the Naive Empirical Bayes model (Yang 1997). Positive selection, as detected by PAML, was further supported by Fast Unbiased Bayesian AppRoximation (FUBAR) and Random Effects Likelihood (REL), implemented through the Datamonkey suite of phylogenetic analysis tools (Delport et al. 2010). TRIM genes were required to exhibit overlapping sites of positive selection by PAML and Datamonkey to be identified as under positive selection. Specific sites of positive selection identified by both PAML and Datamonkey were denoted by underline (table 1).
Table 1.
Primate TRIM Genes Evolving Under Positive Selection
| TRIM Gene | M7vsM8 (2lnλ) | P Value | % of Positively Selected Sites | Average dN/dS for Selected Sites | Positively Selected Sites | No. of Primate Taxa |
|---|---|---|---|---|---|---|
| TRIM2 | 7.31 | 0.026 | 0.64 | 1.54 | 98, 497 | 12 |
| TRIML2 | 6.03062 | 0.049 | 3.63 | 8.74 | 277 | 8 |
| TRIM5 | 73.47 | <0.005 | 20.46 | 3.29 | 7, 139, 175, 182, 213, 215, 228, 257, 258, 310, 311, 317, 324, 379, 381, 382, 418, 421, 423, 471, 483 | 22 |
| TRIM7 | 9.92 | 0.007 | 0.36 | 11.04 | 258 | 10 |
| TRIM10 | 6.06 | 0.048 | 2.00 | 3.62 | 152, 329 | 11 |
| TRIM15 | 10.74 | <0.005 | 5.86 | 2.25 | 18, 42, 150, 460 | 11 |
| TRIM21 | 7.02 | 0.03 | 3.38 | 4.81 | 124, 407 | 10 |
| TRIM22 | 10.20 | <0.005 | 4.89 | 6.17 | 99, 171, 220, 308 | 13 |
| TRIM25 | 19.27 | <0.005 | 9.39 | 2.40 | 58, 259, 297, 338, 377, 415, 418, 420, 435 | 10 |
| TRIM31 | 15.06 | <0.005 | 5.27 | 8.68 | 72, 227, 250 | 7 |
| TRIM38 | 6.51 | 0.039 | 3.66 | 2.94 | 215 | 12 |
| TRIM52 | 16.51 | <0.005 | 6.66 | 5.84 | 75, 111, 149, 153, 221 | 7 |
| TRIM58 | 17.75 | <0.005 | 4.24 | 2.34 | 223, 443, 472, 475, 480 | 10 |
| TRIM60 | 8.00 | 0.018 | 20.43 | 2.15 | 8, 82, 96, 134, 200, 251, 252, 255, 264, 271, 302, 322, 370, 405, 459 | 11 |
| TRIM69 | 6.65 | 0.036 | 19.16 | 2.46 | 14, 158, 192, 226, 246, 261, 285, 353, 371, 473 | 10 |
| TRIM75 | 10.61 | <0.005 | 0.67 | 12.24 | 45, 227 | 10 |
| TRIM76 | 42.29 | <0.005 | 1.71 | 7.94 | 306, 651, 1507, 2727, 2797, 3314 | 10 |
Note.—PAML model M7 (Ns sites model disallowing positive selection) was directly compared with M8 (Ns sites model permitting one extra category of codons evolving under positive selection) to detect positive selection (Yang 2007). We indicate the category of codons that were found to be in the category of positively selected codons and the average dN/dS associated with those codons. Sites underlined were also found to be under positive selection by Datamonkey (Delport et al. 2010).
TRIM52 Restriction Assays
We generated CRFK cell lines that stably express HA-tagged human and rhesus TRIM52 by transduction of a retrovirus vector (LPCX) encoding human and rhesus TRIM52 as described (Sawyer et al. 2005). Stable cell lines, including a negative control empty vector CRFK cell line, were plated on 12-well plates (0.8 × 105 cells/well). These were allowed to incubate overnight and then infected with the following GFP-encoding retroviruses: HIV-1, HIV-2 (ROD9), and FIV. We used a virus titer determined to give us at least 15% infection. Three days after infection, cells were fixed with paraformaldehyde and GFP expression was measured by flow cytometry.
Results
Positive Selection Has Acted on Several TRIM Genes in Primates
To screen the TRIM gene family for signatures of having participated in an evolutionary arms race, we evaluated TRIM orthologs from primates for recurrent positive selection via maximum likelihood analyses using the CODEML program from the PAML package (Yang 2007). We compared TRIM orthologs from human, chimpanzee, orangutan, rhesus, and marmoset reference genomes. In some instances, we were able to identify additional orthologs from other primate genome sequencing projects that are underway via Ensembl (Vilella et al. 2009) or from previous gene-directed sequencing efforts. For a few TRIM genes, we were unable to identify the full complement of orthologs, as the genes were either absent or not intact in the available genome assemblies. Using this collection of orthologs, we identified 17 out of 67 TRIM genes as having evolved under positive selection using a P value cutoff of 0.05: TRIM2, TRIML2, TRIM5, TRIM7, TRIM10, TRIM15, TRIM21, TRIM22, TRIM25, TRIM31, TRIM38, TRIM52, TRIM58, TRIM60, TRIM69, TRIM75, and TRIM76 (table 1 and supplementary table S1, Supplementary Material online). This list includes TRIM5 and TRIM22, restriction factors that were previously reported to show strong evidence of positive selection (Stremlau et al. 2004; Sawyer et al. 2005; Sawyer et al. 2007; Barr et al. 2008).
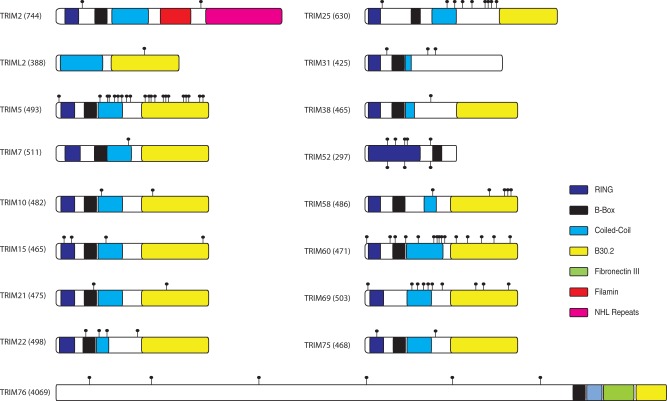
Our screen also recovered known restriction factors TRIM15, TRIM21, TRIM25, and TRIM38 for which no evolutionary analyses had been previously conducted. TRIM15 was discovered in a knockdown screen to inhibit the release of retroviruses (Uchil et al. 2008) and later found to have a role in the RIG-I sensing pathway (Uchil et al. 2013). We find sites of positive selection within the RING, Coiled-Coil, and B30.2 domains of TRIM15 (fig. 1). TRIM21 is able to degrade viruses via an intracellular antibody-mediated mechanism (Mallery et al. 2010). Positive selection was detected within the B-Box and B30.2 domains of TRIM21 (fig. 1). TRIM25 activates RIG-I signaling via ubiquitination (Gack et al. 2007), and TRIM25-mediated signal transduction is known to be inhibited by the direct interaction of influenza A protein NS1 to the Coiled-Coil domain of TRIM25 (Gack et al. 2009). We find that TRIM25 exhibits a number of sites under positive selection, clustered between the Coiled-Coil and B30.2 domains (fig. 1). TRIM38 is known to negatively regulate innate immunity by targeting TRIF (Xue et al. 2012), NAP1 (Zhao, Wang, Zhang, Yuan et al. 2012), and TRAF6 (Zhao, Wang, Zhang, Wang et al. 2012) for ubiquitination and degradation. TRIM38 has also been shown to improve the fitness of HIV-1 during entry by an unknown mechanism (Uchil et al. 2008). Only a single site of positive selection residing between the Coiled-Coil and B30.2 domains was detected (fig. 1).
Fig. 1.—
Architectures of TRIM family members exhibiting positive selection in primates. We present a domain schematic for all the proteins with signatures of positive selection in our evolutionary survey (table 1) along with their total length in amino acids (parentheses); in cases of alternate splicing, we represent the largest possible protein isoform encoded by a given TRIM gene. The schematized protein domains are based on GenBank and Ensembl reports. Sites of recurrent positive selection are marked with lollipops above the protein representation. The sites identified by a more in-depth analysis of TRIM52 are shown as lollipops below the protein representation.
The TRIM genes with known antiviral activity that we recovered in our positive selection screen (TRIM5, TRIM15, TRIM21, TRIM22, TRIM25, and TRIM38) belong to the C-IV family of TRIM genes based on their domain structure (Ozato et al. 2008). Our screen highlighted six additional genes from the C-IV family that have not been previously implicated in antiviral defense: TRIM7, TRIM10, TRIM58, TRIM60, TRIM69, and TRIM75. We found modest signatures of positive selection for three of these genes, TRIM7, TRIM10, and TRIM75. A single site was found to be under positive selection in TRIM7 in the Coiled-Coil domain (fig. 1). Similarly, TRIM75 has a single site of positive selection in its RING domain associated with E3 ubiquitin ligase activity. For TRIM10, positive selection was found in the Coiled-Coil and B30.2 domains, which may reflect changes in target recognition, similar to TRIM5 (Sawyer et al. 2005; Maillard et al. 2010). We further found evidence of robust positive selection for TRIM58, TRIM60, and TRIM69, which have multiple sites with high dN/dS values (fig. 1). Indeed, among all known primate TRIM genes, the signature of positive selection for these three genes appears to be on par with what we see for the bona fide restriction factor TRIM5. For TRIM58, we find a cluster of positively selected sites in the B30.2 domain and a single sight highlighted in the Coiled-Coil domain. TRIM60 has positively selected sites in each of the RING, B-Box, Coiled-Coil, and B30.2 domains (with significant clustering in the latter two domains). TRIM69, similarly, exhibits a cluster of sites under positive selection in the Coiled-Coil and B30.2 domains. Thus, this evolutionary screen of primate TRIM genes identified multiple new candidate restriction factors belonging to the same subfamily (C-IV) already known to harbor known antiviral genes.
We also identified candidate restriction factors outside of the C-IV family of TRIM genes: TRIM2 (C-VII), TRIML2 (UC), TRIM31 (C-V), TRIM52 (C-V), and TRIM76 (UC). TRIM2 contains a Filamin-type immunoglobulin domain and array of NHL repeats. Two sites of positive selection were found in TRIM2, with neither of these residing within the known domains of TRIM2 (fig. 1). TRIML2 is a highly unusual TRIM-like gene. It lacks canonical RING and B-box domains, being solely composed of Coiled-Coil and B30.2 domains. Formally, it does not meet the criteria of being an RBCC-type TRIM gene. However, given the propensity of TRIM proteins to homo- and heterodimerize, we also included such noncanonical genes within our analysis. We find a single site of positive selection within the C-terminus B30.2 domain of TRIML2 (fig. 1). TRIM31 is a suspected retroviral restriction factor that acts at the stages of entry and release for HIV-1 and MLV, respectively (Uchil et al. 2008). We identified three sites exhibiting signatures of positive selection in TRIM31; two of these sites are in the C-terminal region, which is not homologous to any known TRIM-associated domains but may represent an analogous virus-interacting domain (fig. 1). TRIM52 is unique among the restriction factor candidates as it only encodes the RING and B-Box domains. We identified the majority of positive selection within the RING domain and a single site immediately upstream of the B-Box domain. Intriguingly, the RING domain of TRIM52 has expanded and is the largest among the genes recovered by our screen (fig. 1); this expansion also appears to contain the positively selected sites. TRIM76 is the final candidate identified. It encodes for a large ∼3,500 amino-acid protein that contains B-Box, Coiled-Coil, Fibronectin III, and B30.2 domain in its C-terminus region. The remainder of the protein does not contain homology to any annotated domains but contains the six sites of positive selection we identified.
Thus, our evolutionary screen for novel restriction factors among the TRIM gene family identified 15 members not previously known to be under positive selection. Most excitingly, our screen identifies as many as 10 novel candidates for antiviral function. Although all of these TRIM genes may not participate in host–pathogen interactions, several exciting canonical candidates (e.g.,TRIM58 and TRIM60) emerged from our screen. In addition, we identified several noncanonical candidates (e.g., TRIML2 and TRIM52) that may have otherwise been overlooked as potential restriction factors because they are missing some of the key RBCC domains. We decided to pick one of these noncanonical candidates, TRIM52, for a more in-depth analysis to confirm and expand the findings of our initial screen.
Rapid Evolution of the TRIM52 RING Domain in Primates
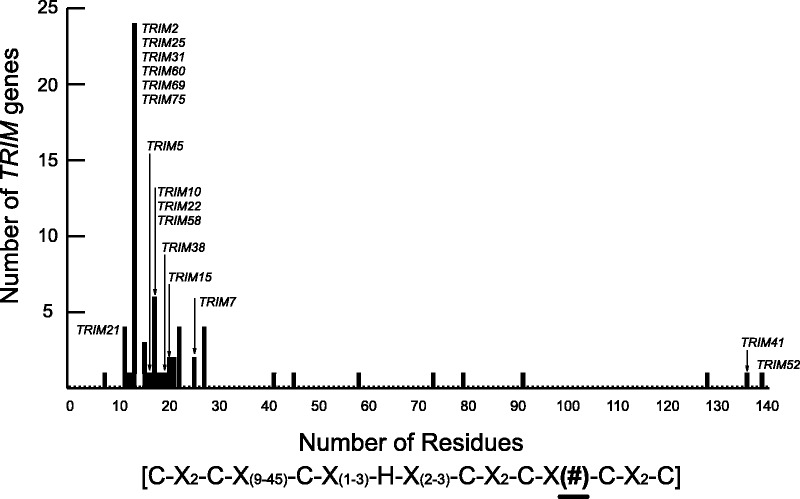
We decided to evaluate TRIM52 in more detail because it structurally deviated the most from the canonical TRIM restriction factors (i.e., TRIM5 and TRIM22). For example, TRIM52 lacks the viral recognition (B30.2) domain and displays signatures of rapid evolution within the RING domain. Moreover, TRIM52 appears to lack an intact Coiled-Coil domain within its coding region (fig. 1). Thus, TRIM52 is comprised solely of the RING and B-Box2 domains, making it a highly unusual member of the TRIM multigene family. Even the RING domain of TRIM52 is highly unusual. RING domains of the TRIM family are generally defined by the consensus sequence Cx2Cx9-45Cx1-3Hx2-3Cx2Cx4-48Cx2[C/D], where eight cysteine, histidine, or aspartic acid residues coordinate two zinc atoms (Meroni and Diez-Roux 2005). The region between the sixth and the seventh coordinating residues is referred to as the “loop 2” region of the RING tertiary structure using the precedent of the human c-cbl RING-containing E3 ubiquitin ligase (Zheng et al. 2000). The majority of TRIM genes encode between 4 and 48 amino acids in their loop 2 region, with the mode being 13 amino acids (fig. 2). However, several TRIM genes were found to deviate from the consensus range. Most notably, TRIM52 encodes 139 amino acids in its loop 2 region (fig. 2). Thus, TRIM52 encodes the largest RING domain of any human TRIM gene. BLAST (Altschul et al. 1990) analysis of this region reveals similarity only to mammalian TRIM52 and TRIM41 genes, both of which have exceptionally large RING domain expansions.
Fig. 2.—
Variability in the length of the RING domain. The RING domains from 67 annotated human TRIM genes were collected from Ensembl (Flicek et al. 2012) and GenBank and evaluated to determine the length of the variable loop 2 region located within the domain. Alignments of homologous regions were built using ClustalX (Larkin et al. 2007) and the number of residues residing in the variable region were counted. The predicted length of this variable region ranges from 4 to 48 amino acids. TRIM52 and TRIM41 have the largest expansion of their RING domains.
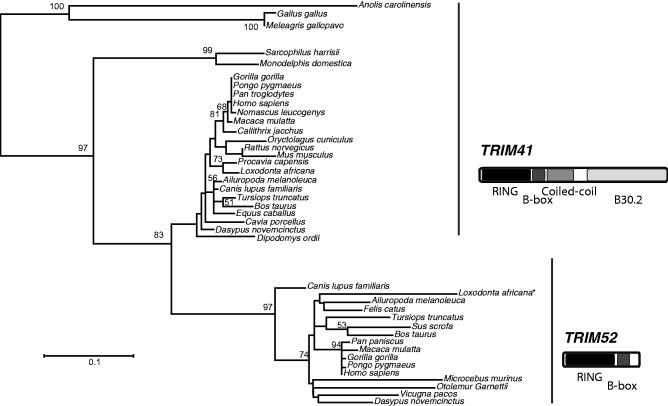
In order to elucidate the evolutionary relationship between TRIM52 and TRIM41 and to deduce when this large loop 2 RING expansion occurred, we carried out phylogenetic analyses of TRIM52 and TRIM41 sequences that were obtained from Blast (Altschul et al. 1990) searches of vertebrate genomes. Our analyses revealed that TRIM52 and TRIM41 are close paralogs that are found in close proximity to each other in most mammalian genomes (fig. 3). We found that the reptile (anole lizard), avian (chicken and wild turkey), and marsupial (Tasmanian devil and opossum) genomes have only single TRIM41-like genes, which are phylogenetic outgroups to both the TRIM52 and TRIM41 clades from eutherian mammals (fig. 3). This suggests that TRIM52 was born in eutherian mammals ∼190 Ma via a partial duplication of TRIM41, having lost both the Coiled-Coil and B30.2 domains at birth (Meredith et al. 2011).
Fig. 3.—
Phylogenetic relationship of TRIM52 and TRIM41. A phylogram of homologous regions of the RING and B-Box2 domains from TRIM52 and TRIM41 orthologs was built using a maximum likelihood based approach via PhyML (Guindon et al. 2010). Statistical support is represented by bootstrap values, collected from 100 iterations. The * symbol denotes the presence of nonsense mutations that result in pseudogenization.
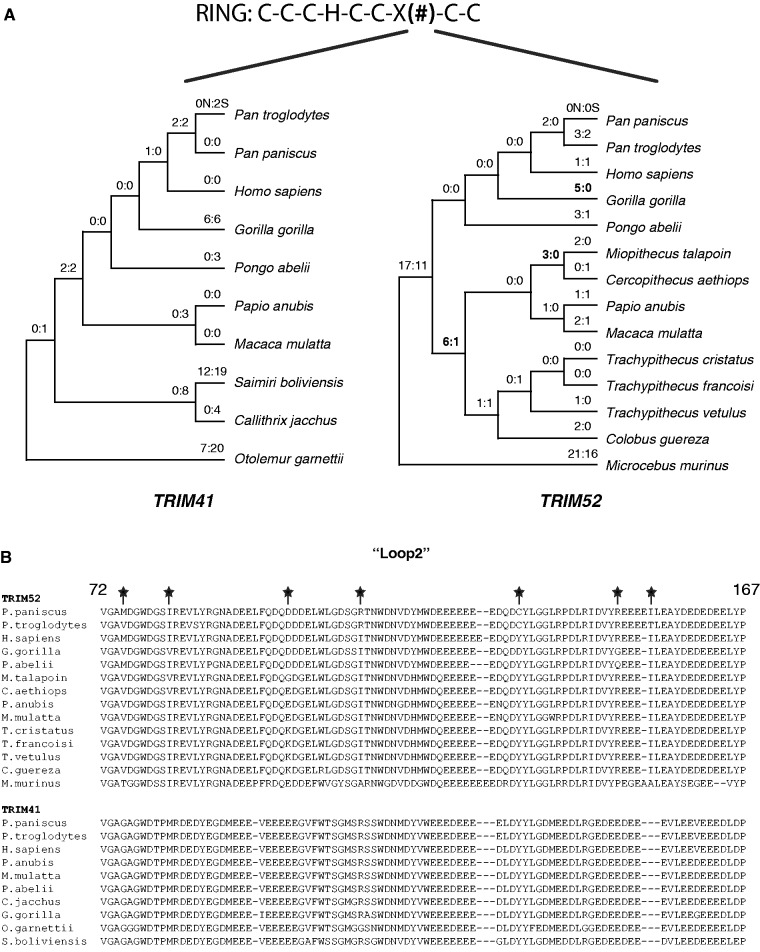
Despite their evolutionary relationship, our screen for positive selection in primates highlighted TRIM52 but not TRIM41. To further evaluate the evolutionary history of TRIM52, we repeated our analysis of recurrent, site-based positive selection via maximum likelihood analyses using primate TRIM52 orthologs obtained by our additional sequencing efforts. From this in-depth analysis, we refined the sites of recurrent, codon-based positive selection (figs. 1 and 4B). The sites of positive selection reside primarily within the expanded “loop 2” region of TRIM52. This rapid evolution of the RING domain is especially evident in an evolutionary comparison focused on the “loop 2” expansion unique to TRIM41 and TRIM52, which highlights the dramatic acceleration of amino acid replacements in TRIM52 (fig. 4A and B). In contrast to TRIM52, we found no evidence of positive selection having acted on the TRIM41 using available primate sequences from databases (fig. 4A). Thus, in stark contrast to TRIM41 and its RING domain that has been evolving under constraint, we find that TRIM52 has been rapidly evolving throughout primate history, with much of that selection acting on the expanded RING domain.
Fig. 4.—
Positive selection within the RING domain of TRIM52. (A) More than half the sites predicted to be evolving under positive selection (fig. 1 and table 1) are located within the RING domain of TRIM52. To further highlight this, we identified the number of synonymous (S) and nonsynonymous (N) substitutions that have occurred in the expanded loop 2 region of TRIM52 in primate evolution (the equivalent domain of TRIM41 is shown for comparison). Examples of dramatic episodes of lineage-specific positive selection in TRIM52’s RING domain are highlighted in bold. (B) The differences in evolutionary signals are further demonstrated by an alignment of a 90 amino acid-long stretch of the loop 2 region from primate TRIM41 and TRIM52. Sites of positive selection are highlighted with a star and boldface.
Repeated Loss/Pseudogenization of TRIM52 in Mammals
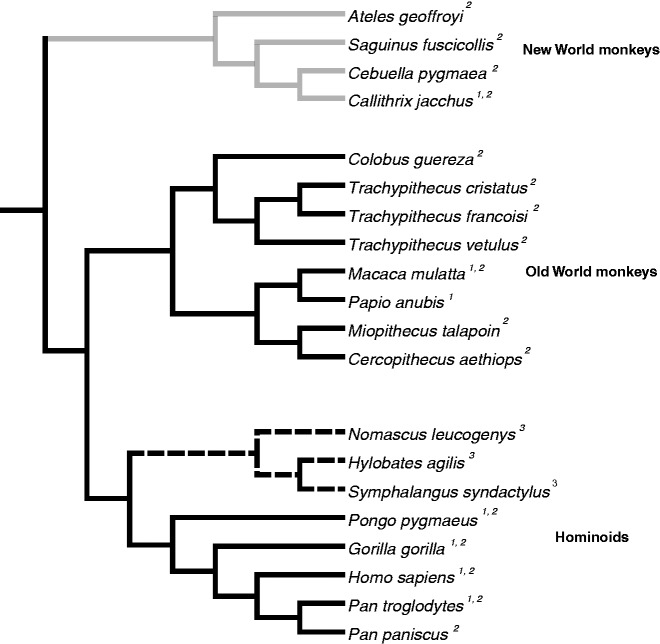
Our sequencing survey also revealed at least two instances of TRIM52 loss or pseudogenization over the course of primate evolution (fig. 5). For instance, within marmoset and other New World monkey genomes, we were only able to identify exon 2 using a combination of Blast (Altschul et al. 1990) and BLAT (Kent 2002) searches (supplementary fig. S1, Supplementary Material online) and our own polymerase chain reaction (PCR) analyses. These analyses suggested that TRIM52 is present but pseudogenized throughout the New World monkey lineage. We were also unable to detect TRIM52 from gibbon genomes (N. leucogenys, Hylobates agilis, and Symphalangus syndactylus) via PCR with genomic DNA, despite using PCR primers that amplified TRIM52 from all other Hominoids and Old World monkeys. Blast (Altschul et al. 1990) and BLAT (Kent 2002) analyses support the absence of TRIM52 from publicly available gibbon genomes.
Fig. 5.—
Presence/absence of TRIM52 in primates. We evaluated TRIM52 from a range of Hominoids, Old World monkeys, and New World monkeys, using sequences collected from 1) Ensembl (Flicek et al. 2012) and GenBank and via 2) PCR. Primates surveyed by our analysis are presented in a guide tree of the well-accepted primate phylogeny (Perelman et al. 2011). 3) We were unable to amplify TRIM52 from the gibbon lineage of Hominoids (represented by dotted branches), despite the use of primers that we used to amplify orthologs from other Hominoids and Old World monkeys. Grayed branches represent lineages where we observed TRIM52 to be pseudogenized.
This pattern of stochastic TRIM52 loss was also evident in other mammalian orders. We identified TRIM52 pseudogenization or loss in African elephant (Loxodonta africana), horse (Equus caballus), microbat (Myotis lucifugus), and megabats (Pteropus vampyrus) (supplementary fig. S1, Supplementary Material online). We were also unable to identify TRIM52 throughout the glires (Rodentia and Lagomorpha) lineage of mammals, suggesting that it has been deleted early within this lineage. However, utilizing UCSC (Kent et al. 2002) and Ensembl (Flicek et al. 2012) predictions, we were able to recover TRIM52 from the genomes of the mouse and rat. Sequence analysis of these predicted mouse and rat TRIM52 revealed that they do not encode a B-Box domain. Therefore, the annotated mouse and rat TRIM52 comprised only a RING domain. Furthermore, the TRIM52 orthologs we did identify in mouse and rat genomes were not located proximal to TRIM41 and are therefore the only nonsyntenic TRIM52 orthologs in mammals (supplementary fig. S2, Supplementary Material online). When we included mouse and rat TRIM52 in our phylogenetic analysis (supplementary fig. S3, Supplementary Material online), branch support at the node separating the TRIM41 and TRIM52 clades was lowered (even though mouse and rat TRIM52 genes localized within the TRIM52 clade). Due to the apparent loss of TRIM52 throughout glires, the truncated structure of mouse and rat TRIM52, and their ambiguous phylogenetic placement, we therefore cannot confidently assign these mouse and rat TRIM genes as bona fide TRIM52 orthologs, labeling them TRIM52-like instead (supplementary fig. S1, Supplementary Material online). Given this uncertainty, we had omitted the mouse and rat TRIM52-like sequences from our phylogenetic analysis (fig. 3). Additional genome sequencing within eutherian mammals may reveal still additional instances of TRIM52 loss or pseudogenization, suggestive of episodes of relaxed selective pressure among individual lineages.
Human and Rhesus TRIM52 Do Not Restrict Lentiviruses
The history of positive selection uncovered among primate TRIM52 orthologs indicates that its function has been adaptively evolving. Although many members of the TRIM family positively and negatively impact retroviruses (Uchil et al. 2008), TRIM52 was not tested in previous analyses. Indeed, the degree of adaptive evolution within the RING domain of TRIM52 suggests that the role of viral recognition has shifted in the absence of the B30.2 domain to the RING domain. Thus, we evaluated human and rhesus TRIM52 orthologs for antiviral activity against a limited panel of lentiviruses (supplementary fig. S4, Supplementary Material online). Although many of these viruses are restricted by other TRIM proteins, we found no evidence of restriction by either human or rhesus TRIM52. Thus, although the evolutionary patterns of positive selection and episodic loss strongly implicate TRIM52 in some form of host defense, the targets of this activity are still unknown and likely not retroviral.
Discussion
TRIM52, A Candidate Antiviral Gene
A screen for positive selection identified 15 new members of the primate TRIM gene family. Of these, TRIM52 was the most unusual. Indeed, in the absence of these evolutionary analyses, TRIM52 might not draw attention as a candidate antiviral factor because it lacks a canonical virus-interaction domain (fig. 1). Although TRIM52 lacks B30.2 and Coiled-Coil domains, the gene bears an ancient expansion of the RING domain that exhibits positive selection. TRIM52 is not the first candidate antiviral TRIM gene that lacks a canonical viral capsid-binding domain, however. In a previous analysis of rodent TRIM5 paralogs, we identified mouse (Mus musculus) TRIM12, which only encodes RING, B-Box2, and Coiled-Coil domains (Tareen et al. 2009). Similar to TRIM52, mouse TRIM12 exhibits signatures of positive selection despite the absence of a recognized interaction interface (i.e., B30.2 domain). Indeed, our finding of positive selection within the RING domain leads to the intriguing model whereby the antiviral interaction interface of TRIM52 may have now shifted to within its RING domain. This is an unusual exception to the highly modular arrangement of the mammalian and fish TRIM gene family in which the target interaction interface is usually restricted to the Coiled-Coil or B30.2 domains, which are also the hotspots for positive selection (Reymond et al. 2001; Meroni and Diez-Roux 2005; Nisole et al. 2005; Song, Gold et al. 2005; Yap et al. 2005; Sawyer et al. 2007; van der Aa et al. 2009).
Despite the strong signature of positive selection, we identified at least six independent losses of TRIM52 within mammals, including two events in primates. The absence of TRIM52 from gibbon genomes may reflect its genomic position, proximal to the telomeric region in Hominoids and Old World monkeys. However, this genomic positioning is not shared in other mammals (supplementary fig. S2, Supplementary Material online) and therefore cannot account for the multiple loss events we have observed. Furthermore, we found no evidence for either loss or pseudogenization of the proximally located TRIM41 gene. This suggests that the parental gene is under strong functional constraint, whereas the episodes of TRIM52 loss strongly suggest that this TRIM gene does not carry out a conserved, housekeeping function in mammalian genomes, further supporting its proposed role as an antiviral factor. Intriguingly, the recurrent loss of TRIM52 is reminiscent of the dynamic evolutionary history observed by other TRIM genes with antiviral function. For instance, the dog TRIM5 ortholog is pseudogenized (Sawyer et al. 2007), whereas cats encode a truncated form of TRIM5 with a disrupted B30.2 domain; both lineages are unable to express TRIM5alpha (McEwan et al. 2009). Both rodent (mouse and rat) and cow genomes lack TRIM22 orthologs but contain expanded sets of TRIM5 paralogs (Sawyer et al. 2007; Tareen et al. 2009). Expansions are not unique to TRIM5. Han et al (2011) identified several TRIM genes that are copy number variable in human genomes. Similar dynamics have also been observed in several teleost species, where unique TRIM genes (fintrims) have expanded and diversified in each lineage (van der Aa et al. 2009). We have previously suggested that positive selection and the expansion of TRIM genes is driven by new or continuous selective pressure, likely provided by viral pathogens (Sawyer et al. 2007; Tareen et al. 2009; Han et al. 2011). Similarly, the loss or relaxation of such a selective pressure could result in the loss of a TRIM gene (Sawyer et al. 2006; Sawyer et al. 2007). Thus, considering the dynamic history of TRIM52 and our evidence of positive selection, we posit that this unusual TRIM gene is involved in genome defense but can be lost either due to relaxed selection or because of the high costs borne by encoding such a defense (Sawyer et al. 2006).
It is also possible that TRIM52 is under positive selection not because of antiviral activity but instead to maintain its interaction with a host target substrate that is also adaptively evolving. However, we find this coevolutionary scenario unlikely because such host–host interaction surfaces are not typically found to evolve under positive selection unless they are challenged by a pathogenic influence (Koyanagi et al. 2010; Daugherty and Malik 2012). Furthermore, this scenario would posit that the many incidences of TRIM52 loss we have documented would have to coincide with the simultaneous loss of the target substrate or the requirement to maintain the interaction.
Positive Selection within the TRIM Gene Family
Based on the unbiased approach of our screen, we predicted the recovery of several known restriction factors. In particular, there was an expectation of identifying TRIM5 and TRIM22, both previously highlighted for their positive selection (Sawyer et al. 2005; Sawyer et al. 2007). In addition to these, we recovered other known or suspected restriction factors: TRIM15, TRIM21, TRIM25, and TRIM38. We detected positive selection occurring all along TRIM25, in particular within the Coiled-Coil and B30.2 domains (fig. 1). TRIM25 plays a role in influenza infection, where its activity is critical for the activation of the RIG-I-dependent signaling cascade (Gack et al. 2007). Specifically, influenza A encodes protein NS1 that directly interacts and inhibits TRIM25 at the Coiled-Coil domain inhibiting the ubiquitination and activation of RIG-I. This is reminiscent of adaptive evolution in other known restriction factors, such as in the case of MAVS to evade protease cleavage by hepatitis C virus (Patel et al. 2012) or in tetherin to evade lentivirus Nef or Vpu (Lim et al. 2010). In both cases, positive selection highlights regions of the host-encoded protein targeted by viral antagonists and provided insight into mechanisms of host evasion. Thus, it is likely that the sites of positive selection exhibited by TRIM25 reveal adaptation during primate history to evade NS1 or NS1-like antagonists. TRIM15 similarly plays a role regulating innate immune signaling, and its positive selection may reflect a similar constraint as TRIM25. In contrast, TRIM21 is able to target cytosolic antibodies bound to viruses and auto-ubiqutinate, leading to the proteasomal degradation of the TRIM21-bound complex (Mallery et al. 2010). As this complex forms via TRIM21 binding to the invariant region of antibodies (James et al. 2007), it is unlikely that the interaction between host-encoded products is responsible for the positive selection we detected. Instead, it is much more likely that a novel viral antagonist targets TRIM21 and that positive selection is reflective of evasion from such an antagonist. Unique among these recovered TRIM genes, TRIM38 has been found to assist HIV-1 during entry (Uchil et al. 2008). TRIM38 has recently been recognized for having a role in negatively regulating innate immunity by targeting components of innate immunity for degradation (Xue et al. 2012; Zhao, Wang, Zhang, Yuan et al. 2012; Zhao, Wang, Zhang, Wang et al. 2012). Positive selection on TRIM38 may therefore also reflect its escape from viral-mediated antagonism of innate immunity. Thus, our analysis of positive selection provides insight into the interface and nature of host–pathogen interactions in cases of known restriction factors (fig. 6). Specifically, we expect these sites of positive selection to be affecting structure, either indirectly altering the host–pathogen interface or at the direct interface contacting viral proteins. In specialized cases like TRIM5, this direct interaction is predicted of a rapidly evolving antiviral TRIM gene. Alternatively, as we predict of TRIM25, positive selection is reflective of evading viral antagonism.
Fig. 6.—
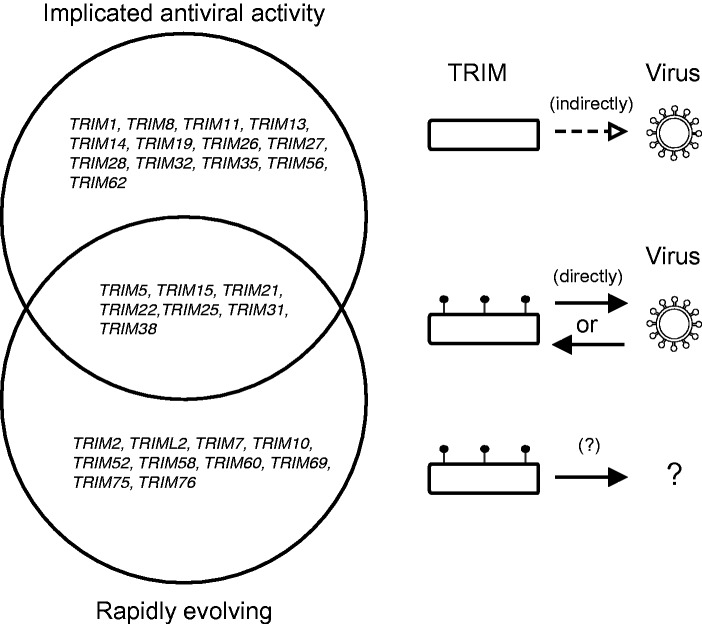
Implicated restriction factors. Of 67TRIM genes, several members have been implicated as restriction factors, either positively or negatively impacting viral fitness. In many of these cases, direct interactions with viral proteins has not been detected (top) (reviewed in Ozato et al. 2008; Kawai and Akira 2011). Seven of these genes have evolved under positive selection in primates—two that were previously published, TRIM5 and TRIM22 (Sawyer et al. 2007; Sawyer et al. 2005), in addition to TRIM25 (Gack et al. 2009), TRIM21 (Mallery et al. 2010), TRIM15, TRIM31, and TRIM38 (Uchil et al. 2008) (Overlap). We believe these restriction factors likely act via a direct interaction interface to recognize or evade viral proteins. In addition, we found ten TRIM genes to be rapidly evolving that represent novel restriction factor candidates which may also act via direct host–virus interactions (bottom).
One TRIM gene that did not show a signature of positive selection at all is TRIM19/PML. This is in agreement with an extended sequencing of primate TRIM19/PML orthologs which concluded that there was no evidence for positive selection of this gene (Ortiz et al. 2006). This may be surprising in light of the evidence that PML functions in antiviral defense (reviewed in Nisole et al. 2005). However, positive selection would only be expected to act on genes encoding proteins that directly interact with viral proteins, and so any upstream or downstream effector may not present such a signal. It is interesting that TRIM1 (MID2) also shows no adaptive signature, given that the human TRIM1 protein has been shown to have anti-MLV activity (Yap et al. 2004). This may reflect a retroviral restriction that is not currently being utilized by humans or chimpanzees, because our screen was especially focused on this lineage of mammals. Nonetheless, it is important to point out that the absence of positive selection does not preclude TRIM genes from being candidate restriction factors, but those TRIM genes that have evolved under positive selection represent the most likely candidates for having an antiviral role via direct interaction (fig. 6).
As many of the TRIM genes remain largely uncharacterized, our evolutionary screen is able to highlight candidate restriction factors based on exhibition of positive selection, a hallmark of antiviral genes at the direct interface of the host–viral pathogen arms race (Daugherty and Malik 2012). Based on the extent of rapid evolution observed among them, we propose that TRIM58, TRIM60, and TRIM69 represent the best uncharacterized candidates for novel restriction factors within the primate TRIM multigene family and should therefore be intensively investigated for antiviral function (fig. 6).
Based on previous studies with APOBEC and TRIM5 restriction genes, it is informative to identify antiviral restriction factors even if they are not currently active against modern viral pathogens. Restriction factors honed against evolutionarily “recent” viral infections might protect us against future viruses or viral variants or might be artificially enhanced to be active against current forms. Genes with partial activity might vary in potency within the human population. Furthermore, such genes serve as barriers to animal models of viral infection (Hatziioannou et al. 2006; Kirmaier et al. 2010). To this end, our evolutionary approach to identify potential restriction factors in the TRIM family has revealed ten canonical and noncanonical members of this gene family that bear previously unrecognized signatures of recent positive selection. These primate TRIM genes are therefore primate candidates to be investigated as novel restriction factors against viruses.
Supplementary Material
Supplementary figures S1–S4 and table S1 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
The authors thank Janet Young and Patrick Mitchell for discussions and comments on the manuscript. This work was supported by amfAR grants 107447-45-RGNT (to S.L.S.), a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (to S.L.S.), NIH R37 AI30927 (to M.E.), and an NSF CAREER award (to H.S.M.). R.M.B. was supported by FHCRC Interdisciplinary Training Grant T32 CA 80416 and a Ruth L. Kirschstein National Research Service Award for Individual Predoctoral Fellowship F31 A1084620. H.S.M. is an Early Career Scientist of the Howard Hughes Medical Institute.
Literature Cited
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SD, Smiley JR, Bushman FD. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 2008;4:e1000007. doi: 10.1371/journal.ppat.1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battivelli E, et al. Modulation of TRIM5alpha activity in human cells by alternatively spliced TRIM5 isoforms. J Virol. 2011;85:7828–7835. doi: 10.1128/JVI.00648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Kodama T, Hu SL. Novel TRIM5 isoforms expressed by Macaca nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthagena L, et al. Human TRIM gene expression in response to interferons. PLoS One. 2009;4:e4894. doi: 10.1371/journal.pone.0004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty M, Malik H. Rules of engagement: molecular insights from host-virus arms races. Annu Rev Genet. 2012;46:675–698. doi: 10.1146/annurev-genet-110711-155522. [DOI] [PubMed] [Google Scholar]
- Delport W, Poon AF, Frost SD, Kosakovsky Pond SL. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26:2455–2457. doi: 10.1093/bioinformatics/btq429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NK, Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat Rev Immunol. 2012;12:687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AJ, Hué S, Schaller T, Pillay D, Towers GJ. Hare TRIM5α restricts divergent retroviruses and exhibits significant sequence variation from closely related lagomorpha TRIM5 genes. J Virol. 2010;84:12463–12468. doi: 10.1128/JVI.01514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicek P, et al. Ensembl 2012. Nucleic Acids Res. 2012;40: D84–D90. doi: 10.1093/nar/gkr991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Gack MU, et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, et al. Hexagonal assembly of a restricting TRIM5alpha protein. Proc Natl Acad Sci U S A. 2011;108:534–539. doi: 10.1073/pnas.1013426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SP. Retrovirus restriction factors. Mol Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Han K, Lou DI, Sawyer SL. Identification of a genomic reservoir for new TRIM genes in primate genomes. PLoS Genet. 2011;7:e1002388. doi: 10.1371/journal.pgen.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, et al. Generation of simian-tropic HIV-1 by restriction factor evasion. Science. 2006;314:95. doi: 10.1126/science.1130994. [DOI] [PubMed] [Google Scholar]
- Huson DH, et al. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci U S A. 2007;104:6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Sawyer SL. Molecular evolution of the antiretroviral TRIM5 gene. Immunogenetics. 2009;61:163–176. doi: 10.1007/s00251-009-0358-y. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol Med. 2011;3:513–527. doi: 10.1002/emmm.201100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier A, et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 2010;8:e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, et al. Diversifying selection and functional analysis of interleukin-4 suggests antagonism-driven evolution at receptor-binding interfaces. BMC Evol Biol. 2010;10:223. doi: 10.1186/1471-2148-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratovac Z, et al. Primate lentivirus capsid sensitivity to TRIM5 proteins. J Virol. 2008;82:6772–6777. doi: 10.1128/JVI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lim ES, Malik HS, Emerman M. Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J Virol. 2010;84:7124–7134. doi: 10.1128/JVI.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21(10):375–382. [PubMed] [Google Scholar]
- Maillard PV, Ecco G, Ortiz M, Trono D. The specificity of TRIM5 alpha-mediated restriction is influenced by its coiled-coil domain. J Virol. 2010;84:5790–5801. doi: 10.1128/JVI.02413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery DL, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci U S A. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, et al. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33:D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, et al. The SPRY domain of SSB-2 adopts a novel fold that presents conserved Par-4-binding residues. Nat Struct Mol Biol. 2006;13(1):77–84. doi: 10.1038/nsmb1034. [DOI] [PubMed] [Google Scholar]
- McEwan WA, et al. Truncation of TRIM5 in the Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J Virol. 2009;83:8270–8275. doi: 10.1128/JVI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16(4):404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- McNab FW, Rajsbaum R, Stoye JP, O'Garra A. Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol. 2011;23:46–56. doi: 10.1016/j.coi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Meredith RW, et al. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger' E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- Meyerson NR, Sawyer SL. Two-stepping through time: mammals and viruses. Trends Microbiol. 2011;19:286–294. doi: 10.1016/j.tim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- Ortiz M, Bleiber G, Martinez R, Kaessmann H, Telenti A. Patterns of evolution of host proteins involved in retroviral pathogenesis. Retrovirology. 2006;3:11. doi: 10.1186/1742-4690-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini LD, Keckesova Z, Towers GJ. Retroviral restriction factors Fv1 and TRIM5alpha act independently and can compete for incoming virus before reverse transcription. J Virol. 2006;80:2100–2105. doi: 10.1128/JVI.80.5.2100-2105.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Loo YM, Horner SM, Gale M, Jr, Malik HS. Convergent evolution of escape from hepaciviral antagonism in primates. PLoS Biol. 2012;10:e1001282. doi: 10.1371/journal.pbio.1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman P, et al. A molecular phylogeny of living primates. PLoS Genet. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm N, et al. Unique spectrum of activity of prosimian TRIM5{alpha} against exogenous and endogenous retroviruses. J Virol. 2011;85(9):4173–4183. doi: 10.1128/JVI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SL, Emerman M, Malik HS. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007;3:e197. doi: 10.1371/journal.ppat.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Akey JM, Emerman M, Malik HS. High-frequency persistence of an impaired allele of the retroviral defense gene TRIM5alpha in humans. Curr Biol. 2006;16:95–100. doi: 10.1016/j.cub.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci U S A. 2005;102:2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller T, Hue S, Towers GJ. An active TRIM5 protein in rabbits indicates a common antiviral ancestor for mammalian TRIM5 proteins. J Virol. 2007;81:11713–11721. doi: 10.1128/JVI.01468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28(1):231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto MH, Liu HL, Zajchowski DA, Whitlow M. Protein fold analysis of the B30.2-like domain. Proteins. 1999;35(2):235–249. [PubMed] [Google Scholar]
- Si Z, et al. Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc Natl Acad Sci U S A. 2006;103:7454–7459. doi: 10.1073/pnas.0600771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Gold B, et al. The B30.2(SPRY) domain of the retroviral restriction factor TRIM5alpha exhibits lineage-specific length and sequence variation in primates. J Virol. 2005;79:6111–6121. doi: 10.1128/JVI.79.10.6111-6121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Javanbakht H, et al. Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J Virol. 2005;79:3930–3937. doi: 10.1128/JVI.79.7.3930-3937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, et al. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Stremlau M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci U S A. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen SU, Emerman M. Human Trim5α has additional activities that are uncoupled from retroviral capsid recognition. Virology. 2011;409:113–120. doi: 10.1016/j.virol.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen SU, Sawyer SL, Malik HS, Emerman M. An expanded clade of rodent Trim5 genes. Virology. 2009;385:473–483. doi: 10.1016/j.virol.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil PD, Quinlan BD, Chan WT, Luna JM, Mothes W. TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 2008;4:e16. doi: 10.1371/journal.ppat.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil PD, et al. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J Virol. 2013;87:257–272. doi: 10.1128/JVI.01804-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aa LM, et al. A large new subset of TRIM genes highly diversified by duplication and positive selection in teleost fish. BMC Biol. 2009;7:7. doi: 10.1186/1741-7007-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella AJ, et al. EnsemblCompara GeneTrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19:327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, et al. TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting TRIF for degradation. PLoS One. 2012;7:e46825. doi: 10.1371/journal.pone.0046825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Chen ZJ. Intrinsic antiviral immunity. Nat Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Lynch C, Stoye JP. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci U S A. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Yap MW, et al. Restriction of foamy viruses by primate Trim5alpha. J Virol. 2008;82(11):5429–5439. doi: 10.1128/JVI.02462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen LM, et al. Isolation of an active Lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals. J Virol. 2006;80:7332–7338. doi: 10.1128/JVI.00516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Hatziioannou T, Perez-Caballero D, Derse D, Bieniasz PD. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology. 2006;353(2):396–409. doi: 10.1016/j.virol.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Yuan C, Gao C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. J Immunol. 2012;188:2567–2574. doi: 10.4049/jimmunol.1103255. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Wang P, et al. Tripartite motif-containing protein 38 negatively regulates TLR3/4- and RIG-I-mediated IFN-β production and antiviral response by targeting NAP1. J Immunol. 2012;188:5311–5318. doi: 10.4049/jimmunol.1103506. [DOI] [PubMed] [Google Scholar]
- Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.