Abstract
Background
All three isoforms of the nitric oxide synthase (NOS) are expressed in the atherosclerotic plaque. To test whether neuronal NOS (nNOS) deficiency affects atherosclerosis, we studied “western-type” diet fed apoE/nNOSα double knockout (DKO) mice, which carry a deletion of nNOS exon 2 and apoE knockout (KO) control animals.
Methods and Results
Mice were fed a “western type” diet for 14 or 24 weeks. After 14 weeks male DKO animals showed a significant 66% increase in lesion-area. Lesion-area in female DKO animals was unchanged at 14 weeks, but significantly increased by 31% after 24 weeks. Moreover, mean arterial blood pressure was significantly reduced in female DKO animals, but was unchanged in male animals. Immunohistochemistry revealed strong nNOS expression in the neointima of KO mice. In DKO animals residual nNOS staining was due to the presence of nNOS splice variants. While nNOSα was present in vessels of KO and absent in DKO animals, nNOSγ was expressed in KO and DKO mice.
Conclusion
nNOSα protects against atherosclerosis as nNOSα deletion leads to an increase in plaque formation in apoE/nNOSα DKO mice. Female DKO mice showed a significant reduction in mean arterial blood pressure which was not observed in male animals. Additionally, we found expression of nNOS splice variants in vessels of apoE KO mice. Our data highlights nNOSα overexpression as a potential therapeutic strategy and naturally occurring splice variants that lack exon 2 of the nNOS gene as a potential risk factor for vascular disease.
Introduction
Neuronal nitric oxide synthase (nNOS) is expressed in early and advanced human atherosclerotic lesions1. Immunolocalization and in situ hybridization revealed nNOS expression in endothelial cells, macrophages and smooth muscle cells. In addition nNOS expression is found in perivascular nitrinergic neurons2-4. Although nNOS and endothelial nitric oxide synthase (eNOS) are termed constitutive NOS isoforms, nNOS is only detectable in intact human vessels using supersensitive methods, suggesting that expression may be induced in atherosclerosis1, 5. The nNOS gene produces multiple mRNA splice variants through various mechanisms, namely alternate promotor usage, alternative splicing, cassette insertion and deletions and varied sites of 3′-UTR cleavage and polyadenylation (For review see6). These mechanisms lead to four different peptides of which two have a PDZ-domain that anchors them to the sarcoplasmic reticulum, while two lack the PDZ-domain, localizing them to the cytosol6. Schwarz et al. reported the presence of small amounts of brain-type nNOSα and muscle-type nNOSμ in the media and adventitia of rat aorta and showed that nNOS may exert an inhibitory effect against a vasoconstrictive response7. Recent studies in a mouse carotid artery ligation model, as well as a rat model of balloon induced vascular injury, demonstrated that nNOS is expressed following vascular injury and inhibits intima proliferation, pointing towards a vasculoprotective role of nNOS8. So far the relevance of nNOS expression in spontaneous plaque formation has not been addressed. To study the relative contribution of nNOS to lesion formation, we combined a genetic model of chronic nNOSα deficiency (nNOS KO) with a mouse model of atherosclerosis, the hypercholesterolemic apoE KO mouse. ApoE KO mice develop progressive endothelial dysfunction which is more pronounced in “western type“-diet fed animals9. As previously published, deletion of eNOS leads to a dramatic increase in lesion formation in apoE KO atherosclerosis10. While eNOS deletion gave rise to an array of vascular complications including abdominal aneurysms, aortic dissections and ischemic heart disease, deletion of the inducible NOS (iNOS) decreased atherosclerosis and plasma levels of lipidperoxides in apoE KO animals11. The latter suggests that iNOS derived NO formation is proatherogenic, partly through increasing oxidative stress11. The current study tests the hypothesis that nNOS expression in atherosclerotic lesions inhibits plaque progression. In this case, genetic deficiency of nNOS would accelerate atherosclerosis. Additionally we studied gender dependence of lesion formation, the influence of nNOS deletion on blood pressure regulation and the expression of nNOS splice variants in atherosclerotic plaques.
Methods
All procedures performed conformed with the policies of the University of Würzburg, the NIH guidelines and an independent governmental committee for care and use of laboratory animals.
Mice
nNOS KO mice were generated by targeted deletion in the laboratory of Paul Huang12. The mice carry a deletion of the flanking region of exon 2, ablating translational start of the full-length brain-spliced nNOSα. ApoE KO animals were purchased from the Jackson Laboratories. All mice were backcrossed for ten generations to the C57BL/6 strain. nNOS KO and apoE KO animals were crossed to generate double heterozygous mice. From the F2-mating, apoE KO animals heterozygous for nNOS, were selected and the offspring were genotyped for nNOS by southern blotting and apoE using a PCR protocol provided by the Jackson Laboratories12. ApoE KO animals, wildtype or knockout for nNOS were weaned and started on a “western-type” diet (42% of total calories from fat; 0.15% cholesterol; Harlan Teklad) at 6 weeks of age and the diet was maintained for 14 or 24 weeks.
Lesion assessment
The aorta was dissected and analyzed as previously described10, 11. Animals were anesthetized with pentobarbital (80 μg/kg i.p.), the aorta was perfused with PBS, pH 7.4 and dissected from the aortic valve to the iliac bifurcation. Adventitial tissue was removed and the aorta was opened longitudinally and pinned onto a black wax surface using micro needles (Fine Science Tools). Serial images of the submerged vessels were captured with a black and white video camera (COHU) mounted on the c-mount of a stereomicroscope (Leica). Lipid rich intraluminal lesions were stained with Sudan IV without removing the pins. Serial color pictures were captured using the same microscope equipped with a Leica 35mm camera and used to identify lesions. Image analysis was performed using Image Pro Plus (Version 4.1; Media Cybernetics). The amount of aortic lesion formation in each animal was measured as percent lesion area per total area of the aorta.
Tissue preparation and histology
The animals were anesthetized with pentobarbital (80μg/kg i.p.) and perfused with 0.9% NaCl using a catheter placed in the left ventricle (LV). For histochemistry and immunohistochemistry tissue of three apoE KO and three apoE/nNOS DKO animals was embedded in Tissue-Tek® (Sakura Finetek) and snap-frozen in liquid nitrogen. Serial sections were taken from the aortic valve and aortic arch and fixed in acetone prior to staining. Sections were stained with hematoxylin/eosin (H&E), Masson’s trichrome and Picric acid. Immunostaining was performed using a monoclonal anti-α smooth muscle actin antibody (Sigma, clone 1A4) and a mouse macrophage/monocyte monoclonal antibody (Chemicon Int.; MOMA-2). A polyclonal anti-nNOS antibody (BD-Biosciences) was used on paraffin sections.
Histomorphometry
Photomicrographs of the aortic valve and aortic arch were taken with a Leitz-Camera mounted on a light microscope (Carl-Zeiss, Jena, Germany). Pictures were digitalized and transferred to a PC for planimetry using Image Pro Plus (Version 4.1; Media Cybernetics). All images were analyzed at 100-fold magnification. Areas of positive staining for SMC’s, macrophages (n=3 apoE/nNOS DKO and 3 apoE KO mice, respectively) and collagen (Picric acid staining, n=4 apoE/nNOS DKO and apoE KO mice) were measured in multiple plaques per animal and results were expressed as % positive staining plaque area.
RT-PCR
First-strand cDNA was synthesized from 2 μg of total aortic RNA, using random primers (Fermentas). PCR amplification was performed for 35 cycles at primer annealing temperature of 56°C. A 240-bp fragment of DNA encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control (sense primer: 5′-TGA TGA CAT CAA GAA GGG GGA A-3′; antisense primer: 5′-TCC TTG GAG GCC ATG TGG GC CAT-3′). Published primers for splice variants were used as indicated or designed according to known cDNA sequences of nNOS variants13. A 553-bp nNOSα fragment was amplified using sense: 5′-ATT AGG ATC CCT TTA CAT CAC ACC TGG AGA CCA-3′ and antisense: 5′-ATT ATT CGA AGA CTG TTC GTT CTC TGA ATA CGG-3′ primers. A 387-bp nNOSβ fragment was amplified using sense: 5′-GTG AAC AGC GTG ATC CAC AG-3′ and antisense: 5′-GAC ATC TTC TGA CTT CCG TAT GTG-3′13. A 159-bp nNOSγ fragment was amplified with sense: 5′-CCC CAG CCA CCT TGC CTT-3′ and antisense: 5′-GAC TGT TCG TTC TCT GAA TAC GG-3′ primers13. A 527-bp nNOS-μ was amplified with sense: 5′-GTC TTC CAC CAG GAG ATG-3′ and antisense 5′-ACC AGA CTG TGG GCT TCA GA-3′14. PCR fragments were analyzed by agarose gel electrophoresis.
Quantification of nNOS mRNA
mRNA expression of nNOSα and nNOSγ variants was quantified by real-time PCR (iCycler, Bio-Rad Laboratories, USA). PCR amplification was performed for 40 cycles at primer annealing of 60°C. nNOS expression was normalized to HPRT signal. nNOS-α sense: 5′-CCC ACC AAA GCT GTC GAT CT-3′ and antisense: 5′-GGA GGT TGG CCT TGG TAT TT. nNOS-γ sense: 5′-CAC CTT GCC TTC CAG AGA CCT-3′ and antisense: 5′-GAC TGT TCG TTC TCT GAA TAC GG-3′. HPRT sense: 5′-GTT GGA TAC AGG CCA GAC TTT GT and antisense: 5′-CCA CAG GAC TAG AAC ACC TGC. Fluorogenic probes for nNOS-α: 5′-6FAM-CAC ACC ATT AGC CTG GGA GAC TGA GCC-DB; nNOS-γ: 5′-6FAM-CCC CAC AGA TCA TTG AAG ACT: CGA TCA T-TMR; HPRT: 5′-6FAM-CTC GTA TTT GCA GAT TCA ACT TGC GC (TIB Molbiol, Germany) were used.
Western blot analysis
Aortas were dissected and samples were snap-frozen in liquid nitrogen. Western blots were performed using a polyclonal anti-eNOS antibody (1:200; Becton Dickinson; BD 30020) and anti-iNOS antibody (1: 200; Santa Cruz; sc-650). To confirm equal loading of protein an anti-actin antibody (Santa Cruz Biotechnologies, sc-8432) was used.
Blood pressure measurements
Mice were intubated and ventilated with a rodent respirator (stroke volume 250 μl, rate 90 min-1). The common carotid artery was cannulated and a Millar catheter (1.4F) was advanced into the left ventricle. Blood pressure was recorded under light isoflurane anesthesia.
Lipids and lipoprotein characterization
Lipoprotein cholesterol distribution of plasma samples was evaluated after fractionation by fast protein liquid chromatography (FPLC) gel filtration. Plasma (200μl) was fractionated on two serial superose-6-columns using an Äkta-FPLC (Amersham Pharmacia Biotech) system. Total cholesterol of plasma samples and FPLC-fractions were measured using Sigma kit 352 and a Spectra MAX 250 photometer (Molecular Devices).
Statistical analysis
Two way ANOVA was used for repeated measures, followed by Scheffe’s F-test (Stat View 4.51, Abacus Concepts, Inc., Berkley, CA). A probability value of p ≤ 0.05 was considered significant. Student’s t test was used for unpaired data. Survival of diet fed animals was expressed by the Kaplan Meier method and survival curves were compared by the log-rank test (Prism 3, GraphPad Software, Inc., San Diego, CA).
Results
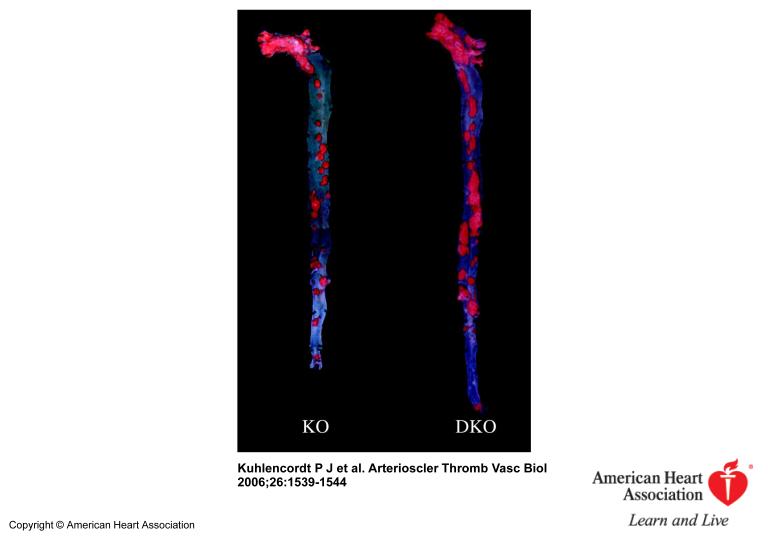
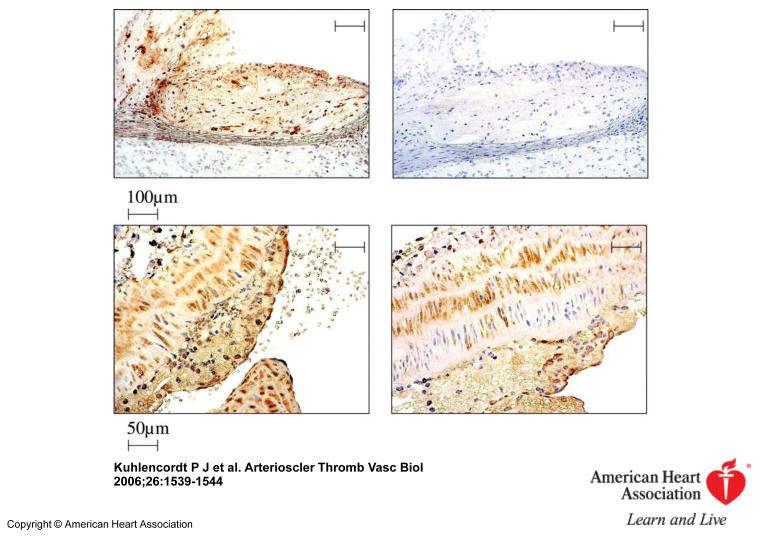
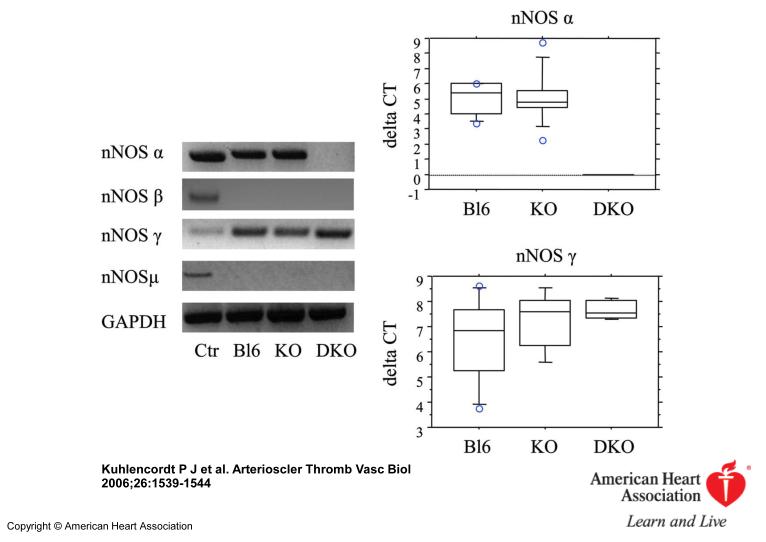
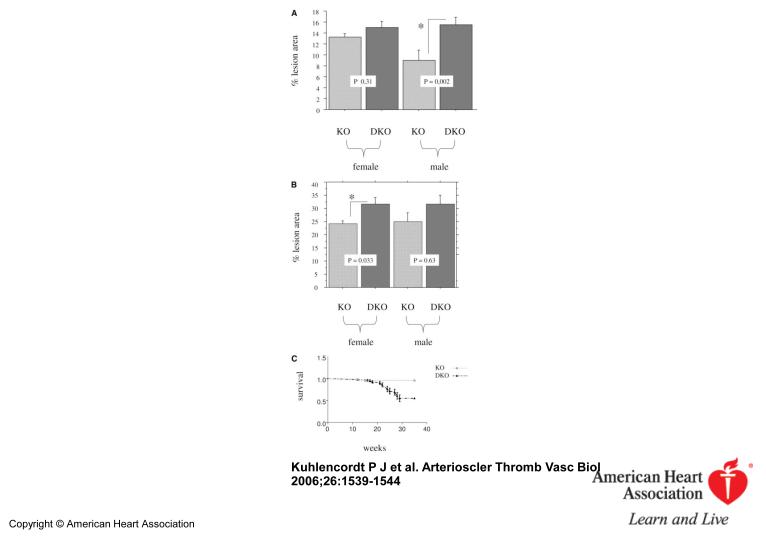
Inspection of the “en-face” dissected aortas revealed that apoE KO and apoE/nNOS DKO animals develop atherosclerostic lesions in areas of disrupted laminar flow, namely in areas of branching off vessels and curvature (Figure 1). Atherosclerotic lesions of “western-type“ diet fed apoE KO animals stained nNOS positive by immunohistochemistry, documenting that nNOS is present in lesions following 14 weeks of diet (Figure 2). However, plaques from apoE/nNOS DKO animals revealed residual nNOS staining (Figure 2). RT-PCR from total aortic RNA documented the presence of nNOSα variant in C57BL6 and apoE KO vessels and its absence in apoE/nNOS DKO mice (Figure 3). In contrast, the nNOSγ variant was present in vessels from C57BL6, apoE KO and apoE/nNOS DKO mice, while the nNOSβ and nNOSμ variant were not detectable in either group. Real time PCR revealed low but equal expression of nNOSα in C57BL6 and apoE KO mice and nNOSγ in vessels from all three genotypes (Figure 3). Following 14 weeks of “western-type“-diet, lesion area in male apoE/nNOS DKO animals (15.56±1.14%, n=10) showed a 66% increase compared to lesions in apoE KO control animals (9.35±1.32%, n=9, p=0.002; Figure 4a). Lesion area in female apoE/nNOS DKO (15.08±0.85%, n=11) and apoE KO animals (12.63±0.7%, n=12, p=0.31, Figure 4a) did not differ at this time point. However, following 24 weeks of “western-type“-diet, female animals showed a significant 31 % increase in lesion area (apoE/nNOS DKO: 31.8±2.3%, n=9; apoE KO: 24.26±1.1%, n=13, p=0.033; Figure 4b), while the increase in lesion area in male animals did not reach statistical significance (apoE/nNOS DKO: 31.64±3.5%, n=11; apoE KO: 24.89±3.5%, n=8, p=0.63). Kaplan Meier analysis revealed a pronounced increase in mortality of DKO animals by log rank test comparison of survival curves (apoE KO: n=31, apoE/nNOS DKO: n=42; p=0.0003). The increase in mortality in apoE/nNOS DKO mice affected male and female animals and was only present following 24 week of “western-type” diet (Figure 4c).
Figure 1.
Sudan IV-stained, longitudinally opened aortas from female apoE KO (KO) and apoE/nNOS DKO (DKO) animals fed a “western-type” diet for 24 weeks. Luminal side facing up, displaying lipid rich (red) atherosclerotic lesions.
Figure 2.
Immunohistochemical staining for nNOS (left, upper row) of an aortic root lesion from an apoE KO animal. Second antibody alone, revealing no background staining (right, upper row). nNOS staining of the common carotid artery of apoE KO (left, lower row) compared to apoE/nNOS DKO mice (right, lower row) showing residual nNOS immunoreactivity in DKO animals.
Figure 3.
RT-PCR for nNOS splice variants showing expected products for nNOSα, β, γ and μ in control RNA. apoE KO aortas express nNOSα- and nNOSγ-variants, while nNOSβ and nNOSμ are absent. In apoE/nNOS DKO aortas only nNOS γ is expressed. Real time PCR reveals equal nNOSα expression in C57BL6 and apoE KO mice (upper box blot) and of nNOSγ in C57BL6, apoE KO and apoE/nNOS DKO mice (lower box blot).
Figure 4.
Mean lesion area expressed as % lesion area of total area of the aorta at 14 weeks (4a; male and female) and 24 weeks (4b; female). *Significant difference (p≤0.05). Kaplan Meier survival curve (4c) of “western-type” diet fed apoE KO (n=31) and apoE/nNOS-DKO (n=42) animals showing a pronounced decrease in late survival of apoE/nNOS DKO animals (p=0.0003).
At both time-points total aortic area and total body weight did not differ between animals of either genotype. In addition total plasma cholesterol (Table 1) and FPLC analysis of lipoprotein profiles (data not shown) did not differ among study groups.
Table I.
Characteristics of apoE KO and apoE/nNOS DKO Mice
| MAP, mm Hg |
No. | HR, beats/min |
No. | T Cholesterol, mg/dL |
No. | Body Weight, g |
No. | |
|---|---|---|---|---|---|---|---|---|
| Female KO | 96.84±2.3 | 10 | 463±16.6 | 10 | 1060±54 | 12 | 26.18±0.84 | 14 |
| Female DKO | 84.13±2.41* | 9 | 517±22.1 | 9 | 847±102 | 8 | 25.81±0.67 | 14 |
| Male KO | 90,04±2.1 | 8 | 480±28.7 | 8 | 1040±123 | 7 | 34.14±1.24 | 9 |
| Male DKO | 88,98±2.4 | 13 | 502±17.9 | 13 | 1080±95 | 10 | 30.74±1.75 | 11 |
Interestingly, mean arterial blood pressure of female apoE/nNOS DKO animals was significantly reduced compared to control apoE KO animals (apoE/nNOS DKO: 84.1±2.73 mmHg, n=9; apoE KO: 96.8±2.3 mmHg, n=10, p=0.013) while blood pressure in male animals of both genotypes did not differ (Table 1). To rule out that nNOS deletion modulated expression levels of iNOS or eNOS, western blot analysis was performed. eNOS protein was present in both genotypes at comparable levels. The iNOS protein level in female mice was higher compared to male animals but no difference was observed between the two genotypes (Figure 5).
To check for differences in plaque composition serial cryosections were taken from the aortic valve area and aortic arch and used for histochemistry and immunohistochemistry. All groups of animals studied developed complex lesions with a variable necrotic core and content of fibrous tissue (Figure 6a/b). No statistical difference of picric acid positive areas between animals of the same gender but different genotype was observed (apoE/nNOS DKO: 38.2% ±2.1 SE, n=19 plaques; apoE KO: 38.0% ±2.7 SE, n=19 plaques, p=0.94). In addition, plaques displayed strong immunoreactivity for macrophages without a gross difference between the groups (apoE/nNOS DKO: 46.4 % ±2.6 SE, n=14 plaques; apoE KO: 49.7 % ±3.9 SE, n=19 plaques, p=0.53; Figure 6a/b). Staining for vascular smooth muscle cells (SMC) revealed a high degree of variability within each animal, regardless of the experimental groups. However, the strongest αSMC-actin-staining was seen in apoE/nNOS DKO animals (apoE/nNOS DKO: 11.4% ±3.1 SE, n=18 plaques; apoE KO: 6.16 % ±0.95 SE, n= 14 plaques, p=0.16, Figure 6b/6c).
Discussion
Nitric oxide has potential atheroprotective effects since it inhibits smooth muscle cell proliferation, leukocyte endothelial interactions, leukocyte adhesion and aggregation of platelets and endothelial exocytosis of granules that mediate vascular inflammation and thrombiosis15-19. On the other hand, NO has proatherogenic properties since it reacts with superoxide to form peroxinitrite which can further react to an array of oxygen- and nitrogen radicals20. Moreover, each NOS isoform is capable of generating superoxide under conditions of substrate and cofactor deficiency21-24. To dissect the role of each isoform in lesion formation, we generated apoE/NOS DKO mice in order to test the relevance of the absence of each isoform on lesion development. Our previously published results show that eNOS is atheroprotective and apoE/eNOS DKO animals developed a dramatic increase in lesion formation10. In contrast, genetic deficiency of iNOS decreases atherosclerosis and lowers plasma lipidperoxides, a marker of oxidative stress, in apoE KO animals11.
In the present study we demonstrate that genetic deficiency of nNOS accelerates atherosclerotic lesion progression. The location of lesions was similar in apoE Ko control and apoE/nNOS DKO animals. Western blot analysis revealed higher iNOS expression in female compared to male animals. However, iNOS expression levels in the female and the male group were similar and do not explain the differences in lesion formation within these gender groups. eNOS expression levels were equal in all experimental groups.
Lesion progression was significantly accelerated in male apoE/nNOS DKO compared to male apoE KO animals following 14 weeks of western type diet. In female apoE/nNOS DKO mice there was a significant increase in plaque area following 24 weeks of western diet. Following 24 weeks of diet, however, the increase in lesion area in male apoE/nNOS DKO animals did not reach statistical significance which may be explained by a higher mortality in apoE/nNOS DKO mice at this time point (Figure 4c).
The slower increase in lesion area in female apoE/nNOS DKO compared to male apoE/nNOS DKO animals was associated with a reduction in mean arterial blood pressure in female DKO mice. Since blood pressure elevation is an independent risk factor for atherosclerosis, the reduced blood pressure in female DKO animals renders a possible explanation for the delayed lesion progression in this group.
The reduction in blood pressure in female animals is not likely to occur at the level of the vessel wall since vascular nNOS would likely cause vasodilation and thus nNOS deletion would be expected to raise the blood pressure7. Instead, nNOS deletion could affect blood pressure regulation in the central nervous system (CNS), at the level of the baroreceptors or through endocrine mechanisms. In this respect, NO, regardless of its source, is known to be involved in the regulation of renin secretion25. While acute inhibition of nNOS does not seem to affect blood pressure, chronic pharmacologic inhibition of nNOS using 7-nitroindazole (7-NI) significantly increased blood pressure in one study26-28. In contrast, acute and chronic nNOS inhibition in eNOS KO animals caused a decrease in blood pressure which may be due to nNOS dependent mechanisms at the level of the CNS or the baroreceptor pathway29. And although nNOS KO animals were reported to have a tendency towards hypotension, gender differences in nNOS dependent blood pressure regulation have not been reported so far 30, 31.
To evaluate potential differences in plaque composition, we stained lesions by immunohistochemistry and histochemistry. Animals of both genotypes developed complex lesions with fibrous caps and necrotic cores. Both genotypes showed strong immunoreactivity for macrophages without an obvious difference between groups. Lesions with the strongest alpha smooth muscle actin staining were seen in apoE/nNOS DKO animals (Figure 6a/b). In the past, NO has been shown to suppress smooth muscle cell proliferation18. Recent data indicated that angiotensin can induce nNOS expression in vascular smooth muscle cells and that this nNOS expression is sufficient to suppress SMC proliferation in vitro8. However, neointimal alpha smooth muscle actin staining in our study was highly variable between animals and even among lesions within each mouse (Figure 6c), preventing statistical significance of our observation. The occurrence of plaques in apoE/nNOS DKO mice that lack any SMC-staining suggests that the absence of nNOS does not by itself trigger SMC-proliferation. Instead, the occurrence of pronounced SMC proliferation in single plaques of apoE/nNOS DKO mice let us speculate that nNOS serves to counterbalance local and systemic factors that would foster SMC proliferation.
It is important to note that nNOSα (exon 2) KO animals used in this study express nNOSβ and nNOSγ mRNA splice variants in brain tissue by utilizing alternate translation start sites13. Therefore, we investigated whether nNOS splice variants are present in vessels of C57BL6, apoE KO and apoE/nNOS DKO animals. Indeed, we find residual nNOS immunoreactivity in the neointima of apoE/nNOS DKO mice and similarly low expression levels of the nNOSγ splice variant in aortas of C57BL6, apoE KO and apoE/nNOS DKO animals. The N-terminal truncated nNOSγ variant lacks the PDZ domain and is localized to the cytosolic fraction13. In vitro assays indicate that nNOSγ has low catalytic activity (3% of nNOSα), whereas nNOSβ possesses activity comparable to nNOSα13. However, nNOSβ and nNOSμ variants were not detectable in mouse aortas in our study. Our study differs from a study by Schwartz et al. who reported the presence of small amounts of nNOS in the media and adventitia of intact rat aorta and the study by Morishita et al. who reported absence of nNOS expression in carotid arteries of untreated C57BL6 mice7, 8. These latter differences may be explained by the use of different species, the study of the arteria carotis vs. the aorta and the use of injury models as opposed to a model of spontaneous arteriosclerosis. The nNOS KO animals used in this study are not expected to have membrane targeted nNOS expression due to deletion of the PDZ targeting motif contained in exon 2. Interestingly, splice variants that lack exon 2 are reported to occur spontaneously in mice, rats and humans 13, 32, 33. Our data highlights nNOSα overexpression as a potential therapeutic strategy. Moreover, the prevalence of splice variants that lack exon 2 of the nNOS gene within each individual may be a risk factor for vascular disease.
In summary, we provide first evidence for the presence of nNOS splice variants in atherosclerotic vessels and show that genetic deficiency of nNOSα increases atherosclerosis development in apoE KO animals. Therefore, our study indicates that nNOSα protects from atherosclerosis. In addition systemic blood pressure was reduced in female apoE/nNOS DKO mice and nNOS deletion led to a significant increase in mortality in DKO animals.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche-Forschungsgemeinschaft (KU-1206(1) u. 1206(2) to P.K., the National Institute of Neurological Disorders and Stroke (NINDS, NS33335) and the National Heart, Lung, and Blood Institute (NHLBI, HL57818) to P.L.H. P.L.H. is an Established Investigator of the American Heart Association. We thank Mrs. Gabriele Riehl and Mrs. Alla Ganscher for excellent technical assistance.
References
- 1.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni AP, Getchell TV, Getchell ML. Neuronal nitric oxide synthase is localized in extrinsic nerves regulating perireceptor processes in the chemosensory nasal mucosae of rats and humans. J Comp Neurol. 1994;345:125–138. doi: 10.1002/cne.903450110. [DOI] [PubMed] [Google Scholar]
- 4.Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–79. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- 5.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. Faseb J. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol. 1999;13:21–43. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz PM, Kleinert H, Forstermann U. Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arterioscler Thromb Vasc Biol. 1999;19:2584–2590. doi: 10.1161/01.atv.19.11.2584. [DOI] [PubMed] [Google Scholar]
- 8.Morishita T, Tsutsui M, Shimokawa H, Horiuchi M, Tanimoto A, Suda O, Tasaki H, Huang PL, Sasaguri Y, Yanagihara N, Nakashima Y. Vasculoprotective roles of neuronal nitric oxide synthase. Faseb J. 2002;16:1994–1996. doi: 10.1096/fj.02-0155fje. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Powell-Braxton L, Ogaoawara AK, Dybdal N, Bunting S, Ohneda O, Jin H. Hypertension and endothelial dysfunction in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 1999;19:2762–2768. doi: 10.1161/01.atv.19.11.2762. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 12.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 13.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 14.Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271:11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- 15.Lefer AM, Ma XL, Weyrich A, Lefer DJ. Endothelial dysfunction and neutrophil adherence as critical events in the development of reperfusion injury. Agents Actions Suppl. 1993;41:127–135. [PubMed] [Google Scholar]
- 16.Bath PM. The effect of nitric oxide-donating vasodilators on monocyte chemotaxis and intracellular cGMP concentrations in vitro. Eur J Clin Pharmacol. 1993;45:53–58. doi: 10.1007/BF00315350. [DOI] [PubMed] [Google Scholar]
- 17.Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine-nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- 18.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- 19.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O’Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 24.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J. Permissive Role of Nitric Oxide in Macula Densa Control of Renin Secretion. Am J Physiol Renal Physiol. 2004 doi: 10.1152/ajprenal.00272.2003. [DOI] [PubMed] [Google Scholar]
- 26.Pajewski TN, DiFazio CA, Moscicki JC, Johns RA. Nitric oxide synthase inhibitors, 7-nitro indazole and nitroG-L-arginine methyl ester, dose dependently reduce the threshold for isoflurane anesthesia. Anesthesiology. 1996;85:1111–1119. doi: 10.1097/00000542-199611000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Zanzinger J, Czachurski J, Seller H. Neuronal nitric oxide reduces sympathetic excitability by modulation of central glutamate effects in pigs. Circ Res. 1997;80:565–571. doi: 10.1161/01.res.80.4.565. [DOI] [PubMed] [Google Scholar]
- 28.Ollerstam A, Pittner J, Persson AE, Thorup C. Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J Clin Invest. 1997;99:2212–2218. doi: 10.1172/JCI119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurihara N, Alfie ME, Sigmon DH, Rhaleb NE, Shesely EG, Carretero OA. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32:856–861. doi: 10.1161/01.hyp.32.5.856. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Inglis FM, Kalb RG. Defective fluid and HCO(3)(-) absorption in proximal tubule of neuronal nitric oxide synthase-knockout mice. Am J Physiol Renal Physiol. 2000;279:F518–524. doi: 10.1152/ajprenal.2000.279.3.F518. [DOI] [PubMed] [Google Scholar]
- 32.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci U S A. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Newton DC, Robb GB, Kau CL, Miller TL, Cheung AH, Hall AV, VanDamme S, Wilcox JN, Marsden PA. RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12150–12155. doi: 10.1073/pnas.96.21.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.