Abstract
Among the many products which influence microglial activation and resulting neuroinflammation, herbal medicine has recently drawn much attention due to its immunomodulatory and neuroprotective activities. The purpose of the current study was to investigate the effects of an extract of Panax notoginseng (NotoG™) on TLR ligand-and IFNγ-induced activation in N9 and EOC20 microglial cells lines. NotoG suppressed microglial activation as measured by reduced expression of accessory molecules (CD40 and CD86), decreased production of inflammatory mediators (IL-6 and TNFα), and diminished release of antibacterial products (nitric oxide). Furthermore, this immunosuppressive activity was neither dependent on the glucocorticoid receptor, nor the result of a single ginsenosides (Rb1, Rg1, or Re), which are the major active constituents of the whole extract. NotoG and select ginsenosides may therefore be of therapeutic benefit in treating or preventing neurodegenerative diseases such as multiple sclerosis and parkinson’s disease.
Keywords: Inflammation, Cytokine, Nitric oxide, Glucocorticoid receptor, Ginsenoside
Introduction
Microglia (MÖ), the resident antigen presenting cell within the central nervous system (CNS), play a significant role in host defense and tissue repair within the CNS. As the CNS’ intrinsic immune effector cell, MÖ rapidly respond to subtle, acute, and chronic pathological stimuli. However, recent evidence has established that MÖ also contribute to neuro-pathological changes associated with several CNS diseases including Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Multiple Sclerosis (MS), Acquired Immune Deficiency Syndrome Dementia (HIV-Dementia), Tramatic Brain Injury (TBI), and Hypoxic-Ischemic Injury (Dheen et al. 2007; Esiri 2007; Glezer et al. 2007; Peterson and Fujinami 2007). Although once considered separate and distinct events in the same disease, inflammation and neurodegeneration are now known to occur in concert within the same CNS pathology (Esiri 2007).
As the primary immune effector cell in the CNS, MÖ migrate to the site of tissue injury where they respond to invading pathogens or other inflammatory signals. Like peripheral monocytes and macrophages, MÖ become activated and participate in propagation of CNS inflammation through antigen presentation and the production of pro-inflammatory and toxic mediators (Suk 2005; Ponomarev et al. 2006). In vitro, the process of MÖ activation is associated with upregulation of cell surface markers such as CD45, MHC class II, CD40, and CD86 (Aloisi et al. 2000; Ponomarev et al. 2005); although this has not been extensively characterized in vivo. Activation of MÖ and the consequent release of inflammatory mediators such as interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), glutamate, nitric oxide (NO), and reactive oxygen species (ROS) may contribute to neurodegenerative processes (Aloisi et al. 2001; Glezer et al. 2007). In vitro studies demonstrated MÖ activation increased neurotoxicity through the production of pro-inflammatory and cytotoxic mediators in mixed neuron-glial cultures stimulated with either toll like receptor (TLR) ligands (e.g. lipopolysaccha-ride, LPS), cytokines (e.g. TNFα or IFNγ) glutamate, N-methyl-D-aspartate (NMDA), or β-amyloid (Aβ) (Dheen et al. 2007; Glezer et al. 2007). Activation of MÖ in the CNS may initially be intended to protect neurons; however, more frequently, activation of these cells and inflammatory products derived from them have been implicated in various disease states (Suk 2005). Therefore, activation of MÖ occupies a key position whereby the immune response may proceed in two directions: one being neuroprotective and the other neurotoxic (or a combination of the two), depending on the stimulus. In this manner, suppression of MÖ activation represents an important immunotherapeutic target.
Among the many endogenous and exogenous factors that might influence MÖ activation and resulting neuroinflam-mation, herbal medicine has recently drawn much attention due to its potential inhibitory effects on inflammatory responses and neuroprotective activity (Radad et al. 2006; Rausch et al. 2006; Rhule et al. 2006). Because of its wide range of pharmacological effects, Panax ginseng has been used in traditional Chinese medicine for more than 5 thousand years as both a tonic and a haemostatic agent (Radad et al. 2006). As such, it continues to occupy a prominent position on the herbal best seller list and is considered the most widely consumed herbal product in the world (Blumenthal 2001; Barnes et al. 2004). Ginseng saponins, also known as ginsenosides, are the principal active components responsible for ginseng’s biological effects. The biological activity of the species Panax Notoginseng is similar to the more widely known Panax ginseng, with differences in activity associated with higher levels of ginsenosides in the Panax Notoginseng species (Zhu et al. 2004). Pharmacological effects of ginseng and ginsenosides have been demonstrated on the CNS, as well as the cardiovascular, endocrine and immune systems (Attele et al. 1999; Radad et al. 2006; Lopez et al. 2007); however, the ability of ginsenosides to act on CNS immune cells has not been widely examined.
CNS effects include increased cell survival, extension of neurite growth, and rescuing of neurons from death in consequence of different insults both in vivo and in vitro (Radad et al. 2006; Rausch et al. 2006). A number of studies have begun to describe the beneficial effect of ginseng and ginsenosides in neurodegenerative disease models (e.g. Parkinson’s disease, spinal cord injury, and Ischemia-reperfusion injury) which are thought to occur via inhibition of inflammation and MÖ activation in particular (Bu et al. 2005; Lin et al. 2007; Joo et al. 2008). Although the ability of ginseng and ginsenosides to modulate some inflammatory and allergic processes has been documented within the peripheral immune system, to date there is a shortage of studies examining ginseng’s ability to modulate neuroinflammatory conditions (Lin et al. 2007; Wu et al. 2007; Joo et al. 2008). We hypothesized that Panax notoginseng may temper MÖ activation processes. In this study, we examined whether an extract of Panax Notoginseng (NotoG™) attenuates TLR ligand- or interferon gamma (IFNγ)-induced activation of MÖ. In vitro activation of murine MÖ by both TLR ligands and IFNγ was suppressed by concomitant NotoG treatment in a dose-dependent and ligand specific manner with regards to cell surface molecules and secreted cytokines. Furthermore, this immunosuppressive activity was neither dependent on the glucocorticoid receptor, nor the results of a single ginsenoside component of the whole extract.
Materials & methods
Plant extracts
Extracts from the plant Panax Notoginseng (Noto-G™) were kindly provided by Technical Sourcing International, Inc. (TSI, Missoula MT). Notoginseng was extracted from the root of the Panax notoginseng plant using ethanol and standardized to contain Rb1 and Rg1 ginsenosides at 35% and 34% of the whole extract, respectively. Quantification of Rb1 and Rg1 content were determined by high performance liquid chromatography analysis (HPLC) by TSI and NotoG extracts were determined to be free of E. coli and S. enterica contamination (data not shown). The lyophilized NotoG extract, purified ginsenosides Rb1 and Rg1 (Rg1 34 μg/ml, Rb1 32 μg/ml, MP Biomedicals Solon, OH), purified Re (9 μg/ml Sigma St Louis, MO), Lipopolysaccharide (LPS) from E.coli (O55:B5, 100 ng/ml, Sigma), CpG ODN 1826 type B specific for mouse TLR9 (0.5 μM, Invivogen San Diego, CA), Poly I:C (a synthetic analog of dsRNA, 250 μg/ml Invivogen), IFNγ (5 ng/ml, RnD Systems Minneapolis, MN), RU486 (5 mM Sigma), and dexamethasone (6.8 mM, Sigma) were dissolved in complete RPMI (see below).
Cell culture and microglial stimulation
The murine N9 MÖ cell line, originally derived from CBA mice, was kindly provided by Dr. P Ricciardi-Castagnoli (University of Milano-Bicocca, Milan Italy). The cells were maintained in complete RPMI 1640 medium supplemented with 3% heat inactivated FBS, 2 mM glutamine, 25 mM HEPES, with antibiotic-antimycotic solution (Sigma). The murine EOC20 MÖ cell line, originally derived from C3H/HeJ juvenile mice, was purchased from ATCC (CRL-2469). The cells were maintained in DMEM with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10%; heat inactivated FBS, 20% LADMAC Conditioned Media (produced from the LADMAC cell line (CRL-2420, ATCC), with antibiotic-antimycotic solution (Sigma). MÖ were seeded at 106 cells/ml/well of a six well plate and immediately exposed to NotoG (1-100 µg/ml, TSI), LPS (endotoxin, 100 ng/ml), CpG (0.5 μM), Poly I:C (250 μg/ml), IFNγ (5 ng/ml), RU486 (5 mM), dexamethasone (6.8 mM), Re (9 μg/ml), Rg1 (34 μg/ml), and Rb1 (32 μg/ml) and allowed to incubate for 24 h at 37°C. To minimize variability MÖ cells were lightly scraped within the spent culture media, collected into 1.5 ml eppendorf tubes, centrifuged, and the media and cells separated for further analysis.
Flow cytometry
MÖ were harvested, washed, and non-specific antibody binding blocked with purified rat anti-mouse CD16/CD32 (BD Pharmingen, San Jose, CA) diluted 1:100 in 30 μg of rat IgG (Jackson ImmunoResearch, West Grove, PA) prior to staining with fluorochrome-conjugated antibodies. One μg of monoclonal antibodies specific to CD40 PE (clone 3/23), CD86 APC clone GL1), BD Pharmingen), CD54 Pacific Blue (clone YN1/1.7.4, Biolegend, San Diego, CA), MHC class II FITC (clone M5/114.15.2), CD38 PE-Cy7 (clone 90), CCR7 PE (clone 4B12), or CCR3 APC (clone 5E8-G9-B4, eBioscience, San Diego, CA) were added and incubated for 30 min on ice. Five μl of 7-AAD (BD Pharmingen) was then added directly to the cells and incubated for 5 min at room temperature. Cells were washed with PBS, re-suspended in 0.3 ml PAB (1% bovine serum albumin, 0.01% sodium azide in PBS) and analyzed immediately. Cell acquisition and analysis was performed on a FACS Aria flow cytometer using FACS Diva software (version 4.1.2). A live cell gate was established using 7-AAD negative cells and 100,000 events captured. Compensation of the spectral overlap for each fluorochrome was calculated using anti-rat/hamster IgG compensation beads (BD Biosciences).
Cytokine ELISA, LDH, and nitrite assay
Acellular supernatants resulting from the experiments described above were collected and analyzed for IL-6 and TNFα production using murine cytokine ELISA kits according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN). In addition, 50 μl of tissue culture supernatants were analyzed for LDH (Biovision, Mountain View, CA) or nitrite levels (NO2-) according to the Griess method following the manufacturer’s instructions (Promega, Madison, WI).
Data analysis
For each parameter, the values for individual experiments were averaged and the standard deviation and standard error calculated. The significance of the differences between the exposure groups was determined by t-test, one-way, or two-way ANOVA, in conjunction with Bonferroni’s post-hoc analysis and Tukey’s test for variance, where appropriate. All ANOVA models were performed with Prism software, version 4. A P value of <0.05 was considered significant.
Results
Notoginseng inhibits LPS-induced activation of N9 microglia in a dose-dependent manner
After microglia (MÖ) encounter pathogens, toll like receptor (TLR) mediated activation results in increased cell surface expression of accessory molecules, as well as increased production of pro-inflammatory cytokines and anti-bactericidal activities which are essential to initiating and maintaining a proper immune response (Olson and Miller 2004). To test whether concurrent exposure of N9 MÖ to endotoxin (LPS, the classical TLR4 ligand) and NotoG resulted in altered expression of accessory molecules and early response cytokines in a dose-dependent manner, cells were stimulated with LPS and varying concentrations of NotoG for 24 h. As anticipated, LPS increased the expression of CD40 on N9 MÖ when compared to unstimulated cells as measured by flow cytometry (Fig. 1a). LPS-induced upregulation of CD40 expression was attenuated by co-exposure with 25, 50, and 100 μg/ml, but not 5 μg/ml NotoG (Fig. 1a). While LPS stimulation slightly reduced CD86 expression compared to unstimulated MÖ, NotoG increase CD86 expression at all concentrations examined compared to LPS alone (Fig. 1b). Cytokine ELISAs established that N9 MÖ increased production of IL-6 and TNFα in response to LPS stimulation (Fig. 1c and d, respectively). This increase in pro-inflammatory cytokine production was diminished by concomitant treatment with 50 and 100 μg/ml NotoG. Although 5 and 25 μg/ml NotoG exhibited a trend towards a reduction in IL-6 and TNFα, this decrease was not statistically significant (Fig. 1c and d, respectively). With regards to accessory molecule expression, only 100 μg/ml NotoG alone altered expressionofCD40 and CD86 compared to unstimulated MÖ. No differences were observed in either IL-6 or TNFα production in response to any of the concentrations of NotoG alone. Furthermore, because the MFI analysis was performed on live cells (gated as 7-AAD negative) (data not shown) and because no variation was detected in either percent cell viability, LDH release, or the Aqeuous One cell viability assay (data not shown), the differences detected in LPS-induced activation by NotoG treatment were not attributed to cytotoxicity. Although 100 μg/ml of NotoG was the most potent of the concentrations examined, 50 μg/ml NotoG was chosen for analysis in the subsequent studies because of its ability to reduce MÖ activation, without any inherent effects of its own.
Fig. 1.
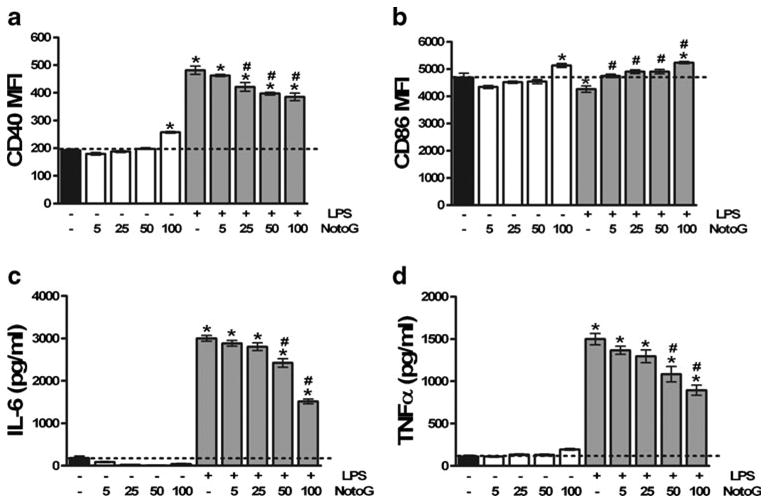
Notoginseng (NotoG) decreased LPS-induced activation of N9 MÖ in a dose-dependent manner. LPS increased expression of CD40 (a), but not CD86 (b) compared to unstimulated MÖ. This increased CD40 expression was dose-dependently reduced by NotoG, and 100 μg/ml of NotoG was the most potent of the concentrations examined. In contrast, LPS and NotoG appeared to act synergistically to increase CD86 expression. As anticipated, LPS activation increased the production of IL-6 (c) and TNFα (d) by N9 MÖ. Similarly, this upregulation of pro-inflammatory cytokines was attenuated by NotoG. n=3–6; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to LPS only control
Notoginseng attenuates TLR ligand and IFNγ-induced nitric oxide production
Nitric oxide (NO) is a major mediator of innate immune responses due to its involvement in bactericidal activity (Delclaux and Azoulay 2003), plays a role in the regulation of cytokine synthesis (Coleman 2002), and promotes neuro-degeneration (Xie et al. 2002). In order to investigate the impact of NotoG on NO production in response to the TLR ligands: LPS, CpG, Poly I:C, and the potent microglial activator interferon gamma (IFNγ), the levels of nitrite (NO2-), which is one of two primary, stable and nonvolatile breakdown products of NO, were measured. Only trace amounts of NO2- were detected in the acellular supernatants collected from unstimulated MÖ. These values were not altered by NotoG alone in either N9 or EOC20 MÖ (data not shown). Exposure to LPS, CpG, and IFNγ, but not Poly I:C, increased NO2- levels compared to unstimulated N9 MÖ. Concomitant exposure to NotoG attenuated LPS-(42.1%), CpG- (21.7%), and IFNγ-induced NO2- production (18.8%) compared to the stimulant alone (Fig. 2a). In contrast, CpG, Poly I:C, and IFNγ, but not LPS, increased NO2- compared to unstimulated EOC20 MÖ. Similarly, simultaneous exposure to NotoG decreased CpG- (30.1%), Poly I:C- (26.6%), and IFNγ-induced NO2- production (10.1%) compared to the stimulants alone (Fig. 2b).
Fig. 2.
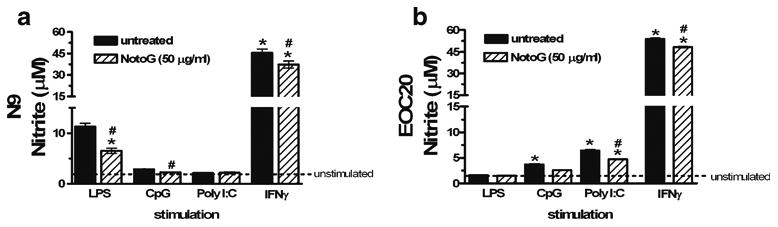
Notoginseng reduced TLR ligand and IFNγ-induced nitrite production by both N9 and EOC20 MÖ. NotoG alone exhibited no stimulatory effect on nitrite levels. a LPS, CpG, and IFNγ, but not Poly I:C, increased nitrite production compared to unstimulated N9 MÖ. This increase was reduced by concomitant exposure to 50 μg/ml NotoG. b CpG, Poly I:C, and IFNγ, but not LPS, increased nitrite production compared to unstimulated EOC20 MÖ. This increase was similarly reduced by concomitant exposure to NotoG. n=3–6; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to appropriate TLR ligand control
CD40 accessory molecule expression is attenuated by notoginseng
Stimulation of MÖ upregulates the expression of cell surface antigens involved in T cell activation. Of these, the CD40 molecule in particular, has an important role in promoting inflammatory responses by macrophages/microglia, since interaction with its cognate ligand, CD154, leads to secretion of cytokines and neurotoxins which contributes to neuro-immunologic pathologies in the CNS. To investigate whether NotoG modulates the expression of CD40 on mu-rine MÖ cell lines, the levels of CD40 were analyzed by flow cytometry on N9 and EOC20 MÖ following co-culture with LPS, CpG, Poly I:C, or IFNγ. NotoG alone slightly augmented CD40 expression on N9 MÖ (Fig. 3a), but had no effect on EOC20 MÖ (Fig. 3b). As anticipated, LPS and IFNγ increased expression of CD40 on N9 MÖ; whereas concomitant exposure to NotoG decreased CD40 expression by 25.0% and 47.5%, respectively (Fig. 3a). In contrast, in the presence of CpG and Poly I:C the expression of CD40 accessory molecules was similar to the unstimulated N9 MÖ, but augmented by the presence of NotoG relative to the stimulant alone (16.8 and 23.1%, respectively) (Fig. 3a). EOC20 MÖ were responsive to CpG-, Poly I:C-and IFNγ-induced activation, but not LPS, and consequently upregu-lated expression of CD40 (Fig. 3b). Similar to the observed response in N9 MÖ, in the presence of NotoG, EOC20 MÖ simultaneously stimulated with CpG- (11.4%), Poly I:C-(20.7%), or IFNγ36.7 showed reduced expression of CD40 compared to the stimulant alone (Fig. 3b).
Fig. 3.
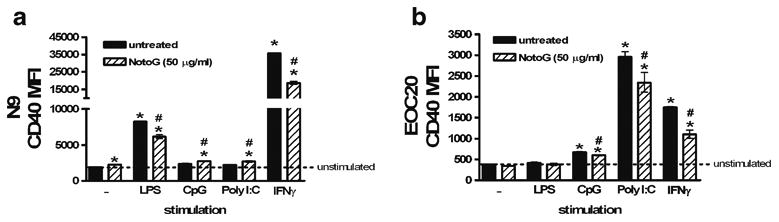
Notoginseng attenuated TLR ligand and IFNγ-induced CD40 expression by MÖ. NotoG alone exhibited slight stimulatory effects on CD40 levels. a LPS and IFNγ, but not CpG or Poly I:C, increased CD40 expression compared to unstimulated N9 MÖ. Concomitant exposure to NotoG reduced LPS- and IFNγ-induced CD40 expression, yet slightly increased CpG- and Poly I:C-induced CD40 expression relative to the stimulant alone. b CpG, Poly I:C, and IFNγ, but not LPS, increased CD40 expression compared to unstimulated EOC20 MÖ. Concomitant exposure to NotoG reduced CpG-, Poly I:C, and IFNγ-induced CD40 expression relative to the stimulant alone. n=3–6; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to appropriate TLR ligand control
Pro-inflammatory cytokine production is reduced by notoginseng
Both TLR ligands and IFNγ stimulate the production of pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα). To determine whether NotoG modifies the production of pro-inflammatory cytokines by MÖ, the amount of IL-6 and TNFα produced by N9 and EOC20 MÖ cell lines was determined following co-culture with LPS, CpG, Poly I:C, or IFNγ. As anticipated, Fig. 4 demonstrates that pro-inflammatory cytokine production is upregulated in response to TLR ligands and IFNγ in both MÖ cell lines. N9 MÖ increased production of IL-6 and TNFα in response to LPS, CpG, and IFNγ, but not Poly I:C (Fig. 4a & b). Consistent with the observed effects on NO2- and accessory molecule expression, concomitant exposure to LPS, CpG, or IFNγ and NotoG attenuated IL-6 and TNFα cytokine production compared to the stimulant alone (Fig. 4a & b). EOC20 MÖ upregulate IL-6 production in response to LPS, CpG, and Poly I:C and this increase is reduced by concomitant exposure to NotoG (Fig. 4c & d). In contrast, TNFα production by EOC20 MÖ in response to CpG and Poly I:C stimulation exhibits augmented levels of TNFα in the presence of NotoG (Fig. 4c & d). The percent change from control values are indicated above the corresponding bars on the graph.
Fig. 4.
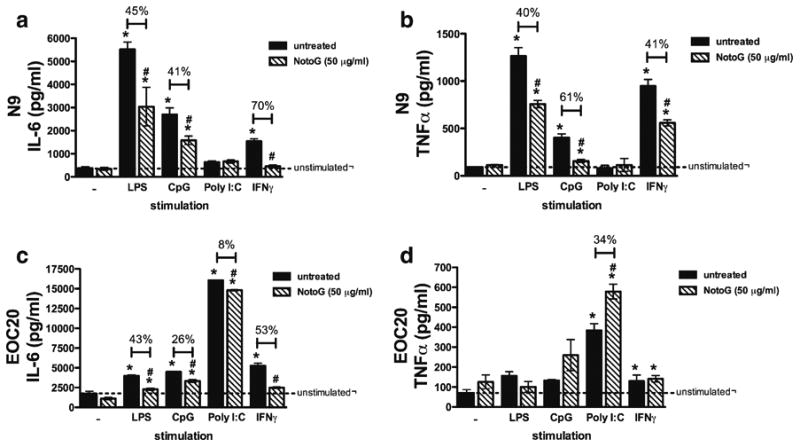
TLR ligand and IFNγ induced IL-6 and TNFα production by MÖ was modified by NotoG. NotoG alone exhibited no effects on either IL-6 or TNFα production. LPS, CpG, and IFNγ, but not Poly I: C increased IL-6 (a) and TNFα (b) compared to unstimulated N9 MÖ. This response was reduced by concomitant exposure to NotoG. c LPS, CpG, Poly I:C, and IFNγ increased IL-6 production compared to unstimulated EOC20 MÖ cells and this increase in IL-6 was reduced by concomitant exposure to NotoG. d In contrast, Poly I:C and IFNγ stimulated TNFα production compared to unstimulated EOC20 MÖ. Concomitant exposure to NotoG increased Poly I:C-induced TNFα production, but had no effect on IFNγ induced TNFα. n=3–6; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to appropriate TLR ligand control
Chemokine receptor expression induced by TLR ligand is not affected by notoginseng
Activated MÖ phagocytose damaged cells and debris, as well as foreign pathogens, and subsequently express surface proteins involved in maturation and migration including chemokine receptors such as CCR7 (Aloisi 2001; Dijkstra et al. 2006). Table 1 demonstrated that chemokine receptors are upregulated in response to LPS on N9 MÖ, but not in response to Poly I:C on EOC20 MÖ. These data show that NotoG alone slightly increased CCR7 and CD38 expression on the cell surface of N9 MÖ, while no effect was observed with CCR3. NotoG augmented CD38 expression in the presence of LPS, but had no effect on either CCR7 or CCR3 expression (Table 1). Interestingly, EOC20 MÖ responded to Poly I:C challenge by reducing expression of CCR7 and CCR3, while no effect was observed on CD38 levels. Of the three molecules examined on EOC20 MÖ, only CCR3 expression was affected by NotoG treatment.
Table 1. Effects of NotoG on MÖ chemokine receptor expression.
| N9 microglia | |||
|---|---|---|---|
| CCR7 (MFI)a | CD38 (MFI) | CCR3 (MFI) | |
| Unstimulatedb | 152.7±1.2 | 17.67±2.4 | 19.67±0.3 |
| NotoG | 178.3±23.0 * | 33.67±0.8 * | 21.33±0.7 |
| LPS | 314.7±3.8 * | 85.67±2.0 * | 77.00±4.6 * |
| LPS + NotoG | 322.3±33.2 * | 96.33±2.3 *# | 81.67±1.7 * |
| EOC20 microglia | |||
| CCR7 (MFI) | CD38 (MFI) | CCR3 (MFI) | |
| Unstimulated | 313.3±7.5 | 241.7±9.8 | 130.0±4.0 |
| NotoG | 294.7±6.0 | 231.7±7.1 | 121.7±2.4 |
| Poly I:C | 132.0±11.0 * | 246.0±31.9 | 74.00±2.0 * |
| Poly I:C + NotoG | 109.3±2.6 * | 217.3±8.8 | 57.00±1.2 *# |
mean fluorescence intensity as determined by flow cytometry
Microglia were either unstimulated or stimulated with LPS (100 ng/ml) or Poly I:C (250 μg/ml) in the presence or absence of NotoG (50 μg/ml) for 24 h
Results are representative of three independent experiments (n=3) ± standard error of the mean.
P<0.05 compared to unstimulated.
P<0.05 compared to LPS or Poly I:C only control
NotoG does not appear to act through the glucocorticoid receptor
Ginseng and ginsenoside extracts exhibit glucocorticoid-like activities in homeostasis and regulation of immunity (Chung et al. 1998; Ling et al. 2005). Because the actions of glucocorticoids are mediated through cytosolic glucocorti-coid receptors (GRs), we hypothesized that NotoG may mediate its immunosuppressive actions by binding to the GR. Concomitant exposure of N9 MÖ cells to LPS or IFNγ and the glucocorticoid receptor antagonist RU486 attenuated CD40 expression compared to the stimulant alone (Fig. 5a and b). Furthermore, the immunosuppressive effects of NotoG against LPS-induced activation were not blocked by RU486, indicating that the action of the whole NotoG extract is not mediated through GR (Fig. 5a). In contrast, the ability of NotoG to reduce IFNγ-induced CD40 expression was not reversed by RU486, but rather synergis-tically suppressed by RU486 (Fig. 5b). In the LPS stimulated cells, dexamethasone was a more potent inhibitor of CD40 expression than NotoG (Fig. 5a); while in IFNg stimulated cells NotoG appeared to be the more potent inhibitor (Fig. 5b). NotoG also appeared to have a greater ability to suppress CD40 expression in response to IFNγ stimulation than dexamethasone (Fig. 5b).
Fig. 5.
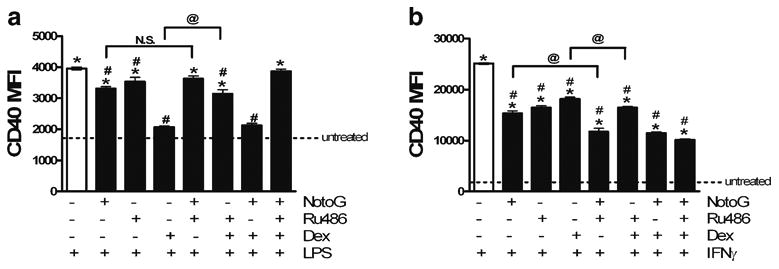
RU486 does not affect the ability of Notoginseng to reduce the expression of CD40 in response to LPS or IFNg stimulation. a NotoG does not appear to be acting through the glucocorticoid receptor to attenuate LPS-induced CD40 expression in N9 MÖ. b Similarly, NotoG does not appear to be acting through the glucocorticoid receptor to attenuate IFNg-induced CD40 expression in N9 MÖ. n=02–3; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to LPS or IFNg. @ P<0.05 comparison indicated
Re, Rb1, and Rg1 ginsenosides exert differential effects LPS- and IFNγ-induced MÖ activation
Ginsenosides, which are triterpene saponins that have a rigid steroidal skeleton with sugar moieties, are purported to be the pharmacologically active component of ginseng (Leung et al. 2006; Leung et al. 2007a, b). In order to determine the contributions of the three major ginsenosides present in NotoG to the observed effects of the whole extract on CD40 expression, the response of N9 MÖ cells exposed to LPS or IFNγ in the presence or absence of Rg1, Rb1 or Re ginsenosides was examined. Purified ginsenosides at comparable concentrations to those found in the whole extract exhibited no effects on CD40 expression (Fig. 6); however, differential effects on MÖ activation were observed dependent on the stimulant. LPS-induced elevations in CD40 expression were attenuated by the ginsenoside Rg1, but not Rb1 or Re (Fig. 6a). In contrast, IFNγ-induced elevations in CD40 expression were attenuated by the ginseno-side Re, but not Rb1 or Rg1 (Fig. 6b). Although none of the major components of our NotoG extract individually attenuated IFNγ-induced nitrite production by N9 MÖ, each of the three ginsenosides (Re, Rb1, and Rg1) may contribute to the observed ability of the whole NotoG extract to reduce IFNγ-induced nitrite production (Table 2).
Fig. 6.
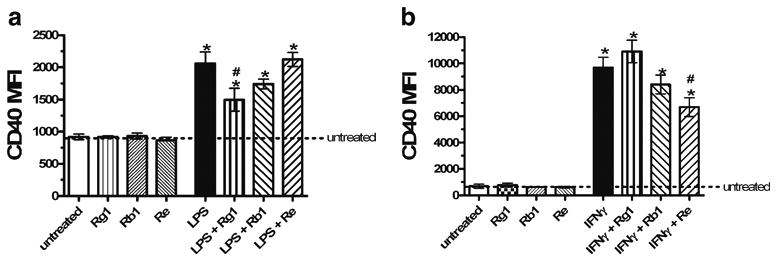
Purified ginsenosides at comparable concentrations to those found in the whole NotoG extract exhibit differential effects on MÖ activation dependent on the stimulant. a The purified ginsenoside Rg1, but not Rb1 or Re, attenuated LPS-induced CD40 expression in N9 MÖ. b In contrast, the purified ginsenoside Re, but not Rg1 or Rb1, reduced IFNγ-induced CD40 expression in N9 MÖ. n=02–3; in triplicate. Error bars indicate the standard error of the mean. *P<0.05 compared to unstimulated control (dashed line). #P<0.05 compared to LPS or IFNγ
Table 2. Effects of purified ginsenosides on IFNγ induced nitrite production.
| N9 microglia | |
|---|---|
|
| |
| Stimulation | Nitrite (NO2-) (μM)a |
| Unstimulated | 2.044±0.13 |
| NotoG | 2.42±0.08 * |
| IFNγ | 45.69±2.55 * |
| IFNγ + NotoG | 37.29±2.42 *# |
| IFNγ + Re | 40.59±1.60 * |
| IFNγ + Rb1 | 38.86±0.88 * |
| IFNγ + Rg1 | 40.50±0.68 * |
micromolar (μM) concentration as determined by Griess Assay
Results are representative of three independent experiments (n=3) ± standard error of the mean
P<0.05 compared to unstimulated.
P<0.05 compared to IFNγ only control
Discussion
Activation of MÖ and the consequent release of inflammatory mediators such as interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), glutamate, nitric oxide (NO), and reactive oxygen species (ROS) contribute to neurodegener-ative processes (Aloisi et al. 2001; Glezer et al. 2007) as demonstrated in pathological lesions from Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Multiple Sclerosis (MS), Acquired Immune Deficiency Syndrome Dementia (HIV-Dementia), Tramatic Brain Injury (TBI), and Hypoxic-Ischemic Injury (Dheen et al. 2007; Glezer et al. 2007; Peterson and Fujinami 2007). Although pharmacological effects of Panax ginseng and ginsenosides have been demonstrated on the CNS, as well as the cardiovascular, endocrine and immune systems (Attele et al. 1999; Radad et al. 2006), the ability of Panax notoginseng to act on CNS immune cells such as MÖ has not been widely examined (Lopez et al. 2007; Wu et al. 2007). Therefore, the present studies were designed to determine whether MÖ activation stimulated via innate immune receptors (e.g. TLR) and interferon gamma (IFNγ) was modulated following exposure to an ethanolic extract of Panax notoginseng (NotoG). NotoG reduced both TLR ligand- and IFNγ-induced activation of MÖ in a dose-dependent manner by reducing cell surface expression of accessory molecules, inflammatory cytokines, and release of bactericidal products (e.g. nitric oxide). The findings of the present study also demonstrate that NotoG reduced TLR ligand- and IFNγ-induced activation was not mediated via glucocorticoid receptors. Furthermore, the contributions of specific ginsenosides (e.g. Re, Rb1, and Rg1) to these inhibitory effects of NotoG on CD40 expression may be dependent on the stimulant utilized.
There exists an urgent need for the generation and development of novel therapies and/or adjuvants to current treatment available for numerous neurodegenerative and neuroimmune diseases. Although a variety of compounds have been used in experimental therapies for these conditions, most of these materials elicit an immune response in the periphery only, exhibit limited ability to cross the blood brain barrier, and/or have undesirable side effects that have limited their potential application. Natural products are currently receiving widespread recognition as having beneficial health effects when consumed on a regular basis as part of a varied diet and at effective levels (Suk 2005). Mounting evidence from epidemiological studies, animal research, clinical trials and research in nutritional biochemistry suggests that natural products may be beneficial in coronary heart disease, cancer, osteoporosis, diabetes, Parkinson’s and Alzheimer’s diseases. These studies indicate that the mechanistic actions of natural products involve a wide array of biological processes, including activation of antioxidant defenses, signal transduction pathways, cell survival-associated gene expression, cell proliferation and differentiation, and preservation of mitochondrial integrity. Furthermore, many natural products exert anti-inflammatory actions through inhibition of oxidative stress-induced transcription factors (e.g., NF-κB, AP-1), cytotoxic cytokines and cyclooxygenase-2 (Mandel et al. 2005). Therefore, natural products and/or their component compounds that target specific pathways involved in MÖ activation may halt or limit the progression of neuroinflammatory and neurodegenerative disorders.
Pharmacological effects of ginseng and ginsenosides have been demonstrated on the cardiovascular, endocrine and immune systems (Attele et al. 1999; Radad et al. 2006). In particular, Panax Notoginseng (commonly referred to as Notoginseng) has been shown to exhibit a wide range of actions in the CNS including increased cell survival, extension of neurite growth, and rescuing of neurons from death following both in vivo and in vitro insults (Jiang and Qian 1995; Chen et al. 2003; Radad et al. 2006; Lopez et al. 2007). Although the ability of Notoginseng to act on immune cells within the CNS has not been widely examined, collectively, scientific evidence supports the hypothesis that microglial-neuonal interactions are critically involved in neuroinflammatory and neurodegenerative disorders, thereby providing a target for therapeutic intervention by Notoginseng (Rock and Peterson 2006). Previous studies and the data presented herein suggest that an ethanolic extract of Panax Notoginseng, NotoG reduces 1) the expression of immune molecules expressed on the cell surface, 2) the production of inflammatory cytokines and nitric oxide production, and 3) modulates signal transduction pathways necessary to mediate innate immune responses by multiple APC types (Rhule et al. 2006). Therefore, Panax Notoginseng represents a novel natural alternative and/or complementary approach to current traditional medicines to reduce the detrimental effects of MÖ activation.
The innate immune response within the CNS is directed at identifying pathogens as non-self by recognizing pathogen associated molecular patterns through various toll like receptors (TLR) (Lee and Kim 2007). The broad expression pattern of mRNA for nine identified TLR in cultured neonatal MÖ and the functional activation of these cells following stimulation with TLR agonists further implicates MÖ as playing an important role in the CNS innate immune system (Olsson et al. 2006). TLR3 recognizes double stranded RNA found primarily in viruses, TLR4 recognizes LPS in association with LPS-binding protein, CD14 and MD-2, and TLR9 recognizes bacterial CpG DNA (Amati et al. 2006). TLR are expressed on a variety of cells; however, most cells have preferential expression of TLR. The results from our study suggest that N9 and EOC20 MÖ differ in their TLR expression based on differential sensitivity to three TLR ligands. This observation may be directly related to the original strain of mice from which the cells were derived. Because of a point mutation at a single residue in the cytoplasmic tail of TLR4, signaling via TLR4 in C3H/HeJ macrophages is deficient; therefore, the murine EOC20 MÖ cell line (originally derived from C3H/HeJ juvenile mice) would not be expected to respond to LPS (Poltorak et al. 1998). Although in the current study, minimal activation of N9 and EOC20 MÖ by CpG was observed, several studies previously demonstrated that primary MÖ, as well as BV-2 and N9 MÖ cell lines express TLR9 and can by activated by CpG-ODN to express different cytokines (Dalpke et al. 2002; Zhang et al. 2005). It would appear that in our hands, EOC20 MÖ preferentially express TLR3 over TLR9, as exhibited by the more robust response to Poly I:C than CpG. Although we have not yet directly measured and compared the expression levels of TLR3, TLR4, and TLR9 in N9 vs.EOC20 MÖ cells lines, studies are currently underway to examine TLR expression levels and determine whether these levels correlate with the biological responses observed.
TLR ligands including LPS, CpG, and Poly I:C, as well as IFNγ, are potent inducers of accessory molecules such as MHC class II, CD40, CD54 and CD86 on immune cells including MÖ. These accessory molecules then function to facilitate the recruitment, interaction, and activation of lymphocytes. When stimulated with TLR ligands or IFNγ, N9 and EOC20 MÖ cell lines respond by upregulating the cell surface expression of these accessory molecules. The current study demonstrates that an ethanolic extract of Panax Notoginseng (NotoG) decreased LPS-induced CD40 accessory molecule expression by N9 MÖ in a dose-dependent manner; yet also appeared to act synergisti-cally with LPS to increase CD86 expression. Subsequent more detailed studies showed that NotoG diminished TLR ligand- and IFNγ-induced CD40 by N9 and EOC20 MÖ. This apparent dichotomy suggests that the NotoG extract may act on multiple, distinct pathways or that different ginsenoside components of the whole NotoG extract may act in opposing manners—which is consistent with other natural products, as well as the constituents of the current NotoG extract (Sengupta et al. 2004; Joo et al. 2005; Mandel et al. 2005). Although previous studies demonstrated that TLR4 and CD14 expression was unaffected by NotoG (Rhule et al. 2006), we have not yet assessed the expression of TLR3 or TLR9 in response to NotoG in murine microglial cell lines.
Several of the secretory products that play a direct or indirect anti-microbial role have been implicated in the pathogenesis of neuroinflammatory and neurodegenerative disorders, including reactive oxygen and reactive nitrogen species (Schell et al. 2007; Wu et al. 2007). Of these, nitric oxide is a key messenger involved in physiological functions, as well as regulation of inflammatory and immune responses (Willenborg et al. 2007). To characterize the ability of NotoG to alter the MÖ antibacterial response to exogenous TLR ligand and IFNγ stimulation, we performed assays to examine nitrite production. NotoG alone exhibited no stimulatory effect on nitrite levels–similar to the effects of purified ginsenosides on LPS induced NO production (Wu et al. 2007). Our results showed that TLR ligand- and IFNγ-dependent increases in nitrite production were reduced by concomitant exposure to NotoG in both cell lines examined. Previous studies using N9 MÖ showed that the ginsenosides Rg1 and Re1, but not Rb2 or Rd, decreased LPS-induced nitrite levels, but did not examine effects on CpG, Poly I:C, or IFNγ induction (Wu et al. 2007). Although neither of the major components of our NotoG extract (Rb1, Rg1, and Re) individually attenuated IFNγ-induced nitrite production by N9 MÖ, we have not yet studied the effects of these ginsenosides alone on TLR ligand-induced nitrite production. It appears that each of the three ginsenosides contribute in small fashion to the ability of the whole NotoG extract to reduce IFNγ-induced nitrite production. Taken together, these data suggest that NotoG may be neuroprotective against various CNS pathologies by limiting the activation of MÖ by microbial pathogens.
Although the exact function of MÖ is not fully understood, several lines of evidence suggest that these cells may sustain and propagate inflammation within the CNS through antigen presentation and/or cytokine/chemokine secretion (Ponomarev et al. 2005; Ponomarev et al. 2006; Muzio et al. 2007). During disease pathology, activated MÖ secrete potent pro-inflammatory cytokines such as TNFα, IL-6 and IL-1β and a recent study demonstrated the ability of specific ginsenosides to reduce LPS-induced TNFa secretion (Wu et al. 2007). We expanded upon these observations to show that NotoG reduces IL-6 and TNFa secretion in response to three different TLR ligands, as well as IFNγ in two distinct MÖ cell lines. Given that transcription factors such as NF-kB are critical regulators of inflammatory genes in MÖ (Bhat et al. 1998; Chan et al. 2001), and that the ginsesno-side Rg3 reduced Aβ42-mediated microglial activation through inhibition of NF-kB p65 binding (Joo et al. 2008), the capacity of NotoG to affect these systems will be examined in further investigations into its molecular mechanisms of action.
Because ginseng and ginsenosides have previously been shown to exhibit glucocorticoid-like activities in homeostasis and regulation of immunity (Chung et al. 1998; Ling et al. 2005) and the biological actions of glucocorticoids are mediated through cytosolic glucocorticoid receptors (GRs), we examined the ability of NotoG to exert its immunosuppressive effects through these receptors. Using the glucocorticoid receptor antagonist RU486, our studies indicated that the action of the whole NotoG extract is not likely mediated through GR. Although the combination exposure of LPS or IFNγ plus or minus NotoG in the presence of absence of RU486 yielded contrasting results, this most likely may be attributed to the nature of the compound itself—which has both partial agonist and antagonist properties.
Ginsenosides, which have a rigid steroidal skeleton with sugar moieties—are purported to be the pharmacologically active component of ginseng (Leung et al. 2006; Leung et al. 2007a; Leung et al. 2007b). Purified ginsenosides at comparable concentrations to those found in the whole extract exhibited no effects on CD40 expression; however, differential effects on MÖ activation were observed dependent on the stimulant. Ginsenoside Rg1 appeared to attenuate LPS-induced elevations in CD40 expression, while ginsenoside Re reduced IFNγ-induced elevations in CD40 expression. Although neither Re, Rb1 or Rg1 individually reduced IFNγ-induced nitrite production by N9 MÖ, each of the three ginsenosides (Re, Rb1, and Rg1) may contribute to the observed ability of the whole NotoG extract to reduce IFNγ-induced nitrite production.
An important aspect of our study is the use of MÖ cell lines, as opposed to primary murine MÖ. While cell lines are an invaluable tool for defining key steps in activation responses, evaluating otherwise unmanipulated, pure primary cultures represents a more powerful tool to resolve the basis of cellular activation in disease pathologies and immunosuppression in response to natural products such as NotoG. Thus, as our studies and understanding progress, the use of primary MÖ will be essential, not only to reveal how altered signaling impacts the eventual CNS immune response, but also to ascertain whether potential differences exist in distinct MÖ populations. Moreover, there exists a need to evaluate the use of Notoginseng and its components in various animal models of disease (Suk 2005).
Collectively, these studies confirm a critical role for MÖ in the innate immune response to CNS pathogens, and more importantly suggests that an ethanolic extract of Panax Notoginseng (NotoG) attenuated CNS immune functions in response to TLR ligands as a model of pathogen exposure. MÖ have been suggested to play a role in various CNS diseases such as Multiple Sclerosis, Alzheimer’s disease, bacterial meningitis, and ischemia-reperfusion injury. In particular, MS has been suggested to be initiated or exacerbated by the presence of underlying infections (Becher et al. 2001). It may be that Notoginseng’s ability to reduce TLR ligand- and IFNγ-induced MÖ activation represents a novel therapeutic target for the modulation of immune functions in MS and other CNS inflammatory or neurodegenerative diseases. Therefore, a better understanding of the mechanism (s) by which Notoginseng modulates MÖ activation using in vitro and in vivo tools may lead to the development of additional therapeutics strategies aimed at attenuating immune activation.
Acknowledgments
This work is supported by NIH grants COBRE P20 RR17670 from the National Center for Research Resources (NCRR) and NRSA fellowship ES-013044 (CAB). The authors wish to thank Pamela Shaw and the CEHS Fluorescence Core, as well as Dr. Darrell Jackson at UM for their expert technical assistance.
Footnotes
Conflicts of interest: The authors have no financial or commercial conflicts of interest.
Contributor Information
Celine A. Beamer, Email: celine.beamer@umontana.edu, Center for Environmental Health Sciences, Department of Biomedical and Pharmaceutical Sciences, The University of Montana, 32 Campus Drive, Skaggs Building Room 285A, Missoula, MT 59812-1552, USA.
David M. Shepherd, Email: david.shepherd@umontana.edu, Center for Environmental Health Sciences, Department of Biomedical and Pharmaceutical Sciences, The University of Montana, 32 Campus Drive, Skaggs Building Room 284, Missoula, MT 59812-1552, USA.
References
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Ambrosini E, Columba-Cabezas S, Magliozzi R, Serafini B. Intracerebral regulation of immune responses. Ann Med. 2001;33:510–515. doi: 10.3109/07853890108995960. [DOI] [PubMed] [Google Scholar]
- Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–4254. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Barnes P, Powell-Griner E, McFann K, Nahin R. National Center for Health Statistics. Centers for disease control and prevention; 2004. Complemetary and alternative medicine use among adults: United States 2002; p. 343. [PubMed] [Google Scholar]
- Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal M. Asian ginseng: potential therapeutic uses. Adv Nurs Pract. 2001;9(26–28):33. [PubMed] [Google Scholar]
- Bu Y, Jin ZH, Park SY, Baek S, Rho S, Ha N, Park SK, Kim H. Siberian ginseng reduces infarct volume in transient focal cerebral ischaemia in Sprague-Dawley rats. Phytother Res. 2005;19:167–169. doi: 10.1002/ptr.1649. [DOI] [PubMed] [Google Scholar]
- Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DW. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways. Infect Immun. 2001;69:2001–2010. doi: 10.1128/IAI.69.4.2001-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxida-tive stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- Chung E, Lee KY, Lee YJ, Lee YH, Lee SK. Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63:421–424. doi: 10.1016/s0039-128x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol. 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Azoulay E. Inflammatory response to infectious pulmonary injury. Eur Respir J Suppl. 2003;42:10s–14s. doi: 10.1183/09031936.03.00420203. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia. 2006;54:861–872. doi: 10.1002/glia.20426. [DOI] [PubMed] [Google Scholar]
- Esiri MM. The interplay between inflammation and neurode-generation in CNS disease. J Neuroimmunol. 2007;184:4–16. doi: 10.1016/j.jneuroim.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Jiang KY, Qian ZN. Effects of Panax notoginseng saponins on posthypoxic cell damage of neurons in vitro. Zhongguo Yao Li Xue Bao. 1995;16:399–402. [PubMed] [Google Scholar]
- Joo SS, Won TJ, Lee DI. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB, Hwang KW, do Lee I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008;31:1392–1396. doi: 10.1248/bpb.31.1392. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- Leung KW, Leung FP, Huang Y, Mak NK, Wong RN. Non-genomic effects of ginsenoside-Re in endothelial cells via gluco-corticoid receptor. FEBS Lett. 2007a;581:2423–2428. doi: 10.1016/j.febslet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology. 2007b;52:827–835. doi: 10.1016/j.neuropharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lin WM, Zhang YM, Moldzio R, Rausch WD. Ginsenoside Rd attenuates neuroinflammation of dopaminergic cells in culture. J Neural Transm Suppl. 2007:105–112. doi: 10.1007/978-3-211-73574-9_13. [DOI] [PubMed] [Google Scholar]
- Ling C, Li Y, Zhu X, Zhang C, Li M. Ginsenosides may reverse the dexamethasone-induced down-regulation of glucocorticoid receptor. Gen Comp Endocrinol. 2005;140:203–209. doi: 10.1016/j.ygcen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lopez MV, Cuadrado MP, Ruiz-Poveda OM, Del Fresno AM, Accame ME. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim Biophys Acta. 2007;1770:1308–1316. doi: 10.1016/j.bbagen.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Mandel S, Packer L, Youdim MB, Weinreb O. Proceedings from the “Third international conference on mechanism of action of nutraceuticals”. J Nutr Biochem. 2005;16:513–520. doi: 10.1016/j.jnutbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Muzio L, Martino G, Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. J Neuroimmunol. 2007;191:39–44. doi: 10.1016/j.jneuroim.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Olsson T, Jagodic M, Piehl F, Wallstrom E. Genetics of autoimmune neuroinflammation. Curr Opin Immunol. 2006;18:643–649. doi: 10.1016/j.coi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Peterson LK, Fujinami RS. Inflammation, demyelination, neuro-degeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;184:37–44. doi: 10.1016/j.jneuroim.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Dittel BN. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J Immunol. 2006;176:1402–1410. doi: 10.4049/jimmunol.176.3.1402. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- Rausch WD, Liu S, Gille G, Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars) 2006;66:369–375. doi: 10.55782/ane-2006-1625. [DOI] [PubMed] [Google Scholar]
- Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notogin-seng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006;106:121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rock RB, Peterson PK. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroim-mune Pharmacol. 2006;1:117–126. doi: 10.1007/s11481-006-9012-8. [DOI] [PubMed] [Google Scholar]
- Schell JB, Crane CA, Smith MF, Jr, Roberts MR. Differential ex vivo nitric oxide production by acutely isolated neonatal and adult microglia. J Neuroimmunol. 2007;189:75–87. doi: 10.1016/j.jneuroim.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- Suk K. Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases A focus on traditional medicines and flavonoids. Neurosignals. 2005;14:23–33. doi: 10.1159/000085383. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Staykova M, Fordham S, O'Brien N, Linares D. The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:16–25. doi: 10.1016/j.jneuroim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX, Wang JH, Wang JM, Zhang R, Li X. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7:313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1-42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Guo K, Schluesener HJ. The immunostimulatory activity of CpG oligonucleotides on microglial N9 cells is affected by a polyguanosine motif. J Neuroimmunol. 2005;161:68–77. doi: 10.1016/j.jneuroim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Zhu S, Zou K, Fushimi H, Cai S, Komatsu K. Comparative study on triterpene saponins of Ginseng drugs. Planta Med. 2004;70:666–677. doi: 10.1055/s-2004-827192. [DOI] [PubMed] [Google Scholar]


