Abstract
Cues repeatedly paired with rewards often themselves become imbued with enhanced motivational value, or incentive salience. During Pavlovian conditioned approach procedures, a cue repeatedly preceding reward delivery often elicits conditioned responses at either the reward delivery location (“goal-tracking”) or the cue itself (“sign-tracking”). Sign-tracking behavior is thought to reflect the individual differences in attribution of incentive salience to reward-paired cues that may contribute to addiction vulnerability. Adolescent rats typically demonstrate less sign-tracking behavior than adult rats, a surprising finding given that adolescence is hypothesized to be a time of heightened addiction vulnerability. Given evidence that adult sign-tracking behavior can be influenced by environmental conditions, the present study compared the effects of isolate housing and food deprivation on expression of sign-tacking and goal-tracking behavior in adolescent and adult male rats across eight days of a Pavlovian conditioned approach procedure. Pair-housed adults exhibited more sign-tracking behavior than pair-housed adolescents; however, this age difference was not apparent in isolate-housed subjects. Adolescents often appeared more sensitive than adults to both food restriction- and isolate housing-induced changes in behavior, with food restriction promoting an increase in sign-tracking among isolate-housed adolescents and an increase in goal-tracking among pair-housed adolescents. For adults, food restriction resulted in a modest increase in overall expression of both sign-and goal-tracking behavior. To the extent that sign-tracking behavior reflects attribution of incentive salience to reward-paired cues, results from the present study provide evidence that reactivity to rewards during adolescence is strongly related to the nature of the surrounding environment.
Keywords: Pavlovian conditioned approach, Incentive salience, Adolescent, Rat, Sign-tracking, Isolate-housing, Food restriction
1. Introduction
Cues repeatedly paired with rewards often themselves become imbued with incentive value. Incentive salience refers to the enhanced motivational value of stimuli repeatedly paired with reward delivery (see Robinson and Berridge, 1993). Attribution of incentive salience to reward-paired cues may reflect individual differences that underlie addiction vulnerability (reviewed by Flagel et al., 2009; Tomie et al., 2008). Pavlovian conditioned approach (PCA) procedures (sometimes referred to as ‘autoshaping’) have gained popularity as a means to assess the attribution of incentive salience to reward-paired cues in rodents (e.g., Anderson and Spear, 2011; Beckmann et al., 2011; Flagel et al., 2007; Tomie, 1996). A typical PCA procedure involves repeated pairings of a cue (conditioned stimulus; CS) and a reward (unconditioned stimulus; US); such pairings often eventually elicit one or more conditioned responses (CR) during cue presentation. One CR involves approach to the reward delivery location (typically a food trough or liquid dipper arm area); this reward-directed response is referred to as goal-tracking (Boakes, 1977). An alternative CR involves approach and interaction with the CS itself, a response referred to as sign-tracking (Hearst and Jenkins, 1974). Sign-tracking behavior is hypothesized to reflect attribution of incentive salience to a reward-paired cue, resulting in the cue serving as a “motivational magnet” (see Flagel et al., 2009).
Evidence supports the hypothesized relationship between sign-tracking and addiction vulnerability: animals that exhibit high levels of sign-tracking show greater sensitization to cocaine-induced psychomotor activation (Flagel et al., 2008), more rapid acquisition of cocaine self-administration (Beckmann et al., 2011), higher breakpoints in progressive ratio operant responding for cocaine (Saunders and Robinson, 2011), and enhanced reinstatement of cocaine self-administration (Saunders and Robinson, 2011). Likewise, prior amphetamine sensitization increases sign-tracking behavior (Doremus-Fitzwater and Spear, 2011). Sign-tracking behavior is also positively correlated with other behaviors related to addiction vulnerability, including impulsive action (Flagel et al., 2010; Lovic et al., 2011) and novelty preference (Beckmann et al., 2011). Release of corticosterone (CORT) also appears to correlate with sign-tracking behavior: animals that exhibit high levels of sign-tracking behavior during PCA sessions have significantly higher post-session CORT levels than animals that exhibit low levels of sign-tracking (Flagel et al., 2009; Tomie et al., 2000). Evidence also strongly supports a role for CORT in addiction vulnerability (see Piazza and Le Moal, 1996; Marinelli and Piazza, 2002), with high levels of CORT associated with greater drug self-administration and psychomotor activation (Piazza et al., 1991; Prasad and Prasad, 1995; Deroche et al., 1994; Marinelli et al., 1997).
Adolescence is the developmental period that encompasses the transition from youth to maturity, during which individuals experience a host of neural, hormonal, and behavioral alterations that include increased peer affiliation, impulsivity, risk taking, and novelty seeking/preference (Hartup and Stevens, 1997; Primus & Kellogg, 1989; Varlinskaya & Spear, 2008; Adriani et al., 1998; Douglas et al., 2003; Laviola et al., 2003; Adriani and Laviola, 2003). These behavioral characteristics may contribute to the initiation of substance use and abuse that is prevalent during adolescence. Results from the 2011 Monitoring the Future study revealed that 70% of high school seniors have consumed alcohol, 40% have smoked cigarettes or marijuana, and 25% report having used other illicit drugs (Johnston et al., 2012). Indeed, adolescence is often considered a critical period for addiction vulnerability (see Chambers et al., 2003; Crews et al., 2007). Adolescents demonstrate greater neural activation (indexed by c-fos protein expression) than adults in the nucleus accumbens in response to a cue previously paired with a food reward (Friemel et al., 2010). Among adult animals, elevated c-fos mRNA expression in the nucleus accumbens in response to presentation of a cue previously paired with a food reward is seen only in animals that exhibit high levels of sign-tracking behavior (Flagel et al., 2011). Given the hypothesized relationship between sign-tracking behavior and heightened drug abuse vulnerability, as well as the other behavioral and neurobiological correlates of sign-tracking, one might expect adolescents to exhibit more sign-tracking behavior than adults. Previous evidence from our lab, however, has revealed an opposite ontogenetic profile: adults typically exhibit greater levels of sign-tracking behavior than adolescents (Anderson and Spear, 2011; Doremus-Fitzwater and Spear, 2011).
Although evidence supports a strong genetic component in sign-tracking and goal-tracking behavior (e.g., Flagel et al., 2010), early environmental manipulations such as isolation rearing and deprivation of natural maternal care have recently been demonstrated to increase expression of sign-tracking behavior in adulthood (Beckmann and Bardo, 2012; Lomanowska et al., 2011). These studies support a role for early life experiences in shaping attribution of incentive salience to reward-paired cues later in life, potentially contributing to differences in addiction vulnerability. The present study was designed to assess the effects of environmental manipulations on sign-tracking and goal-tracking behavior in adolescent and adult rats. Food restriction and isolate-housing were selected as the experimental manipulations due to evidence that each can influence drug reward and/or sensitivity (Ahmed et al., 1995; Bell et al., 1997; Carr, 2002; Carroll and Meisch, 1979; Phillips et al., 1994). Isolate-housing in particular may have different consequences for adolescents and adults (see Hall, 1998).
2. Materials and Methods
2.1 Subjects
A total of 64 male Sprague-Dawley rats bred in our colony at Binghamton University were used in the present study. On postnatal day (P) 1, litters were culled to 8 to 10 pups, keeping a ratio of 6 males to 4 females when possible. Subjects were weaned on P21, at which time they were pair-housed with same-sex littermates and maintained in a temperature-controlled vivarium on a 12:12-hr light:dark cycle (lights on at 7 AM), with ad libitum access to food (Purina lab chow, Lowell, MA) and water (except as specified below). All animals were treated in accordance with guidelines established by the National Institute of Health (Laboratory Animal Resources, Commission on Life Sciences, 2011) and protocols approved by the Binghamton University Institutional Animal Care and Use Committee. Eight subjects were assigned to each of the groups defined by the 2 age (adolescents, adults) × 2 housing (isolated, paired) × 2 food condition (food-restricted, free-feeding) factorial design. In order to avoid confounding litter effects, no more than one animal per litter was assigned to the same experimental condition (see Holson and Pearce, 1992; Zorrilla, 1997). All testing was conducted between 1000 and 1600 hrs.
2.2 Apparatus
Twelve operant chambers measuring 30.5 × 24.1 × 21 cm (Med Associates, St. Albans, VT) housed within sound-attenuating boxes measuring 55.9 × 38 × 35.6 cm were used. A food receptacle with a dispenser for banana pellets (45 mg dustless precision banana-flavored pellets, Bio-Serv, Frenchtown, NJ) was mounted on the right wall of each chamber, along with a retractable illuminated lever on either the left or right side of the receptacle. Levers were illuminated only when extended out into the chamber, and not while retracted into the chamber wall. For the adults, the lever measured 4.8 cm wide, whereas a mouse-sized lever measuring 1.6 cm was used for adolescent animals. The receptacle and the lever were mounted 2.5 cm from the floor of the chamber for adolescents and 4.5 cm from the floor of the chamber for adults. Photosensors within the food receptacle were used to count nosepokes into the receptacle area. A red house light was mounted in the top right corner of the left wall and was illuminated throughout each session.
2.3 Procedure
Eleven days before the start of PCA testing, subjects were re-housed either alone or with a same-sex non-littermate on P21 (adolescents) or P65 (adults) in standard acrylic breeder tubs with wood shavings. Animals remained either isolate- or pair-housed for the duration of the study. Whenever possible, subjects were housed with a counterpart of similar body weight, resulting in average weight differences of 7.6 grams (12% total body weight) among adolescents and 15.1 grams (4% total body weight) among adults. To reduce potential neophobia to the banana pellets used during training, approximately 6.5 g of banana pellets were placed in the home cage of each animal (or 13 g per pair of animals) beginning on P28 or P72, for 2 consecutive days prior to pre-training. All animals assigned to free-feeding conditions had ad libitum access to food and water. Subjects in the food-restricted conditions had ad libitum access to water, but were given daily food allotments as described below.
2.3.1 Food restriction
Beginning the day prior to pre-training, adult subjects assigned to the food-restricted group were given 3–3.5 g of rat chow daily until they reached 85% of their free-feeding (pre-restriction) weight. When they reached this point, they were given approximately 14 g per day, with this amount increased as needed to maintain their target body weight. Adolescents assigned to the food-restricted group were given approximately 7–7.5 g of food initially, such that they gained little weight overnight (approximately 1–2 g). Each day thereafter, this amount was increased as needed to allow for 5–8 g of weight gain, thereby permitting maintenance of approximately 85% of the normal growth trajectory determined from the weights of their free-feeding counterparts. Food-restricted subjects received food each day after testing.
2.3.2 Pre-training
On each of the 2 days prior to onset of the PCA procedure (P30 or P74), animals were placed in the operant chambers with the levers in the retracted position. During each pre-training session, 25 pellets were delivered on a variable interval (VI) 90 s schedule over the course of 35 to 40 minutes.
2.3.3 Pavlovian Conditioned Approach
Beginning on P32 or P67, subjects were given daily PCA sessions for 8 days. Each session consisted of 25 8-s presentations of the lighted lever conditioned stimulus (CS) on a VI 90 s schedule, followed by delivery of a pellet (US) as the lever retracted. Sessions lasted for approximately 35 to 45 minutes, with the CS presentations provided on a VI 90-s schedule and with the 25 CS-US pairings occurring independently of the subjects’ behavior. Number of nosepokes and lever presses were recorded during each 8-s lever presentation as measures of goal-tracking (GT) and sign-tracking (ST), respectively. Any remaining banana pellets after each daily session were counted and removed from the chamber. By day 8 of the PCA procedure, food-restricted adolescents and all adults consumed all banana pellets whereas free-feeding adolescents had an average of 1.87 (pair-housed) and 4.5 (isolate-housed) leftover pellets. Immediately following the final PCA session, subjects were sacrificed and trunk blood was collected and centrifuged. Plasma was stored at −80°C until assayed for CORT using radioimmunoassay (see Willey et al., 2012).
2.4 Dependent variables and data analysis
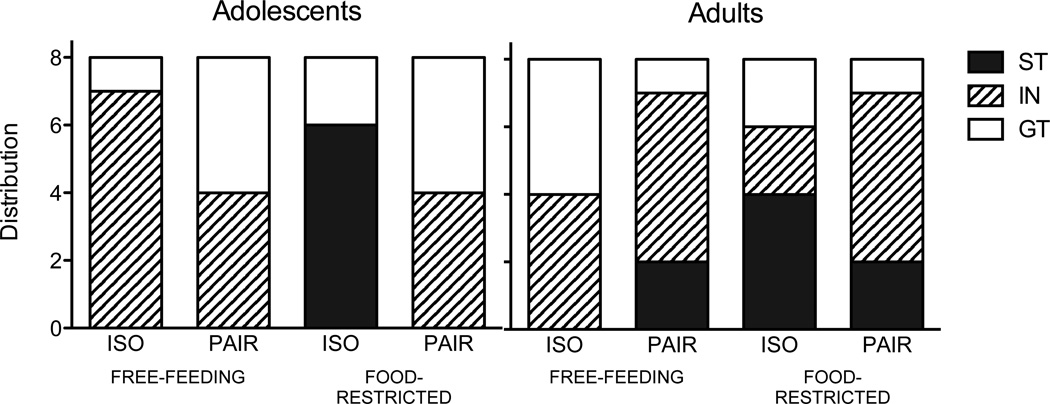
Behavioral measures were used to generate daily PCA scores for each subject (described in Meyer et al., 2012). For each animal, three different coefficients of approach (each reflecting the relative tendency to engage in either ST or GT behavior) were determined each day and were averaged to calculate a daily PCA score for each animal: response bias (difference in lever presses and nosepokes in relation to total responses), difference in probability (percent of trials with lever presses – percent of trials with nosepokes), and difference in latency to approach the lever and food receptacle. Values ranged from −1.0 (indicating behavior directed exclusively at the goal; GT) to +1.0 (indicating behavior directed exclusively at the cue; ST). The average PCA score on days 4–8 of the PCA procedure was used to categorize individual subjects as sign-trackers (scores of +0.5 to +1), goal-trackers (scores of −0.5 to −1), or intermediates (−0.49 to +0.49). Given the relatively small sample size, categorization was not included as a factor in any analyses, but the phenotypic distribution is depicted in Figure 1. A more thorough description and discussion of the PCA score is provided by Meyer and colleagues (2012). PCA scores were analyzed using 2 age × 2 housing × 2 food condition × 8 day repeated measures analyses of variance (ANOVAs). On the last day (day 8), the extent to which alterations in lever presses (index of ST) versus nosepokes (index of GT) may have contributed to the composite score was evaluated with ANOVAs using behavioral measure (ST and GT) as a repeated measure, as described later. Significant interactions were further explored using Fisher’s LSD tests.
Figure 1.
Distribution of behavioral phenotypes. Within each experimental condition (n=8), average PCA scores on sessions 4–8 were used to categorize individuals as sign-trackers (ST), goal-trackers (GT), or intermediates (IN).
Body weight data were analyzed to ensure that food restriction was similar across housing conditions. Twelve days of body weights (including two days prior to food restriction) were analyzed separately for each age via 2 housing condition × 2 food condition × 12 day repeated measures ANOVAs. CORT data collected after the final PCA sessions were analyzed using a 2 age × 2 housing × 2 food condition factorial ANOVA.
3. Results
3.1 PCA scores
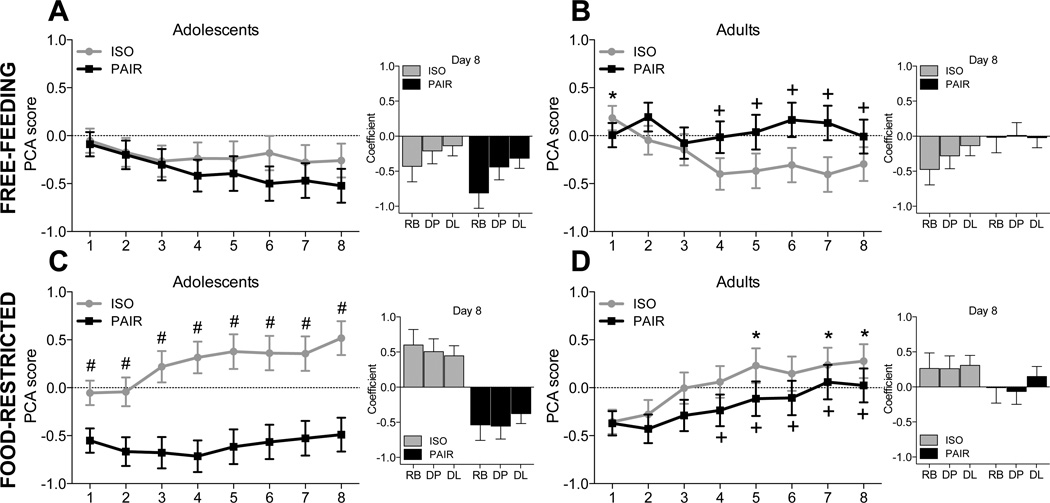
The overall analysis of the PCA scores revealed a number of significant effects, including a day × age × housing interaction [F(7,392) = 3.1, p < .01]. Among pair-housed subjects, adults had higher PCA scores than adolescents on days 4–8, whereas no age differences were seen in isolate-housed subjects. Data are shown in Figure 2. Day 8 values for each of the three components contributing to the PCA score are also included in the figure for reference.
Figure 2.
PCA scores across 8 days of testing. Positive values indicate more ST than GT behavior whereas negative values indicate more GT behavior than ST behavior. Among pair-housed subjects, adults had higher PCA scores than adolescents on days 4–8 (indicated by +s). No age differences were seen among isolate-housed rats. Among adolescents, isolate-housed, food-restricted subjects had higher PCA scores all other groups (indicated by #). Among isolate-housed adults, food-restricted subjects had lower PCA scores on day 1 but higher PCA scores on days 5, 7, and 8 relative to free-feeding subjects (indicated by *). No effects of food restriction were seen in pair-housed subjects of either age. Offset graphs show the day 8 values of each component of the PCA score: Response bias (RB), Difference in probability (DP), and Difference in latency (DL).
To better explore the effects of housing and food restriction in adolescents and adults, data were analyzed separately by age. Analysis of adolescent PCA scores revealed a housing × food condition interaction [F(1,28) = 6.0, p < .05]. Isolate-housed adolescents that were food restricted had higher PCA scores than all other adolescent groups. No effects of food restriction were seen in pair-housed adolescents. Analysis of adult PCA scores revealed a day × housing × food condition interaction [F(7,196) = 2.7, p < .05]. Among isolate-housed adults, free-feeding rats had higher PCA scores than food-restricted rats on day 1, with lower PCA scores on days 5, 7, and 8. No effects of food restriction were seen in pair-housed adults.
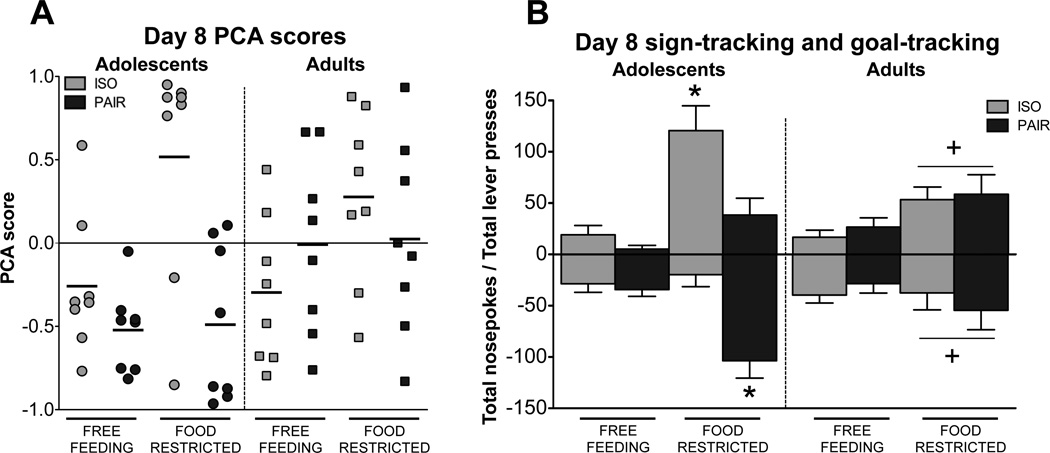
3.2 Day 8 sign-tracking and goal-tracking
To explore individual differences in PCA scores, day 8 values for all subjects are displayed in Figure 3a. Changes in PCA scores may reflect a change in incidence of ST, GT, or both behaviors resulting from the experimental manipulations. Thus, we have shown lever presses (ST; positive values) and nosepokes (GT; expressed as negative values) from day 8 to illustrate the specific behavioral differences between experimental groups (Figure 3b). These day 8 data were analyzed with behavior (i.e., lever press or nosepoke) as a repeated measure. This analysis revealed a number of interactions involving age, including an age × behavior × housing interaction [F(1,56) = 8.8, p < .01] and an age × food condition interaction [F(1,56) = 6.9, p < .05], with food-restricted adolescents exhibiting more behavior overall than food-restricted adults.
Figure 3.
(A) Individual PCA scores on day 8. Horizontal bars indicate mean PCA score for each group. (B) Lever presses (sign-tracking; positive numbers) and nosepokes (goal-tracking; expressed as negative numbers) for each experimental condition on day 8. Among adolescents, significant differences in total lever presses and nosepokes are indicated by *. For adults, an overall increase in both lever presses and nosepokes in food-restricted relative to free-feeding subjects is indicated by +.
In order to better characterize the interactions with age, effects of housing and food deprivation were examined using separate analyses at each age. Analysis of the adolescent data revealed a behavior × housing × food condition interaction [F(1,28) = 10.1, p < .01]. Isolate-housed, food-restricted adolescents demonstrated more ST than GT behavior whereas pair-housed, food-restricted adolescents demonstrated more GT than ST behavior. The same analysis of the adult day 8 data revealed only a main effect of food condition [F(1,28) = 13.1, p < .01], with post-hoc tests conducted on data collapsed across the two behaviors showing that food-restricted rats demonstrated an overall increase in expression of these behaviors.
3.3 Body weight gain
As expected, analysis of the adolescent data revealed an interaction of day and food condition [F(11,308) = 191.5, p < .001], with food-deprived adolescents weighing significantly less than their free-feeding counterparts on the last 8 days. Similarly, following analysis of adult body weight data, an interaction of day and food condition emerged [F(11,308) = 385.6, p < .001], with food restricted adults weighing less than their free-feeding counterparts on all 12 days, and these differences being more marked on days 3–12. Body weight did not differ across housing condition for any groups. Summary body weight data are provided in Table 1.
Table 1.
Body weight gain.
| Adolescents | Pre-restriction (g) | PCA-8 (g) | % Pre-restriction | % Free-feeding gain |
|---|---|---|---|---|
| Free-feeding | 109.6 ± 1.7 | 200.8 ± 2.5 | 183% | |
| Food-restricted | 113.6 ± 2.7 | 167.1 ± 3.2 | 147% | 80% |
| Adults | ||||
| Free-feeding | 405.3 ± 4.9 | 436.5 ± 5.8 | 108% | |
| Food-restricted | 389.7 ± 5.1 | 334.4 ± 4.3 | 86% | 80% |
Body weights (Means ± SEMs) before the onset of food restriction and on the final day of testing are shown in grams. The percent of pre-restriction weight was used to calculate the percent difference in body weight gain between free-feeding and food-restricted subjects.
3.4 Corticosterone levels
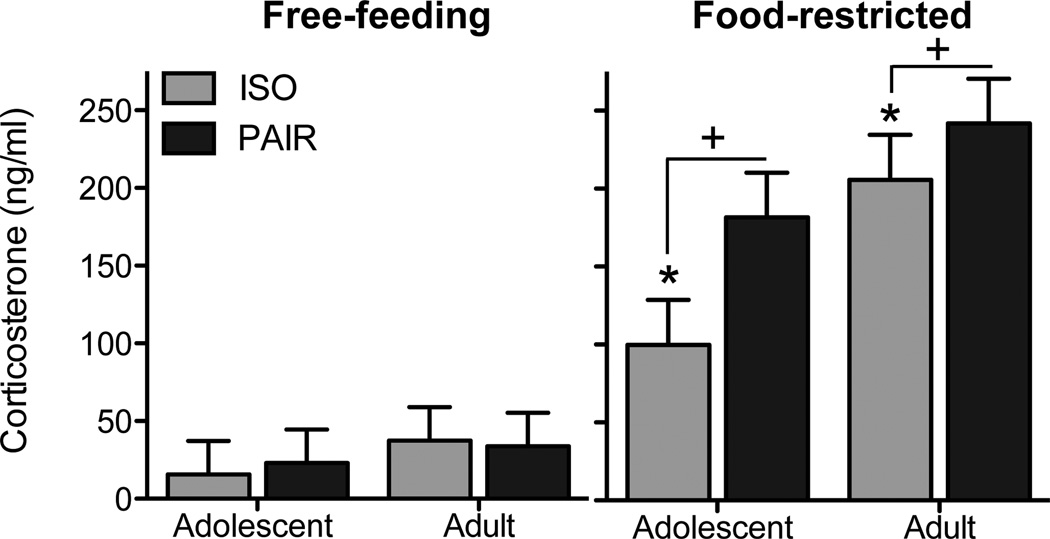
Given dramatic differences in CORT levels between free-feeding and food-restricted subjects [main effect of food condition: F(1,56) = 103.0, p < .001], CORT values were analyzed separately across food condition. Analysis of free-feeding subjects indicated no significant effects of age or housing on post-session CORT levels. Analysis of food-restricted subjects revealed main effects of age [F(1,28) = 8.4, p < .01] and housing [F(1,28) = 4.2, p < .05], with adults having higher post-session CORT values than adolescents and with pair-housed subjects having higher CORT levels than isolate-housed subjects (see Figure 4).
Figure 4.
Post-session CORT levels on day 8 of the Pavlovian conditioned approach testing. No age or housing differences were observed among free-feeding subjects. For food-restricted subjects, adolescents had lower CORT levels than adults (indicated by *) and isolate-housed subjects had lower CORT values than pair-housed subjects (indicated by +).
The relationship between post-session CORT levels and expression of sign-tracking and goal-tracking behavior (i.e., lever presses and nosepokes on day 8) was assessed within each age using Pearson’s r correlation. For adolescents, no significant correlation between CORT levels and ST (r = .32, p > .05) emerged, whereas for adults, CORT levels were correlated with ST behavior (r = .64, p < .05). The correlation between post-session CORT levels and GT behavior revealed the opposite pattern. For adolescents, CORT levels were slightly but significantly correlated with GT (r = .50, p < .05), whereas for adults, no correlation emerged (r = −.07, p > .05).
4. Discussion
Our laboratory has previously reported lower levels of sign-tracking behavior in adolescents relative to adults under pair-housed, free-feeding conditions (Anderson and Spear, 2011; Doremus-Fitzwater and Spear, 2011). Among subjects assigned to similar conditions in the present study, the same pattern of behavior was observed: pair-housed adolescents showed a reduced propensity for sign-tracking (indexed via PCA scores) than adults on most days of PCA testing. Environmental manipulations, however, were effective in elevating expression of sign-tracking in adolescents. Among isolate-housed subjects, no age differences in PCA scores were apparent, and adolescents often appeared more sensitive than adults to both food restriction- and isolate housing-induced changes in sign-tracking.
4.1 Effects of food restriction and housing conditions on sign-tracking behavior
Adolescents had lower PCA scores relative to adults under pair-housed conditions, but not when isolate-housed. In isolate-housed adolescents, food restriction induced notable increases in sign-tracking behavior as reflected by higher PCA scores, increased number of lever presses in the day 8 assessment, and an apparent increase in the sign-tracking phenotype. When adolescents were pair-housed, they were resistant to this effect of food restriction. A similar but less dramatic pattern was seen in adult PCA scores, but not day 8 lever press behavior. These results perhaps suggest a “social buffering” effect (Kikusui et al., 2006) of pair-housing against the effects of food restriction. That is, the pair-housed subjects may have been more resistant to the stress of food-deprivation, whereas this stressor may have been sufficient to promote sign-tracking behavior in isolate-housed subjects who may already have been somewhat stressed by social isolation. Indeed, free-feeding, isolate-housed adolescents consumed fewer banana pellets (i.e., a highly palatable reward) than their pair-housed counterparts during PCA testing, an anhedonic-like effect often evident in stressed animals (Papp et al., 1991). The post-session CORT data revealed an opposite pattern, however. As discussed further below, isolated-housed adolescents were found to have lower post-session levels of this stess-related hormone than their group-housed counterparts.
The increase in PCA scores seen in isolate-housed relative to socially-housed adolescents is reminiscent of a previous study that reported a greater propensity for sign-tracking behavior among young rats reared in isolation relative to rats reared in an enriched environment (Beckmann and Bardo, 2012). The effects of isolate housing observed in adolescent subjects may reflect a consequence of early social deprivation (see Hall, 1998). Not only are social interactions more rewarding for adolescents than adults (Douglas et al., 2004), but adolescence is a critical period during which social deprivation has long-lasting effects (Einon and Morgan, 1978). Because subjects in the present study were only isolated 11 days prior to the start of PCA testing, adult subjects experienced typical social interactions during adolescence. Thus, the relatively brief period of isolate housing in adulthood may have been sufficient to increase the PCA score on some days (Figure 2) but less effective in elevating sign-tracking per se (at least in the day 8 lever press data; Figure 3b).
Our results also complement the findings of a recent study that reported higher levels of sign-tracking among animals subjected to an early environment with inadequate maternal care relative to those reared more naturally (Lomanowska et al., 2011). Adolescents assigned to the food-restricted/isolate-housed condition in the present study also experienced impoverished conditions and likewise demonstrated enhanced sign-tracking relative to subjects in less distressing conditions.
Adolescents were more sensitive to food restriction-induced increases in sign-tracking and goal-tracking behavior than adults (Figure 3b), although the specific changes were dependent on housing condition. Food restriction notably increased lever press behavior in isolate-housed adolescents while as dramatically increasing nosepoke behavior in pair-housed adolescents. For adults, food restriction modestly elevated expression of both behaviors, regardless of housing condition.
4.2 Corticosterone and sign-tracking
Post-session CORT levels in the present study were dramatically enhanced in food-restricted subjects, perhaps not surprising given that food restriction typically results in elevated CORT release (Stamp et al., 2008; Beck and Luine, 1999). Age differences in CORT were seen among food-restricted subjects, with adults having higher CORT levels than adolescents.
Sign-tracking behavior and CORT have been previously reported to be correlated in adult rats and mice (Flagel et al., 2009; Tomie et al., 2000; Tomie et al., 2012), although this relationship has not been previously explored in adolescent animals. In the present study, the relationship between CORT and sign-tracking was found to differ between adolescents and adults. Among adults, CORT levels correlated with sign-tracking behavior, findings consistent with prior reports. This association was not apparent, however, in adolescents where CORT was instead correlated with goal-tracking behavior in adolescents, an effect not evident in adults. To the extent that CORT might reflect general arousal and attention during the PCA procedure (see Tomie et al., 2008; Merali et al., 1998), this pattern may occur as a result of an inherent propensity for adolescents to exhibit the goal-tracking CR.
It is surprising that although isolate housing generally increased sign-tracking behavior in food-deprived animals, higher CORT levels were seen among pair-housed subjects in the food restricted condition. It seems possible that different factors contributed to the post-session CORT levels that are difficult to parse out without basal CORT values. For example, the high CORT levels seen among pair-housed, food-restricted subjects may reflect increased arousal/attention to the task potentiated by the anticipation of being reunited with their cagemate. Without prior assessments of CORT, it is impossible to determine the extent to which the CORT response habituated across days. It is possible that a different pattern of CORT levels might have emerged if we had collected samples after the first PCA session (e.g., Flagel et al., 2009).
4.3 Dopamine and sign-tracking
Numerous research reports support the role of dopamine in the nucleus accumbens in attributing stimuli with incentive salience (Flagel et al., 2007; Flagel et al., 2011; Saunders and Robinson, 2012). Interestingly, both food restriction and isolate-housing have been shown to produce alterations in the dopamine system. For example, food restriction was reported to reduce baseline extracellular dopamine levels in the nucleus accumbens, an effect hypothesized to amplify phasic dopamine released in response to a stimulus such as food (Pothos et al., 1995). Food restriction has also been reported to increase dopamine D1 receptor function in the nucleus accumbens and caudaute putamen (Carr et al., 2003). Similarly, isolate-housing leads to an upregulation of dopamine in the striatum and nucleus accumbens, although decreased D2 densities in the NAC/striatum have also been reported (e.g., Bean and Lee, 1991; Hall et al., 1998; Rilke et al., 1995; but see also Bardo and Hammer, 1991). Thus, the increased sign-tracking behavior seen among isolate-housed, food-restricted animals could reflect altered dopaminergic function associated with a combination of potentially enhanced D1 and attenuated D2 activity. Of course, additional mechanistic exploration would be necessary to confirm or refute such speculations.
Chronic stress has been reported to sensitize stimulant-induced dopamine release in the nucleus accumbens and locomotor activity (Kalivas and Stewart, 1991; Deroche et al., 1995). Evidence suggests that sensitization to stimulant psychomotor effects induced by food restriction, isolate housing and stress are all dependent on corticosterone release (Deroche et al., 1992; 1993; 1995). The effects of food restriction on isolate-housed adolescents may reflect a stress sensitization effect, with the application of both stressors producing alterations in dopaminergic transmission that result in enhanced attribution of incentive salience to reward-paired cues.
5. Conclusions and implications for adolescent addiction vulnerability
Although adolescents typically demonstrate less sign-tracking than adults, the current study clearly demonstrates that adolescent rats are capable of expressing sign-tracking behavior under some conditions. Thus, the neural systems and pathways involved in promoting sign-tracking behavior appear to be easily and dramatically influenced during adolescence by environmental manipulations that exert their effects at least in part via alterations in dopaminergic pathways.
Given that expression of sign-tracking behavior is postulated to reflect individual differences in addiction vulnerability, results from the present study suggest that the adolescent period may be especially susceptible to stress-induced enhancement of such vulnerability. In conjunction with other studies reporting similar effects in adult animals that experienced various impoverished environments early in development, these findings emphasize the harmful and long-lasting consequences of stressors during development and the potential for these stressors to contribute to addiction vulnerability.
Highlights.
Food restriction enhanced sign-tracking behavior in isolate-housed adolescents.
Food restriction increased goal-tracking behavior in pair-housed adolescents.
Food restriction increased overall sign- and goal-tracking behavior in adults.
As seen before with pair housing, adults sign-tracked more than adolescents.
Acknowledgments
The work presented in this manuscript was supported by the NIH grant AA 019972 to LPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachel I. Anderson, Email: randers1@binghamton.edu.
Peter C. Bush, Email: pbush1@binghamton.edu.
Linda P. Spear, Email: lspear@binghamton.edu.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117(4):695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Stinus L, Le Moal M, Cador M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1995;117(1):116–124. doi: 10.1007/BF02245106. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Spear LP. Autoshaping in adolescence enhances sign-tracking behavior in adulthood: Impact on ethanol consumption. Pharmacol Biochem Behav. 2011;98(2):250–260. doi: 10.1016/j.pbb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Hammer RP., Jr Autoradiographic localization of dopamine D1 and D2 receptors in rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience. 1991;45(2):281–290. doi: 10.1016/0306-4522(91)90226-e. [DOI] [PubMed] [Google Scholar]
- Bean G, Lee T. Social isolation and cohabitation with haloperidol-treated partners: effect on density of striatal dopamine D2 receptors in the developing rat brain. Psychiatry Res. 1991;36(3):307–317. doi: 10.1016/0165-1781(91)90029-o. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830(1):56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behav Brain Res. 2012;226(1):331–334. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131(1):1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Boakes R. Performance in learning to associate a stimulus with positive reinforcements. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Erlbaum; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76(3):353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119(4):1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205(4403):319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, et al. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102(17):6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15(11):7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon H. Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993;611(2):352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598(1–2):343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Le Moal M, Simon H. Social isolation-induced enhancement of the psychomotor effects of morphine depends on corticosterone secretion. Brain Res. 1994;640(1–2):136–139. doi: 10.1016/0006-8993(94)91867-8. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behav Neurosci. 2011;125(4):661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80(2–3):317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10(2):123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Flagel S, Watson S, Robinson T, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35(2):388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behavioural Brain Research. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Front Behav Neurosci. 2010;4:39. doi: 10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12(1–2):129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hartup WW, Stevens N. Friendships and adaptation in the life course. Psychological Bulletin. 1997;121(3):355–370. [Google Scholar]
- Hearst E, Jenkins H. Sign-tracking: the stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society, Austin TX. 1974 [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan. 2012 [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220(1):91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural Brain Research. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16(3):387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rouge-Pont F, Deroche V, Barrot M, De Jesus-Oliveira C, Le Moal M, et al. Glucocorticoids and behavioral effects of psychostimulants. I: locomotor response to cocaine depends on basal levels of glucocorticoids. J Pharmacol Exp Ther. 1997;281(3):1392–1400. [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl. 1991;104(2):255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berl. 1994;115(3):407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88(6):2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obes Res. 1995;3(Suppl 4):525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Prasad C, Prasad A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol. 1995;12(1):59–63. doi: 10.1016/0741-8329(94)00070-t. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rilke O, May T, Oehler J, Wolffgramm J. Influences of housing conditions and ethanol intake on binding characteristics of D2, 5-HT1A, and benzodiazepine receptors of rats. Pharmacol Biochem Behav. 1995;52(1):23–28. doi: 10.1016/0091-3057(95)00093-c. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of pavlovian-conditioned responses. Eur J Neurosci. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20(3):505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology. 1998;139(4):376–382. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual Differences in Pavlovian Autoshaping of Lever Pressing in Rats Predict Stress-Induced Corticosterone Release and Mesolimbic Levels of Monoamines. Pharmacology Biochemistry and Behavior. 2000;65(3):509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Lincks M, Nadarajah SD, Pohorecky LA, Yu L. Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behav Brain Res. 2012;226(2):571–578. doi: 10.1016/j.bbr.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Silberman Y, Williams K, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone levels in rats. Pharmacology Biochemistry and Behavior. 2002;72(3):507–513. doi: 10.1016/s0091-3057(01)00781-x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]