SUMMARY
Oct4A is a core component of the regulatory network of pluripotent cells, and by itself can reprogram neural stem cells into pluripotent cells in mouse and humans. However, its role in defining totipotency and inducing pluripotency during embryonic development is still unclear. We genetically eliminated maternal Oct4A using a Cre-lox approach in mouse and found that the establishment of totipotency was not affected, as shown by the generation of live pups. After complete inactivation of both maternal and zygotic Oct4A expression, the embryos still formed Oct4-GFP– and Nanog–expressing inner cell masses, albeit non-pluripotent, indicating that Oct4A is not a determinant for the pluripotent cell lineage separation. Interestingly, Oct4A-deficient oocytes were able to reprogram fibroblasts into pluripotent cells. Our results clearly demonstrate that, in contrast to its role in the maintenance of pluripotency, maternal Oct4A is crucial for neither the establishment of totipotency in embryos, nor the induction of pluripotency in somatic cells using oocytes.
Following fertilization, the genomes of the differentiated oocyte and spermatozoa are epigenetically reprogrammed to instruct the zygote to differentiate into all types of somatic cells in a highly organized manner and generate the entire organism from a single cell, a feature referred to as totipotency1. After 3 cell divisions, early embryos compact, and their blastomeres become polarized. Thereafter, they form blastocysts and differentiate into the first two lineages—the inner cell mass (ICM) and the trophectoderm (TE). The ICM gives rise to the entire embryo proper—i.e., all cell types except for the TE, an ability defined as pluripotency. Oct4 (Pou5f1), a POU family transcription factor, is expressed specifically in germ cells2, the ICM, and embryonic stem (ES) cells3, and has been deemed a critical regulator in the pluripotency as shown by a zygotic Oct4-knockout study4. Oct4 can activate its own expression with its partner Sox2 through a positive autoregulatory loop in ES cells5. Interestingly, Oct4 is listed as one of the 27 proven maternal-effect genes and is regarded to be functionally important for zygotic genome activation6, which provides the first step in the establishment of totipotency-pluripotency. Functionally, Oct4 is required for the binding of two key components of the BMP and LIF signaling pathways, Smad1 and STAT3, to their respective targets, and plays a pivotal role in stabilizing the transcription factor complex7. Among the core components of the pluripotency circuitry formed by Oct4, Nanog, and Sox2, Nanog is directly regulated by Oct4 and Sox28, Sox2 is actually dispensable for the activation of Oct-Sox enhancers, and the forced expression of Oct4 could rescue Sox2-null ES cells9. Hence, Oct4 is considered to be the genetic "master switch" in the establishment of totipotency-pluripotency during the life cycle of mammals10, and presumed to be the most upstream gene in the molecular circuitry of pluripotency11. In this study, however, we provide solid evidence that challenges that viewpoint.
In the present study, we used Oct4floxed mice, in which two LoxP motifs had been inserted that span the proximal promoter and the Oct4A unique first exon12 (Supplementary Fig. 1a). As the other 4 exons shared by Oct4B were not mutated, the Oct4 studied is hereafter referred to as Oct4A. Conditional removal of maternal Oct4A in oocytes was done by crossing the Oct4flox/flox mice with ZP3Cre transgenic mice as shown in Supplementary Fig. 1b. Genotyping for Oct4A deletion in single germinal vesicle (GV) oocytes (47/47) (Fig. 1a), mature metaphase II (MII) oocytes (28/28), preimplantation embryos (665/666), and offspring (95/97) confirmed the efficient deletion of the floxed Oct4A allele by ZP3cre. Depletion of the maternal Oct4A mRNA was also demonstrated, without a significant reduction in the expression of the oocyte-specific genes Sall4, Stella, and Dazl by real-time RT-PCR with TaqMan probe-primer sets, using both the ABI PRISM 7900 Sequence Detection System (Fig. 1b) and the Fluidigm Biomark 48.48 Dynamic Array system (Supplementary Fig. 1e). Elimination of the maternal Oct4A protein was confirmed by Western blot on pools of more than 400 oocytes per sample (Supplementary Fig. 1d).
Figure 1. Generation of oocytes lacking maternal Oct4A and their effect on embryo development.
(a) Genotyping of individual oocytes at the GV stage by nested PCR showed highly efficient deletion of the floxed Oct4A allele. (b) Knockout of maternal Oct4A correlates with elimination of Oct4A transcript as evidenced by real-time RT-PCR on pools of 10 oocytes each. Ctr: wild-type oocytes; KO: Oct4A-null oocytes; ES: ES cells. (c) Immunocytochemistry on E3.5 blastocysts with antibody directed against Cdx2 combined with Oct4 antibody. Elimination of both maternal and zygotic Oct4A does not induce Cdx2 expression in the ICM. WT: wild-type; KO: Oct4A-knockout; M-Z KO: maternal- and zygotic-Oct4A knockout. (d) Immunocytochemistry on E3.5 blastocysts with antibody directed against Nanog combined with Oct4A antibody. Elimination of maternal Oct4A does not interrupt zygotic activation of the pluripotent genes Oct4 and Nanog, while loss of both maternal and zygotic Oct4A does not prevent Nanog expression. WT: wild-type; KO: Oct4A-knockout; M-Z KO: maternal- and zygotic-Oct4A knockout. (e) Real-time RT-PCR on single E3.5 blastocysts confirms that elimination of both maternal and zygotic Oct4 does not affect Nanog and Cdx2 expression. Ctr: Oct4A+/Δ; KO: maternal and zygotic knockout. The number (1, 2, and 3) right after the abbreviation refers to the biological replicate. The scale bars represent 30 µm in c and d. Each bar represents the mean from 3 technical replicates, a result representative of one (a) and 2 (e) biological replicates. The uncropped version of a is shown in Supplementary Fig. 5.
However, using the Taqman Oct4 primers spanning exon 2 and exon 3, real-time reverse transcription polymerase chain reaction (RT-PCR) data consistently showed 1–3% of the wild-type (Wt) expression level in the Oct4A-null oocytes and embryos (Fig. 2a). Such a low level of Oct4 expression was also observed previously in samples with genetically inactive Oct4 loci using the same Oct4floxed mice and was considered to be the background noise of detection methods, the expression of pseudogenes, or the expression of other POU-domain family members13. However, no detectable background signal was found in our Oct4A-null samples using Oct4A-specific primers spanning exons 1 and 2 (Fig. 2b). To identify the background signal, we sequenced the amplicons obtained with the Taqman primer set (Figs. 2c, d), along with another primer set spanning exons 3 and 4 (Figs. 2e, f). As shown in Supplementary Tables 1 and 2, both primer sets amplified sequences matching to the Oct4 mRNA. These results suggested that low levels of Oct4B were expressed in the Oct4A-null oocytes and embryos. Regardless, even though the exact function of Oct4B in mouse oocytes and embryos remains unknown, numerous studies demonstrated that the Oct4B exists in somatic cells14, 15, acts as a stress response factor16, or an antiapoptotic factor in cancer cells17, and only the Oct4A has the ability to confer and sustain pluripotency15. Combined with Sox2, Klf4 and c-Myc, Oct4B was not able to reprogram somatic cell into induced pluripotent stem cells (iPSCs) unlike Oct4A15. Our data showed that low level of Oct4B expression in Oct4A-null embryos could not maintain pluripotency in vitro and in vivo. Therefore, we consider it highly unlikely that the low levels of Oct4B expression in oocytes, embryos, and ES cells can compensate for the critical roles of Oct4A in the establishment of totipotency and pluripotency.
Figure 2. Expression of Oct4 isoforms in Oct4A-knockout oocytes and embryos.
(a) Real-time RT-PCR Ct values of Oct4 together with the internal control Hprt1. The single-oocyte samples were run with preamplification for 18 cycles and tested with the Fluidigm system. The rest of the groups were tested with the ABI PRISM Sequence Detection System 7900 after preamplification for 10 cycles. The Oct4 Taqman probe-primer set from ABI (Mm00658129_gH, spanning exon 2 and 3) consistently detected background signals in all Oct4A-knockout embryos of all preimplantation stages. (b) Further testing using equally efficient Oct4A specific primers (spanning exon 1 and exon 2) eliminated such background signals in all 3 biological replicates of Oct4A-knockout oocytes (pool of 10 per sample) and 2 replicates of single blastocyst samples, indicating expression of truncated Oct4 transcripts. (c) Gel image of RT-PCR using the same Exon2–3 Oct4 Taqman probe–primer set as a and using the same samples as b. The amplicons from the bright bands were cut out and sequenced. They were found to match the Oct4 reference sequence as showed in d. (e) The same samples were tested by PCR using different primers spanning exons 3 and 4, and again the amplicons were found to match the Oct4 reference sequence as showed in f, further confirming the presence of Oct4 isoforms in Oct4-knockout oocytes and embryos. Values represent the means of 2–5 biological replicates, with error bars representing S.D. in a and the means from 3 technical replicates, a result representative of one biological replicates in b. Uncropped versions of c and e is shown in Supplementary Fig. 5.
Previous reports have speculated that maternal Oct4 is a key regulator of oocyte developmental competence, based on microarray gene profiling on MII oocytes with minor GV morphological variation18. Oct4 was also claimed to be a critical regulator of the maternal-embryonic transition as embryos arrested in development after being invasively injected with Oct4-antisense morpholino oligonucleotides at the zygote stage without validation of efficiency and specificity, while ignoring the existing maternal Oct4 protein was ignored19. Here we investigated the functionality of Oct4A-null oocytes in vivo by crossing Oct4flox/flox/ZP3Cre/+ female mice with Wt male mice. To our surprise, we found that these Oct4flox/flox/ZP3Cre/+ female mice were fully fertile, with a normal litter size averaging 7.9 pups (Supplementary Fig. 1f); all these offspring had deletion of the maternal Oct4A allele as confirmed by PCR genotyping (Supplementary Fig. 1g), demonstrating that maternal Oct4A is not critical for totipotency-pluripotency.
Next, we analyzed gene expression using immunocytochemistry and real-time RT-PCR in Oct4A-null blastocysts obtained by crossing Oct4flox/flox–ZP3Cre/+ female mice with Oct4A+/Δ male mice. In total, we obtained 264 maternal and zygotic-knockout embryos and 302 maternal-knockout and zygotic–Wt embryos. As maternal-knockout and zygotic–Wt embryos underwent normal full-term development and had the same genetic background, they were used as control for maternal and zygotic-knockout embryos. Although the maternal and zygotic-knockout blastocysts had a reduced size on embryonic day (E4.5) (102.4 µm ± 19.6 in diameter, n=33 vs. Control 124.7 µm ± 24.4, n=60; P<0.01), they exhibited normal cavitation and formed a distinct ICM (Supplementary Video 1). Notably, on E3.5, the ICM of maternal/zygotic-knockout embryos did not express the TE markers Cdx2 (Figs. 1c, 3a) and Troma-1 (Supplementary Fig. 2d). Instead, they expressed Nanog, another core factor involved in the regulatory network of ES cell self-renewal and pluripotency20, at protein (Figs. 1d, 3a) and mRNA levels (Fig. 1e) comparable to those of the zygotic mutant, although the Nanog+ cells were always found to scatter apart in the ICM (Figs. 1d, 3b). Activation of Nanog expression was observed as early as at the 8-cell stage at mRNA (Supplementary Fig. 2b) and protein levels (Supplementary Fig. 2c), like in control embryos. On E4.5, although the ICM of maternal and zygotic-knockout embryos maintained Nanog expression in 35 of the 41 blastocysts examined, coexpression of Cdx2 in some of the Nanog+ ICM cells (Fig. 3b, Supplementary Fig. 2e) was observed in 73.1% (30/41) of the knockout embryos, while such co-localization was rarely seen in control littermate embryos (3.3%, 2/61). Moreover, we examined the expression of the Oct4-GFP transgene in maternal and zygotic-knockout embryos by crossing Oct4flox/flox–ZP3Cre/+ female mice with Oct4+/Δ GOF18+/+ male mice. Green fluorescent protein (GFP) of Oct4-GFP transgene in GOF18 mice is expressed under the control of Oct4 regulatory elements and is active only in pluripotent cells and cells of the germ cell lineage21, and as such it has been used as a convenient indicator for the acquisition of pluripotency. The time-lapse observation revealed that Oct4-GFP expression was activated in all maternal-knockout embryos (#2, 6, 7, and 10–14) and maternal and zygotic-knockout embryos (#5 and 9) in a timely fashion, as in Wt embryos (#1, 3, 4, and 8) (Fig. 3c, Supplementary Video 2). The genotype of each individual embryo was determined by nested PCR (Supplementary Fig. 3c) at the end of time-lapse observation, with its position tracked in the video with bright field microscopy (Supplementary Fig. 3a, Supplementary Video 3). This result is consistent with a report showing the maintenance of β-galactosidase activity from the Oct4 promoter in the ICM of zygotic Oct4–knockout embryos4. While expression of Nanog and Oct4-GFP is persistent, expression of Cdx2 in Oct4-null E4.5 embryos was upregulated at the mRNA level as assessed by real-time RT-PCR analysis on immunosurgically prepared ICMs (Fig. 3d), indicating a reciprocal interaction between Oct4 and Cdx2 at this stage.
Figure 3. Gene expression of Oct4A-null blastocysts produced by crossing Oct4flox/flox–ZP3Cre/+ female mice with Oct4A+/Δ male mice.
(a) On E3.5, lineage separation in maternal and zygotic-knockout embryos (Oct4A KO) was clearly demonstrated by the activation of Nanog in the ICM and expression of the TE marker Cdx2 in the TE. (b) On E4.5, although the ICM of maternal and zygotic-knockout embryos (Oct4A KO) maintained Nanog expression, coexpression of Cdx2 in some of the Nanog+ ICM cells was observed as indicated by arrowheads, while such co-localization was rarely seen in control littermate embryos. (c) Snapshots of time-lapse confocal observation showed that Oct4-GFP expression was activated in all maternal-knockout (Δ/+: #2, 6, 7, and 10–14) embryos and maternal and zygotic-knockout embryos (Δ/Δ: #5 and 9) in a timely fashion at about E2.5, like in Wt embryos (+/+: #1, 3, 4, and 8). (d) Real-time RT-PCR analysis on immunosurgically prepared ICMs (lysed TE debris was used for genotyping). The persistent expression of Nanog and Oct4-GFP and the upregulation of Cdx2 expression in Oct4A-null E4.5 embryos (KO) were confirmed at the mRNA level. (e) and (f) A comparison of Sall4 (e) and Nr5a2 (f) mRNA levels at the blastocyst stage by real-time RT-PCR in siControl- (GFP-targeting siRNA) and siSall4- (Sall4-targeting siRNA) (e), or siNr5a2- (Nr5a2-targeting siRNA) (f) injected embryos demonstrated robust downregulation of Sall4 without significant effect on Oct4 expression. Expression levels of Hprt1 were used as the internal control to normalize the data. *P<0.05; **P<0.01. The scale bars represent 20 µm in a and b, and 50 µm in c. Values represent mean±S.D. of 5 biological replicates in d and 3 biological replicates in e and f.
Previous studies have suggested that lineage commitment is controlled by the expression level of Oct44, 22. Repression of Oct4 expression was shown to induce the differentiation of ES cells into TE, and a less than two-fold increase in Oct4 expression was shown to cause differentiation of ES cells into cells of primitive endoderm and mesoderm22. In the absence of Oct4, embryos could not form an ICM—i.e., the inner cells of morula-stage embryos were rather driven into trophoblast differentiation4. Therefore, these results indicated that Oct4 plays a critical role in sustaining stem cell self-renewal and that up- or downregulation of Oct4 induces divergent developmental programs, suggesting that Oct4 is the master regulator of pluripotency and that it may also control lineage commitment during early embryonic development4. This hypothesis was further strengthened by the finding of the reciprocal interaction between Oct4 and Cdx2 in determining TE differentiation23 and by the interaction between Oct4 and the histone H3–specific methyltransferase ESET in restricting the extraembryonic trophoblast lineage potential of pluripotent cells24. However, coexpression of Oct4 and Cdx2 was found in normal morula-stage embryos25 and expression of Cdx2 in ES cells was found to be rapidly initiated by Ras activation (i.e., within 24 hours), without previous or simultaneous downregulation of Oct4 expression26. Moreover, Cdx2 deficiency was not shown to interrupt TE-ICM lineage separation27–29. Meanwhile, zygotic Oct4 expression was shown not to be required for initial repression of the TE genes Cdx2 and Gata3, indicating that other mechanisms must lead to the restriction of these genes to the TE30. Now with our maternal Oct4A–knockout results, we added more solid evidence against the Oct4-Cdx2 interaction model of lineage commitment in early mouse embryos23. Our results clearly demonstrated that ICM-TE lineages were separated in maternal and zygotic Oct4A–knockout embryos, suggesting that first lineage separation of the ICM-TE is not determined by the reciprocal interaction between Oct4 and Cdx2. Instead, such a reciprocal interaction between Oct4 and Cdx2 is established subsequently to maintain the ICM fate. Most importantly, we found that maternal Oct4A is not at the root of pluripotency as a determinant in initiating the pluripotent cell lineage, contrary to previous assumptions and views.
Notably, in contrast to ES cells, wherein upstream Oct4 and Sox2 regulate Nanog expression8, 31, we found that activation of Nanog and Oct4-GFP expression occurred in the absence of both maternal and zygotic Oct4A, further suggesting that a unique Oct4A-independent pluripotency-initiating regulatory network is active in early embryos.
By crossing Oct4flox/flox–ZP3Cre/+ female mice with Oct4+/Δ OG2+/− male mice, in which the Oct4-GFP transgene lacks the proximal enhancer and activates GFP expression through the distal enhancer and promoter (GOF18ΔPE)32, we demonstrated that GFP is still activated in Oct4A-null embryos (Supplementary Fig. 3b). This result indicates that in oocytes and embryos, such an unknown Oct4-independent reprogramming engine activates Oct4 expression through the distal enhancer, probably through a completely different network than that shown by Yamanaka’s 4-factor reprogramming33–35.
In an attempt to identify the factors critical for driving Oct4 and Nanog expression, we knocked down 6 reported Oct4-regulating factors, Sall4, Tpt1, Zscan4, Esrrb, Utf1, and Nr5a2, by microinjecting gene-specific siRNA duplexes into maternal Oct4A–deficient zygotes—to avoid any possible effect of maternal Oct4A as a positive autoregulator. The efficient knockdown of Sall4, Tpt1, Zscan4, Esrrb, and Utf1 was observed at the blastocyst stage. However, we did not observe any significant change in either Oct4 expression (Figs. 3e, Supplementary Fig. 3e) or Oct4-GFP activation (Supplementary Fig. 3d). Interestingly, knockdown of Nr5a2 showed a mild, but consistent downregulation of Oct4 expression (P<0.0001) (Fig. 3f). Nr5a2, an orphan nuclear receptor, was found to maintain Oct4 expression at the epiblast stage of embryonic development, by binding to the proximal enhancer and proximal promoter regions of Oct4, but to play no evident role in ES cell self-renewal36. However, Nr5a2 can induce epiblast stem cells into ground state pluripotency37 and replace Oct4 in the reprogramming of somatic cells into pluripotent cells34. Our results indicate the involvement of Nr5a2 in vivo in assisting Oct4 activation in preimplantation embryos. Further studies to elucidate how exactly oocytes activate the pluripotent genes Oct4A and Nanog on top of the Oct4-Sox2 autoregulatory loop will lead to a better understanding of the establishment of totipotency in zygotes and in transplanted somatic cells, and to a marked improvement in the efficiency of iPSC techniques and eventually support the generation of Oct4-independent induced totipotent stem (iTPS) cells to achieve organized differentiation into an intact organism without the use of oocytes through fertilization or nuclear transfer (NT).
On the other hand, we found low levels of Tbx1 expression in Wt E3.5 blastocysts (Fig. 1e) and E4.5 ICM (Fig. 3d) by real-time RT-PCR with a Ct value of 33 and 28–31, respectively, but we could not detect Tbx1 mRNA in any Oct4A–null samples even after 40 cycles of PCR amplification. Tbx1 encodes a T-box transcription factor and is expressed in different components of the mesoderm or mesoderm-endoderm during gastrulation38. Mutation of TBX1 causes the DiGeorge syndrome phenotype, which manifests as defects in the pharyngeal arch and in cardiovascular and craniofacial development39. Chip-seq data has shown that Oct4 binds to the promoter region of Tbx17. Further study on how Oct4A activates Tbx1 expression might reveal another role of Oct4A in the initiation of mesoderm differentiation by promoting pluripotent cells in a “poised” state.
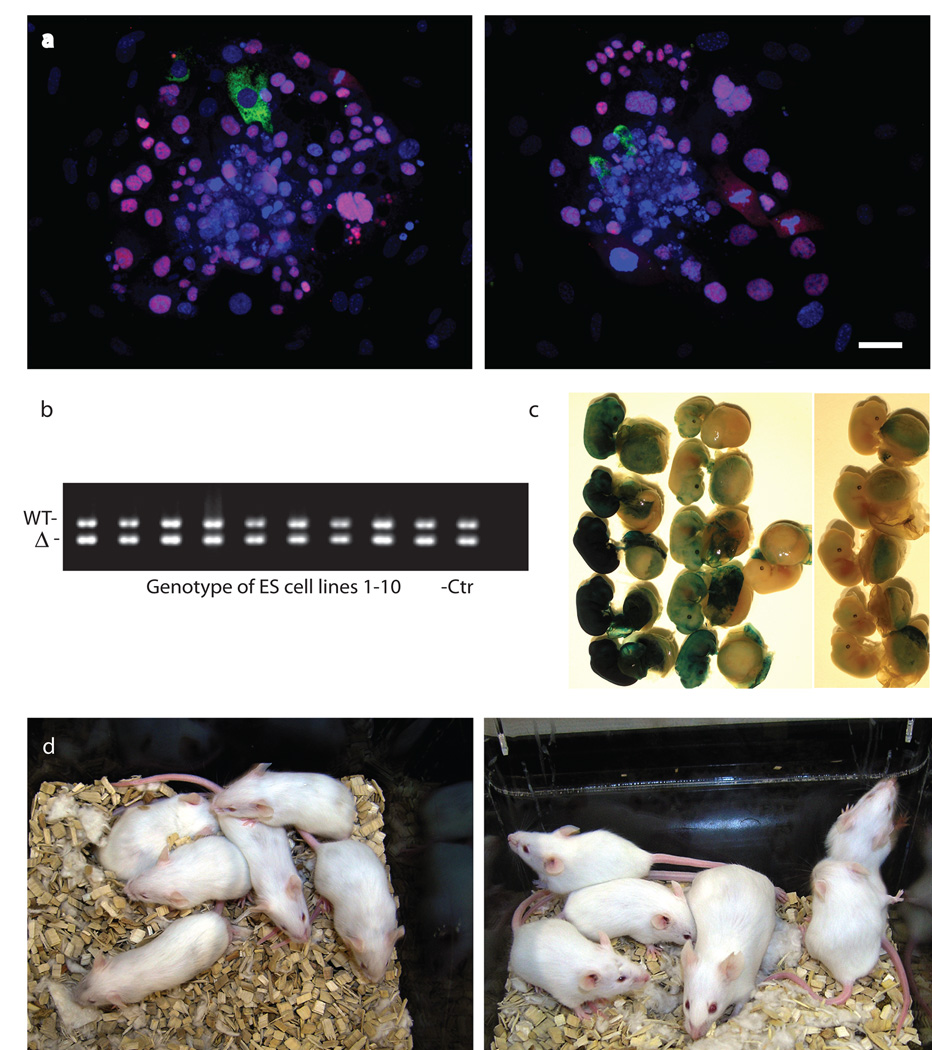
Next, we evaluated the pluripotency of maternal-zygotic Oct4A–knockout embryos in vitro by plating them onto mouse embryonic fibroblasts (MEFs) and attempting to derive pluripotent ES cells. None of the 10 ES cell lines derived from the 43 embryos were homozygous for the Oct4-knockout allele (Fig. 4b), indicating loss of pluripotency in Oct4A–null embryos. Immunocytochemical analysis of the mutant outgrowths revealed many condensed and fragmented Cdx2− nuclei along with Cdx2+ TE cells (Fig. 4a). Interestingly, Nanog was still detectable in some Cdx2− cells, but it was only localized to the cytoplasm—instead of the nucleus.
Figure 4. Fate of Oct4A-null embryos in vitro and in vivo.
(a) Confocal image of 2 typical Oct4A-null outgrowths with immunostaining for Nanog (green) and Cdx2 (red). Nuclear counterstaining with DAPI (blue). Note the cytoplasmic localization of Nanog. (b) Genotyping of 10 established ES cell lines from embryos obtained by crossing Oct4flox/flox–ZP3Cre/+ female mice with Oct4A+/Δ male mice shows all lines had the Oct4 Wt allele (415 bp) and no ES cells were derived from Oct4AΔ/Δ (245 bp) embryos, confirming that the pluripotent phenotype of cells is dependent on the presence of Oct4. (c) Oct4A-null embryos marked by ROSA26 transgene (right) contribute to the formation of only extraembryonic tissues in chimeric embryos, whereas the majority of control embryos (left) contribute to both embryonic and placental tissues. Blue: LacZ staining. (d) After aggregating with CFW embryos (albino), Oct4A-null embryos (agouti) did not contribute to the formation of the adult chimeric mice as shown by coat color, whereas 4 of 5 control embryos did contribute to adult chimeric mice. The scale bar represents 20 µm in a. The uncropped version of b is shown in Supplementary Fig. 5.
As expression of Nanog and Oct4-GFP was persistent in the Oct4A-null embryos between E2.5 and E4.5, we postulated that these defective embryos could be rescued by Wt embryos for differentiation into certain types of somatic cells. Accordingly, we aggregated Rosa26+/− Oct4A-null 8-cell embryos with Wt embryos. After 24 hours of culture, chimeric embryos with homozygous (Oct4A KO) and heterozygous (control) mutants were separately transferred into pseudopregnant mice. E13.5 to E14.5 fetuses were collected and subjected to LacZ staining (Fig. 4c). Consistent with our in vitro study, Oct4A-null embryos contributed exclusively to the placental tissue—and not to the embryo proper (0/7 vs. 10/11 in control). Furthermore, after aggregating with embryos of ICR mice, Oct4A-null embryos also did not contribute to the formation of adult mice, as shown by the coat color, while 4 of 5 control embryos did (Fig. 4d). These results demonstrated that Oct4A-depleted cells lost pluripotency and could only form extraembryonic tissues, supporting the importance of Oct4 as the gatekeeper of pluripotency3.
Studies have shown that the nuclei of terminally differentiated somatic cells can be reverted back to a totipotent state after nuclear transplantation into enucleated oocytes40. To explore whether maternal Oct4A is critical in the reprogramming of somatic cells using oocytes, NT experiments were conducted with the Oct4A-null oocytes from Oct4flox/flox–ZP3Cre/+ female mice. By using both immunocytochemistry (Supplementary Fig. 4a) and real-time RT-PCR (Supplementary Fig. 4b), we observed that Oct4A-null oocytes still activated the expression of pluripotent genes (Oct4 and Nanog) in the ICM and the TE marker gene (Cdx2) in the TE in all 28 cloned embryos analyzed.
To further assess whether maternal Oct4A-null oocytes can induce pluripotency in somatic cells, we derived ES cells from NT embryos using these oocytes as recipient oocytes and CAG-mRFP and Oct4-GFP double transgenic male MEFs as donor cells. Of 135 reconstructed embryos, 78 developed to the morula stage and expressed both CAG-mRFP and Oct4-GFP on day 3 of culture (Fig. 5a). These morulae were plated on irradiated MEFs (Fig. 5b) and gave rise to 9 ES cell lines (Supplementary Fig. 4c). Chromosome counts from metaphase spreads showed that 7 of the 9 NT-ES cell lines were normal, with 40 chromosomes. Four of the 7 NT-ES cell lines—RG1, RG5, RG6, and RG8—were tested with the tetraploid complementation assay, the most stringent test for pluripotency (Fig. 5c). All ES cell-tetraploid embryo aggregates developed to the blastocyst stage and had ES cells integrated into the ICM (Fig. 5d). After transfer into the uterus of pseudopregnant female mice, the RG5 ES cell line did not support any of the 30 aggregates to full-term development. RG6 gave rise to 2 pups of 50 transferred (1 dead; 1 alive initiated breathing but died shortly thereafter). RG1 and RG8 ES cell lines did very well with the test. On E19, we recovered 17 full-term male pups of 31 aggregates transferred (54.8%) in total from RG1 (Supplementary Fig. 4e) and 18 pups of 45 aggregates transferred (40%) in total from RG8. These offspring expressed CAG-mRFP ubiquitously in the body (Fig. 5e) and Oct4-GFP specifically in the gonads (Fig. 5f). Of these 17 pups from line RG1, 11 initiated breathing and were set up with foster mothers, and 5 survived to adulthood (Fig. 5g) and were fertile. Twelve of the 18 pups from RG8 initiated breathing but were rejected by their foster mothers. The pluripotency of RG6 and RG8 ES cell lines was further confirmed by differentiation into cells of all 3 embryonic germ layers in the teratoma assay (Supplementary Fig. 4d). Our NT data unequivocally demonstrated that the reprogramming engine in oocytes could work effectively to reprogram somatic cells into cells of a pluripotent state without Oct4A.
Figure 5. Oct4A-null oocytes reprogram somatic cells to full pluripotency as shown by generation of complete NT–ES cell–derived mice via tetraploid complementation.
(a) Morulae from NT with Oct4A-null oocytes expressed CAG-mRFP and Oct4-GFP on day 3. (b) An outgrowth from NT embryos showed a small group of cells expressing Oct4-GFP along with ubiquitous expression of mRFP. (c) CAG-mRFP and Oct4-GFP–expressing ES cells were aggregated with 2 tetraploid embryos. (d) A blastocyst with CAG-mRFP and Oct4-GFP–expressing ES cells integrated into the ICM after overnight culture. (e) A full-term, E19.5, all ES cell–derived newborn mouse with ubiquitous expression of mRFP. (f) A gonad from an all ES cell–derived newborn mouse expressing mRFP in all cells and Oct4-GFP in germ cells. The insert shows the Oct4-GFP–expressing germ cells in the seminiferous tubules at higher magnification. (g) Adult all ES cell–derived mice. The scale bars represent 50 µm in a, b, c, and d, and 20 µm in f.
In conclusion, our results demonstrated that Oct4A is not the master regulator responsible for initiating totipotency-pluripotency in natural reprogramming by maternal factors in oocytes. The maternal and zygotic Oct4A–null blastocysts maintained the ability to activate Nanog and Oct4-GFP expression, indicating that unknown pathways other than the Oct4-centered pluripotency-regulating network are active in embryos at both the totipotent and pluripotent stages of development and function upstream of Oct4A in driving pluripotency.
METHODS
Induction of Recombination in Oct4flox/flox Mice by Cre recombinase
A knockin 129/svj strain containing a functional Oct4 allele flanked by lox P motifs (Oct4flox/flox)12 was crossed with a ZP3-Cre transgenic C57BL/6J mouse41. The ZP3 promoter–driven Cre expression supports the inactivation of maternally expressed genes in growing oocytes41 as early as day 5 after birth42. DNA extraction and PCR genotyping were performed as reported previously12. As shown in Supplementary Fig. 1a, the intact Oct4 allele and the lox P site were detected by primer pair B (forward = GOF-D1, reverse = GOF-R1; amplicon size: Wt = 415 bp, floxed = 449 bp), and Oct4 allele was found to be deleted by primer pair C (forward = GOF-AatII, reverse = GOF-ApaL1; amplicon size = 245 bp). A representative PCR genotyping result is shown in Supplementary Fig. 1c.
Nested PCR was used to genotype individual oocytes and blastomeres as described43 with combinations of the above primers: GOF-AatII, GOF-D1, and GOF-R1 as outer primers for the primary amplification, and GOF-AatII, GOF-D1, and GOF-ApaL1 as inner primers for the nested amplification. Three genotypes of Oct4 alleles were distinguished by amplicons of different size: Wt (362 bp), floxed (396 bp), and deleted (245 bp). For genotyping cells with Oct4-GFP, another set of primers (outer: GOF-AatII, GOF-HindIII, and GOF-R1; inner: GOF-AatII, GOF-ApaL1, and Oct4R344: 5’-GAGAAGGCGAAGTCTGAAGC-3’) with amplicons of different size: Wt (345 bp), floxed (379 bp), and deleted (245 bp) were used.
A protocol for animal handling and maintenance for this study was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (LANUV NRW) under the supervision of a certified veterinarian in charge of the Max Planck Institute (MPI) animal facility.
Embryo Collection, Culture, and Immunocytochemistry
Embryos from various matings were flushed out of the mouse oviducts or uteri in M2 medium as described by Hogan et al.44 at 2.5, 3.5, or 4.5 days post coitum (dpc) to collect embryos at stages of development as required for specific experiments.
Whole-mount immunostaining was carried out as described29. Briefly, embryos were fixed in 4% paraformaldehyde (20 min), permeabilized with 0.1% Triton X-100 (Sigma-Aldrich Inc., St. Louis, MO, USA) for 1 hour, and blocked with 3% BSA in PBS for 1 hour. This was followed with binding of primary antibodies (overnight at 4°C) and secondary antibodies (Alexa 488–conjugated goat anti-rabbit IgG, Alexa 488–conjugated goat anti-mouse IgG, Alexa 488–conjugated donkey anti-goat IgG, Alexa 568–conjugated donkey anti-rabbit IgG, and Alexa 647–conjugated donkey anti-mouse IgG (Invitrogen, Karlsruhe, Germany), Cy3-conjugated goat anti-rat IgG, or Cy3-conjugated goat anti-mouse IgG (Jackson Laboratory, Bar Harbor, ME, USA) at a 1:400 dilution for 1 hour. The following primary antibodies were used: rabbit anti-Nanog IgG (1:200; Cosmo Bio Company Ltd., Tokyo, Japan, REC-RCAB0002PF), mouse anti-Oct4 IgG (1:100; Santa Cruz Biotech., Santa Cruz, CA, USA, Clone C-10, SC-5279), rabbit anti-Oct4 IgG (1:2,500; generated in our lab) (Palmieri et al., 1994), goat anti-Oct4 IgG (1:100; Santa Cruz Biotechnology, Inc., SC-8628), mouse anti-Cdx2 (1:100; BioGenex, San Ramon, CA, USA, Clone CDX2-88, MU392-UC), and rat anti-Troma-1 (1:100; DSHB, Iowa City, USA). Some samples were counterstained with 5 µM DRAQ5 (Biostatus Limited, Leicestershire, UK) for 30 min. Samples were examined under a laser scanning confocal microscope (UltraVIEW; PerkinElmer Life Sciences, Inc., Jügesheim, Germany) with 488 nm, 568 nm, and 647 nm lasers. Immunocytochemistry experiments were repeated 2–3 times with 30–100 embryos in each experiment.
RNA Extraction, cDNA Synthesis, and Real-Time RT-PCR
For real-time analysis of gene expression, embryos were harvested in RLT buffer (QIAGEN GmbH, Hilden, Germany) and processed as previously described45. Briefly, total RNA was extracted from individual blastocysts using the MicroRNeasy Kit (QIAGEN GmbH) and complementary DNA (cDNA) synthesis was performed with the High Capacity cDNA Archive Kit (Applied Biosystems GmbH, Darmstadt, Germany) according to the manufacturer’s instructions.
Real-time PCR was carried out on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems GmbH) using TaqMan probes from Applied Biosystems. All primers used are listed in Table S3. Two to 6 biological replicates were used, and each sample was run with 3 technical replications; an RT-blank and a no-template blank served as negative controls. The cycle threshold (CT) values were collected using Applied Biosystems SDS v2.0 software and transferred to a Microsoft Excel spreadsheet for further relative quantification analysis using the ΔΔCT method (User Bulletin #2, ABI Prism 7700 Sequence Detection System, 1997). Single-oocyte real-time RT-PCR using the Biomark 48.48 Dynamic Array system (Fluidigm) was performed as previously described19. All real-time RT-PCR source data can be found in supplementary materials.
Microinjection of siRNA into zygotes
Zygotes were collected in the morning after mating from superovulated or natural ovulated Oct4flox/flox–ZP3Cre/+ female mice. Microinjection of siRNA duplexes into zygotes was performed using an Eppendorf FemtoJet microinjector and Narishige micromanipulators as previously described29. Approximately 2 pl of siRNAs (20 µM) was injected into cytoplasm of zygotes. Scrambled siRNA duplexes or siRNA duplexes against GFP was used as Control. The injected zygotes were then cultured with KSOMAA (potassium simplex optimized medium plus 19 natural amino acids)46 in a humidified 5% CO2 in air at 37°C. Expression of the target genes was analyzed at the blastocyst stage (3.5 dpc) in pools of 10–20 embryos per sample. The target sequences of siRNA are listed in Table S4.
Somatic Cell Nuclear Transfer
Seven- to 9-week–old Oct4flox/flox (control oocytes) and Oct4flox/flox–ZP3Cre/+ (Oct4-null oocytes) female mice were used to collect oocytes and cumulus cells after superovulation. Superovulation and collection of oocytes and cumulus cells, nuclear transfer using ovarian cumulus cells, and activation of reconstructed oocytes were all performed essentially as previously described45, 47. Six hours after the onset of activation, the constructs were washed carefully in a 4-well dish (Nunc, Roskilde, Denmark, 176749) with 0.5 ml of alpha minimal essential medium (MEM α, Invitrogen, 12571-063) supplemented with 0.4% of BSA (Sigma-Aldrich Inc, A3311) without cytochalasin B under 5% CO2 in air for 4 days. The blastocysts obtained were subjected to immunostaining, genotyping, and real-time RT-PCR.
For derivation of ES cells from NT embryos, 8- to 12-week–old Oct4Δ/flox–ZP3Cre/cre female mice were used to collect Oct4-null oocytes after superovulation. MEFs of passages 2–4, derived from male E13.5 embryos by crossing CAG-mRFP48 male mice with Oct4-GFP female mice (GOF-18)21, were used as nuclei donors. The NT procedures were the same except that MEF cells were briefly exposed to HVJ-E Sendai virus extract (www.cosmobio.co.jp) and then placed into the perivitelline space of recipient Oct4-null-enucleated oocytes on the side opposite to the 1st polar body49. The constructs were transferred into KSOMAA and cultured for 1–2 hours in an incubator with 5% CO2 in air at 37°C to induce fusion prior to activation. The morulae obtained on day 3 of culture were subjected to the ES cell derivation procedure according to a previous report50.
Western Blotting
Western blot was performed as described previously51. Briefly, 400 Wt and 400 maternal Oct4A-null oocytes were collected in PBS. Oocytes, 10,000 ES cells as well as cumulus cells were lysed in RIPA buffer (50 mM Tris, pH 8.0, 300 mM NaCl, 1% IGEPAL CA 630, 0.5% sodium deoxycholate, 1 mM EDTA, 1× protease inhibitors [Roche], and 1× phosphatase inhibitors [Sigma-Aldrich Inc.]) containing 50 units of Benzonase (Merck, Darmstadt, Germany) on ice for 1 hour. The lysate was cleared by centrifugation and supernatants were collected. An amount of supernatant equivalent to 250 oocytes was resolved using gel electrophoresis. Primary antibody used for western blot was mouse monoclonal anti-Oct4 IgG (Santa Cruz Biotechnology, Inc., clone: C-10, SC-5279). The western bot experiment was repeated 2 times.
Sequencing
Products of qPCR were gel purified and ligated into PCR2.1-TOPO vector (Invitrogen) according to manufacture’s instruction. For each sample, 6 colorless colonies were picked and sent to GATC Biotech AG (Konstanz, Germany) in 96-well format for sequencing using M13 reverse primer.
Generation and Analysis of Chimeric Embryos
To assess the pluripotency of Oct4-inactivated embryos in vivo, we aggregated mutated 8-cell embryos (obtained from crossing Oct4flox/flox–ZP3Cre/+–ROSA26+/+ female mice with Oct4+/Δ–ROSA26+/+ male mice)—after removal of the zonae pellucidae by acidic Tyrode’s solution (Sigma) and biopsy for genotyping by nested PCR—with 8-cell embryos from Wt B6C3F1 mice for LacZ evaluation of chimerism in the fetus or for fur coat color evaluation in albino CFW mice. Following 24 hours of culture in KSOMAA, successfully fused and genotyped chimeric blastocysts carrying heterozygous (69 in total as control) and homozygous mutants (60 in total as Oct4 knockout) were transferred separately into the uterus of 10 ICR pseudopregnant recipients. Eleven fetuses carrying heterozygous mutants and 7 fetuses carrying homozygous mutants were recovered on E13.5 from 8 recipients and subjected to whole-mount LacZ staining as previously described52. Five full-term pups from each of the controls and the knockout mutants were obtained from 2 recipients for evaluation of coat color chimerism.
Derivation of Embryonic Stem Cell Lines
E2.5 embryos were cultured on MEFs to establish ES cell lines with a standard procedure47 after removal of the zonae pellucidae (as described above). All ES cells lines were newly derived in the present study and tested negative for mycoplasma contamination. The complete ES cell medium composition was as follows: 4 mM L-glutamine, 100 units ml−1 penicillin, 100 µg ml−1 streptomycin, 0.1 mM β-mercaptoethanol, 0.1 mM nonessential amino acids, 1,000 units ml−1 LIF (Chemicon), and 15% FBS (Invitrogen, 10165-185) in DMEM (GIBCO, 31885-023).
Tetraploid Embryo Complementation Assay for NT-ES Cells
The assay was conducted as described previously53. Briefly, 2-cell embryos were flushed 20 hours post-hCG from the oviducts of B6C3F1 mice and fused with a peak pulse of 50V for 35 µs in 0.3 M Mannitol to make tetraploid embryos. The tetraploid embryos were cultured overnight in KSOMAA. Then clumps of 15–20 trypsin-treated ES cells were transferred into individual depressions in drops of KSOMAA under mineral oil. Meanwhile, batches of 30–50 embryos were briefly incubated in acidified Tyrode's solution44 to remove zona pellucida. Two embryos were placed on each ES cell clump and cultured in an incubator with 5% CO2 in air at 37°C. After 24 hours of culture, 10–12 embryos at blastocyst stage were transferred into one uterine horn of a 2.5-dpc pseudopregnant recipient for full term development.
Statistical Analysis
Real-time PCR results were analyzed using a program from Applied Biosystems as recommended. For other experiments, statistical analysis was performed using the Student’s t-test and Fisher’s Exact Test. A P value of less than .05 was considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jeanine Mueller-Keuker, Margit Preusser, and Nina Stengel for assistance in preparing the manuscript and Areti Malapetsas in proofreading the manuscript. We thank Karin Huebner for her kind technical help on immunocytochemistry and Bärbel Schäfer for her excellent assistance on histology work. The authors of this manuscript bear sole responsibility for the content presented, which does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. This research was supported by the Max Planck Society, DFG grants DFG SI 1695/1-2 (SPP1356) and SCHO 340/7-1, and grant NIH R01HD059946-01 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
AUTHOR CONTRIBUTIONS G.W. designed and executed experiments as well as wrote the manuscript. D.H., Y.G., V.S., L.G., N.S., K.A., G.F., C.O., M.S., M.R. and A.T. executed experiments, data collection, prepared reagents. H.R.S. provided study concept, provision of funding and edited the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol. 2009;114:185–199. doi: 10.1007/10_2008_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholer HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 4.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 5.Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Rodda DJ, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 9.Masui S, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 10.Pesce M, Marin Gomez M, Philipsen S, Scholer HR. Binding of Sp1 and Sp3 transcription factors to the Oct-4 gene promoter. Cell Mol Biol (Noisy-le-grand) 1999;45:709–716. [PubMed] [Google Scholar]
- 11.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehler J, et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengner CJ, et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281:33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 15.Guo CL, et al. A novel variant of Oct3/4 gene in mouse embryonic stem cells. Stem Cell Res. 2012;9:69–76. doi: 10.1016/j.scr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Farashahi Yazd E, et al. OCT4B1, a novel spliced variant of OCT4, generates a stable truncated protein with a potential role in stress response. Cancer Lett. 2011;309:170–175. doi: 10.1016/j.canlet.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Asadi MH, et al. OCT4B1, a novel spliced variant of OCT4, is highly expressed in gastric cancer and acts as an antiapoptotic factor. International journal of cancer. Journal international du cancer. 2011;128:2645–2652. doi: 10.1002/ijc.25643. [DOI] [PubMed] [Google Scholar]
- 18.Zuccotti M, et al. Maternal Oct-4 is a potential key regulator of the developmental competence of mouse oocytes. BMC Dev Biol. 2008;8:97. doi: 10.1186/1471-213X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foygel K, et al. A novel and critical role for Oct4 as a regulator of the maternal-embryonic transition. Plos One. 2008;3:e4109. doi: 10.1371/journal.pone.0004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers I, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimizu T, et al. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ. 1999;41:675–684. doi: 10.1046/j.1440-169x.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 22.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 23.Niwa H, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Yuan P, et al. Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev. 2009;23:2507–2520. doi: 10.1101/gad.1831909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 26.Lu CW, et al. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet. 2008;40:921–926. doi: 10.1038/ng.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blij S, Frum T, Akyol A, Fearon E, Ralston A. Maternal Cdx2 is dispensable for mouse development. Development. 2012;139:3969–3972. doi: 10.1242/dev.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 29.Wu G, et al. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development. 2010;137:4159–4169. doi: 10.1242/dev.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralston A, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137:395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda T, et al. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo PE, Hubner K, Scholer H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115:157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 33.Adachi K, Scholer HR. Directing reprogramming to pluripotency by transcription factors. Curr Opin Genet Dev. 2012;22:1–7. doi: 10.1016/j.gde.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Heng JC, et al. The Nuclear Receptor Nr5a2 Can Replace Oct4 in the Reprogramming of Murine Somatic Cells to Pluripotent Cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Gu P, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25:3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–3192. doi: 10.1242/dev.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman DL, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Yagi H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 40.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 41.de Vries WN, et al. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- 42.Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- 43.el-Hashemite N, Wells D, Delhanty JD. Single cell detection of beta-thalassaemia mutations using silver stained SSCP analysis: an application for preimplantation diagnosis. Mol Hum Reprod. 1997;3:693–698. doi: 10.1093/molehr/3.8.693. [DOI] [PubMed] [Google Scholar]
- 44.Hogan B. Manipulating the mouse embryo : a laboratory manual. Edn. 2nd. Plainview, N.Y.: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 45.Boiani M, et al. Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: distinct developmental potentials in different culture environments. Stem Cells. 2005;23:1089–1104. doi: 10.1634/stemcells.2004-0352. [DOI] [PubMed] [Google Scholar]
- 46.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–238. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 47.Nagy A. Manipulating the mouse embryo : a laboratory manual. Edn. 3rd. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 48.Long JZ, Lackan CS, Hadjantonakis AK. Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein. BMC Biotechnol. 2005;5:20. doi: 10.1186/1472-6750-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tachibana M, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc. 2006;1:2082–2087. doi: 10.1038/nprot.2006.355. [DOI] [PubMed] [Google Scholar]
- 51.Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 52.Wu G, et al. Efficient Derivation of Pluripotent Stem Cells from siRNA-Mediated Cdx2-Deficient Mouse Embryos. Stem Cells Dev. 2011;20:485–493. doi: 10.1089/scd.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G, et al. Generation of healthy mice from gene-corrected disease-specific induced pluripotent stem cells. PLoS Biol. 2011;9:e1001099. doi: 10.1371/journal.pbio.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.