Abstract
Background
Methamphetamine (METH) use and human immunodeficiency virus (HIV) infection are highly comorbid, and both are associated with increased prevalence of affective distress. Delineating the trajectory of affective distress in the context of METH dependence and HIV infection is important given the implications for everyday functional impairment, adverse health behaviors, and increased risk for adverse health outcomes.
Methods
We conducted a five-year longitudinal investigation involving 133 METH-dependent (74 HIV seropositive) and 163 non-METH-dependent (90 HIV seropositive) persons to examine both long-standing patterns and transient changes in affective distress. Mixed-effect regression models with random subject-specific slopes and intercepts evaluated the effect of METH dependence, HIV serostatus, and related variables on affective distress, as measured by the Profile of Mood States.
Results
Transient changes in affective distress were found to be greater among those with a diagnosis of current MDD, briefer durations of abstinence from METH, and higher quantity of METH consumed. Weak associations were observed among static (time-independent predictors) covariates and long-standing patterns in affective distress.
Limitations
Study lacked data pertaining to the participants’ involvement in METH treatment and relied on respondent-driven sampling.
Conclusions
Our longitudinal investigation of the trajectory of affective distress indicated that specific and dynamic indices of current METH use were associated with greater transient changes in mood. In the evaluation and treatment of affective distress, recency and quantity of current METH use are important to consider given their association with heightened affective distress and mood instability over time.
Keywords: Affective distress, Methamphetamine, Dependence, HIV, Longitudinal
Introduction
Affective distress is associated with increased symptom burden, functional impairment, adverse health behaviors, and poor adherence to medical care regimens among persons managing chronic medical conditions (Katon, 2003). In the context of human immunodeficiency virus (HIV), methamphetamine (METH) abuse is a highly prevalent comoribidity with physiological and neurocognitive effects that are associated with impairment in everyday functioning abilities [e.g., medication adherence (Hinkin et al., 2004; Marquez et al., 2009; Moore et al., 2012)] and increased risk for negative health outcomes [e.g., faster disease progression (Ellis et al., 2003)]. Both METH dependence and HIV infection are associated with increased affective distress that may further impair the ability to prioritize and manage chronic health conditions (Carrico et al., 2007).
The long-term associations among affective distress and both METH abuse and HIV warrant considerable attention given the implications for health outcomes. Investigations of the temporal relationship between METH use and mood outcomes have thus far yielded discordant results. For example, one investigation found depressive symptoms persisted when measured two to five years after substance use treatment despite a reduction in self-reported METH use (Rawson et al., 2002). Another study, however, reported a positive association between recent METH use (i.e., within a five-day period) and increased depression ratings (Peck et al., 2005). The discrepant findings may be due to a host of factors, such as differences in evaluation periods, assessment measures, and characteristics of the participants. Although these studies have begun to elucidate the association between METH abuse and mood outcomes, these investigations were restricted to in-patient clinical samples, which tend to yield higher rates of depression than samples of community volunteers (Rabkin, 2008). Longitudinal investigations of affective distress in the context of HIV infection suggest an association between longstanding HIV disease and increased likelihood of incident mood disorder (e.g., Atkinson et al., 2008). Moreover, major depressive disorder (MDD) in the context of HIV infection has been linked to poorer health-related quality of life (Ickovics et al., 2001) and more rapid progression of HIV disease (Atkinson et al., 2008; Ironson et al., 2005).
To complement investigations of affective distress in the context of METH abuse or HIV infection alone, recent studies have examined the independent and combined effects of these two conditions on affective distress. A cross-sectional study found an additive effect of METH and HIV on mood disturbance, as evidenced by more severe depression in a dually concordant group (i.e., METH+/HIV+) relative to single-risk groups (Bousman et al., 2009). A two-wave longitudinal study (Iudicello et al., 2010) observed a significant improvement in affective distress over time among a METH-abstinent group, which was not significantly associated with HIV status. Although these studies offer preliminary results regarding affective distress in the context of both METH dependence and HIV infection, research utilizing longitudinal designs with an increased number of data collection waves may help better characterize changes and variability in affective distress that may be attributable to the dynamic and fluctuating courses of METH use and HIV disease. This improved characterization of the trajectory of affective distress has the potential to enrich the identification of specific dynamic variables that may be critical targets of intervention to improve health outcomes.
The extent to which the temporal associations among affective distress, METH use, and HIV disease covary, or are spurious, over time is difficult to ascertain without longitudinal designs with multiple waves of data collection. Multi-wave longitudinal designs are advantageous in comparison to two-wave studies because they permit investigation of individual growth curves, thus affording description and explanation of individual patterns of change (Willett et al., 1998). Although most longitudinal studies focus solely on the trajectories of change over time (i.e., the long-standing patterns captured by the estimated slope), longitudinal data are also abundant with information about transient changes (i.e., the changes in individual growth trajectories that are not accounted for by the specified model). This current study aimed to provide a robust analytical approach by examining both the long-standing patterns and transient changes in affective distress in relation to METH dependence, HIV disease, and specific METH use characteristic variables and HIV disease indicators.
The aim of this longitudinal study was to better characterize the trajectory of affective distress in the context of METH dependence and HIV infection. This analysis also aimed to delineate variation in trajectories of affective distress that may be better accounted for by dynamic METH use (e.g., quantity of METH consumed between study visits) and HIV disease (e.g., current CD4 count) indices than by METH dependency and HIV statuses alone. The Profile of Mood States (POMS) (McNair et al., 1981) was selected as a measure of affective distress because it assesses multiple indicators of emotional disturbance. Given the additive effects of METH dependence and HIV infection observed in a prior cross-sectional study (Bousman et al., 2009), we hypothesized that the METH+/HIV+ group would report greater affective distress at the baseline assessment and would demonstrate greater instability in self-reported affective distress over time in comparison to all other groups. Additionally, we hypothesized that dynamic METH use and HIV disease indices would be significant predictors of transient changes in affective distress. The temporal relationship between affective distress and variables related to both METH dependence and HIV serostatus warrants consideration given their interplay and associations with sexual risk behaviors (Nakamura et al., 2011; Semple et al., 2002), which may lead to the transmission of HIV (Halkitis et al., 2006).
Methods
Participants
Participants included 296 English-speaking individuals enrolled in a five-year longitudinal NIDA-funded program project at the University of California, San Diego studying the individual and combined neurobehavioral effects of METH dependence, HIV, and hepatitis C (HCV). This study was approved by the Institutional Review Board of the University of California, San Diego. Participants were recruited from the San Diego community, HIV clinics, and substance abuse treatment programs. After providing written, informed consent, each participant underwent an extensive structured substance use interview and a comprehensive evaluation of other factors (e.g., neuropsychological, medical, and psychiatric) at annual visits. The structured substance use interview was conducted at each study visit and obtained a detailed history of cumulative and between-visit use of METH and other recreational substances. The variables derived from this interview were age of first use, recency of use (i.e., number of days since last use), quantity (in grams) and duration of use (in days). HIV infection was indicated by enzyme linked immunosorbent assays and a Western Blot confirmatory test. General exclusion criteria were prior histories of neurological (e.g., seizure disorders, closed head injuries, and cerebrovascular accidents) or severe psychiatric (e.g., schizophrenia, mental retardation) conditions.
Diagnoses for psychiatric disorders common in METH-using populations, including MDD, Bipolar Disorder (BD), and Antisocial Personality Disorder (ASPD) were established using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996). Although not an exclusionary criterion for the overall program project, participants with a BD diagnosis were excluded from this current investigation because analyses were interested in examining changes in affective distress that were disentangled from a disorder of which mood instability is a cardinal feature. Participants who met Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV) criteria for METH-dependence during their lifetime and within 18 months of their first visit based on the SCID were included in the METH+ sample (n=133). A sample of participants who may have used METH in their lifetime but did not meet criteria for lifetime or current METH abuse or dependence was included in the METH− sample (n=166). Due to the high prevalence of cannabis and alcohol use among METH+ individuals, participants with a history of abuse or dependence for alcohol and/or cannabis were included. Individuals meeting criteria for abuse of other substances (e.g., cocaine, opioids, hallucinogens, and sedatives) within the last 12 months or dependence within five years of their initial evaluation were excluded.
As part of the overall program project protocol, most participants were followed with annual study visits, although some participants were seen every six months. In this sample, participants returned after an average of 411 days (SD=170) for visit 2 (n=296); 395 days (SD=166) for visit 3 (n=228); 369 days (SD=118) for visit 4 (n=165); 353 days (SD=91) for visit 5 (n=110); 337 days (SD=94) for visit 6 (n=37); 286 days (SD=103) for visit 7 (n=6); and 249 days (SD=68) for visit 8 (n=3). Unlike the rigid underpinnings of repeated measures analysis of variance (ANOVA), the general random-coefficient growth curve model employed in this study allowed covariates and was insensitive to imbalances in the time sampling between participants. Therefore, inclusion of visits with fewer participants was less of a statistical issue because a fitted growth trajectory utilized all available data points for a participant.
Procedure
Each participant was administered the Profile of Mood States (POMS) (McNair et al., 1981) in order to assess acute affective distress within the sample. The POMS is a 65-item self-report measure of current (i.e., the week prior to evaluation) mood states in which participants rate various adjectives (e.g., “restless”) and brief phrases (e.g., “unable to concentrate”) on a five-point Likert-type scale ranging from 0 (“not at all”) to 4 (“extremely”). Items on the POMS comprise a Total Mood Disturbance score and six subscale scores (i.e., Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activation, Fatigue-Inertia, and Confusion-Bewilderment). Higher scores on the POMS Total Mood Score indicate greater affective distress. For the POMS subscales, an elevated score also indicated greater affective distress, with the exception of POMS-Vigor-Activation for which the opposite is true. Analyses were conducted using raw scores on the POMS Total Mood Disturbance and subscales.
Data analyses and statistical considerations
Sample and substance use characteristics at the baseline assessment visit were compared between four groups (METH−/HIV−, METH−/HIV+, METH +/HIV−, and METH +/HIV+). ANOVA or Welch’s ANOVA for unequal variances was used for comparison of continuous outcomes, and chi-square or Fisher exact test was used for comparison of categorical outcomes. Benjamini-Hochberg adjustment was used to correct for multiple significance testing (Benjamini and Hochberg, 1995).
The variables involved in this longitudinal study fall into two primary groups: response and predictor. Affective distress measures (i.e., POMS total and the six subscale scores) were regarded as the response variables. The response variables were transformed by square root to achieve normality in residuals in the analyses, with exception of POMS vigor score, for which the non-transformed values provided a better fit. Predictors were subdivided into time-independent (T-I) and time-dependent (T-D) predictors. The T-I predictors consisted of the two classifiers of interest (i.e., METH dependency and HIV infection statuses), demographic variables, and variables measured at the baseline visit (e.g., lifetime MDD, and substance use- and HIV disease-related indices). The T-D predictors were variables measured and changeable at each visit and corresponded to specific study visits.
The analysis consisted of two phases, according to a standard statistical approach described by Abramson and Wolfson (2002). The first phase involved the production of derived data based on a repeated-measures fit of each response variable to time alone. Each subject’s record (whose length varies) was reduced by standard fitting methods to slopes and intercepts (corresponding one-to-one with the subjects), and residuals (corresponding one-to-one with the subjects’ visits). These derived data were the inputs to the next and more novel phase of the analysis.
Of note, the employed fitting technique, while likelihood-based, was not a least-squares method. Rather, the fitting method produced best linear unbiased predictions (BLUPs) that were guided by a more suitable optimality criterion for random-effects modeling (Abramson and Wolfson, 2002). The ensemble of slopes, for instance, generally achieves closer approximation to the true value than a method fitting a separate least-squares line to each subject. This approach did not require balance or regularity of the design. Long subjects’ records, in general, were considered more reliable, while short and relatively unreliable records were reduced more strongly to a central point where they exerted appropriately less influence on the subsequent analyses performed. Slopes and residuals are a natural separation of the two main components of information in longitudinal designs. That is, slopes encoded “chronic” information (i.e., the long-standing pattern of change in the response variable) while residuals encoded “acute” information (i.e., transient changes in the response variable unexplained by the basic time fit). The slopes were valuable for correlation analyses with the T-I predictors while the residuals were valuable for correlation analyses with T-D predictors.
In the second phase of the analysis, slopes were modeled in a multiple regression with T-I predictors, and residuals were modeled in a multiple regression with T-D predictors. The T-I predictors consisted of variables measured at baseline and included age; gender; lifetime abuse or dependence diagnosis for alcohol and/or cannabis; age of first use and cumulative quantity and duration of METH, alcohol, and cannabis use; HCV status; lifetime MDD diagnosis; ASPD diagnosis; mood stabilizer use; antidepressant use; antipsychotic use; nadir CD4; antiretroviral (ARV) status; and ARV regimen type. The T-D predictors were variables measured at each study visit and included days since last METH, alcohol, and cannabis use; interval METH, alcohol, and cannabis quantity and duration; current MDD status; current CD4; HIV cerebrospinal fluid (CSF) viral load; and HIV plasma viral load.
The modeling was guided by scientific intent and parsimony to avoid over-fitting of all possible available data, to which the highly mutivariate dataset might have lent itself. Prior to model selection, the variable inflation factor was used to check for multicollinearity, and model selection was re-run to include only one of the correlated variables at a time. Some regressions were performed on a subset of the participants, based on the availability of the variables (e.g., CD4 nadir was measured in HIV+ participants only). Once the initial predictive models were built, possible interactions were tested (e.g., lifetime MDD by HIV status). With appropriate statistical cautions observed and guided by the Akaike Information Criterion for model selection, multiple regression models were fitted for the response variables. All statistical analyses were conducted using R [version 2.10.0 (2009); http://www.R-project.org].
Results
Sample characteristics
The sample consisted of 296 participants at the baseline assessment visit. In general this was a sample of mostly white, high school educated men in their mid-30s. At the baseline assessment visit, the groups statistically differed by age and gender (p’s<.05) but not by education or ethnicity (p’s>.05). Groups significantly differed at the baseline assessment visit by proportion with HCV (p<.05). In terms of HIV status, a substantial proportion had advanced disease (AIDS), most were not on antiretroviral treatment, and virologic control was marginal. The two HIV groups did not differ on any of the HIV disease indicators (p’s>.05). Relative to psychiatric illness, there were high lifetime frequencies of MDD, especially in HIV+ groups, which were also reflected in elevated scores on scales of Tension-Anxiety, Depression-Dejection, and Fatigue-Inertia. At the baseline assessment visit, there were statistically significant group differences for proportion with lifetime MDD diagnosis, proportion with lifetime ASPD diagnosis, scores on the POMS Total Mood Disturbance scale and all subscales except Anger-Hostility (p’s<.05). However, there were no statistically significant group differences for current MDD (p>.05). Demographic and clinical characteristics of the study sample are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study sample at baseline assessment (N = 296 participants).
| Variable | Mean (SD) or n (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Demographic Characteristics | A. METH−/HIV−(n = 73) | B. METH−/HIV+ (n = 90) | C. METH+/HIV−(n = 59) | D. METH+/HIV+ (n = 74) | Significant Differencesb |
| Age (years) | 33.9 (11.1) | 39.9 (8.5) | 36.4 (9.4) | 37.2 (6.8) | A<C,D<B |
| Gender (%male) | 45 (62%) | 76 (84%) | 45 (76%) | 70 (95%) | A<B,D; C<D |
| Education (years) | 13.2 (2.2) | 12.9 (2.5) | 12.2 (2.1) | 12.2 (2.5) | |
| Ethnicity | |||||
| Caucasian | 46 (63%) | 51 (57%) | 43 (73%) | 48 (65%) | |
| Hispanic | 14 (19%) | 20 (22%) | 11 (19%) | 19 (26%) | |
| Black | 9 (12%) | 16 (18%) | 3 (5%) | 6 (8%) | |
| Other | 4 (5%) | 3 (3%) | 2 (3%) | 1 (1%) | |
| HCV (%) | 0 (0%) | 10 (11%) | 11 (19%) | 24 (32%) | A<B,C,D; B<D |
| HIV Disease Characteristics | |||||
| Estimated duration of HIV infection (years) | - | 8.1 (5.2) | - | 7.2 (5.6) | |
| Proportion with AIDS | - | 42 (47%)c | - | 28 (38%)d | |
| Proportion on cART | - | 24 (27%) | - | 22 (31%)e | |
| Current CD4 Counta | - | 397 [273, 571]f | - | 358 [217, 560] | |
| CD4 Nadir Counta | - | 200 [48, 312]c | - | 219 [92, 302]d | |
| Proportion with detectable HIV plasma viral loada | - | 44 (52%)g | - | 40 (54%) | |
| Proportion with detectable HIV CSF viral loada | - | 25 (42%)h | - | 22 (37%)i | |
| Psychiatric Characteristics | |||||
| Current MDD | 3 (4%) | 13 (14%) | 3 (5%) | 11 (15%) | |
| Lifetime MDD | 17 (23%) | 44 (49%) | 19 (32%) | 37 (50%) | A<B,D |
| ASPD | 0 (0%) | 3 (3%) | 15 (25%) | 10 (14%) | A<C,D; B<C |
| POMS Total Mood Disturbance | 44.6 (33.1) | 62.6 (37.5) | 59.9 (37.3) | 70.1 (33.8) | A<B,C,D |
| Tension-Anxiety | 7.4 (6.1) | 10.0 (7.0) | 9.7 (6.1) | 11.4 (6.6) | A<B,D |
| Depression-Dejection | 7.0 (10.2) | 10.9 (11.5) | 11.6 (12.9) | 14.0 (10.5) | A<D |
| Anger-Hostility | 6.6 (8.7) | 7.3 (8.6) | 7.8 (7.7) | 8.9 (8.2) | |
| Vigor-Activation | 18.5 (6.2) | 15.2 (6.8) | 16.1 (5.9) | 15.0 (6.8) | A>B,D |
| Fatigue-Inertia | 4.8 (4.7) | 9.7 (6.6) | 7.1 (6.0) | 9.8 (6.3) | A<C<B,D |
| Confusion-Bewilderment | 5.2 (4.7) | 8.0 (5.3) | 7.8 (5.6) | 9.0 (5.4) | A<B,C,D |
Note: AIDS = Acquired Immune Deficiency Syndrome; ASPD = Antisocial Personality Disorder; cART = Combination Antiretroviral Therapy (current); CD4 = Cluster of Differentiation 4; CSF = Cerebrospinal Fluid; HCV = Hepatitis C Virus; HIV = Human Immunodeficiency Virus; METH = Methamphetamine; MDD = Major Depressive Disorder; POMS = Profile of Mood States
Median (Interquartile Range)
Statistics are based on observed data. Pairwise group comparisons were performed with Benjamini-Hochberg correction with a significance alpha level of .05. Group labels: A = METH−/HIV−, B = METH−/HIV+, C = METH+/HIV−, D = METH+/HIV+.
CD4 Nadir count variable was unavailable for eleven METH−/HIV+ participants. Proportion is based on n = 79.
CD4 Nadir count variable was unavailable for six METH+/HIV+ participants. Proportion is based on n = 68.
cART status was unavailable for two METH+/HIV+ participants. Proportion is based on n = 72.
Current CD4 Count was unavailable for two METH−/HIV+ participants. Median (interquartile range) is based on n = 88.
Plasma HIV RNA/μl load was unavailable for five METH−/HIV+ participants. Proportion is based on n = 85.
CSF HIV RNA/μl load was unavailable for 31 METH−/HIV+ participants. Proportion is based on n = 59.
CSF HIV RNA/μl load was unavailable for 14 METH+/HIV+ participants. Proportion is based on n = 60.
Substance use characteristics of the study sample at the baseline assessment are shown in Table 2. The METH+ groups demonstrated significant higher levels of cumulative METH, alcohol, and cannabis use as compared to the METH− groups. At the baseline assessment visit, groups differed on all METH use characteristics (p’s<.05).
Table 2.
Substance use characteristics of the study sample at baseline assessment (N = 296)
| Variable | Mean (SD) or n (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| A. METH−/HIV−(n = 73) | B. METH−/HIV+ (n= 90) | C. METH+/HIV−(n = 59) | D. METH+/HIV+ (n = 74) | Significant Differencesb | |
| Current METH | 0 (0%) | 0 (0%) | 3 (5%) | 17 (23%) | A,B,C<D |
| Dependence | |||||
| Lifetime | |||||
| Substance | |||||
| Dependence | |||||
| METH | 0 (0%) | 0 (0%) | 59 (100%) | 74 (100%) | A,B<C,D |
| Alcohol | 5 (7%) | 7 (8%) | 25 (42%) | 17 (23%) | A,B<D<C |
| Cannabis | 3 (4%) | 4 (4%) | 13 (22%) | 15 (20%) | A,B<C,D |
| METH Use Characteristics a | |||||
| Age of First Use | 24 (9) | 29 (10) | 22 (7) | 24 (7) | B>C,D |
| Cumulative Duration of Use (days) | 54 (62) | 131 (238) | 4413 (2424) | 2632 (2382) | A,B<D<C |
| Cumulative Quantity of Use (grams) | 5 (6) | 22 (63) | 4451 (3676) | 2525 (3673) | A,B<D<C |
| Recency of Use (days) | 2552 (2504) | 3593 (3037) | 121 (91) | 142 (168) | A,B>C,D |
| Alcohol Use Characteristics a | |||||
| Age of First Use | 16 (4) | 17 (4) | 14 (4) | 14 (4) | A,B>C,D |
| Cumulative Duration of Use (days) | 832 (936) | 1596 (1620) | 2933 (2586) | 1950 (1854) | A<B,D<C |
| Cumulative Quantity of Use (grams) | 3277 (6769) | 7720 (15949) | 22890 (23742) | 15649 (23993) | A<B<C,D |
| Recency of Use (days) | 610 (1351) | 545 (1517) | 627 (1470) | 380 (842) | |
| Cannabis Use Characteristics a | |||||
| Age of First Use | 18 (4) | 19 (5) | 15 (3) | 16 (5) | A>C; B>C,D |
| Cumulative Duration of Use (days) | 594 (1334) | 758 (1397) | 3322 (3306) | 3182 (3463) | A,B<C,D |
| Cumulative Quantity of Use (grams) | 196 (412) | 345 (828) | 2196 (3535) | 3741 (14531) | |
| Recency of Use (days) | 2810 (3622) | 2676 (3260) | 1433 (2393) | 1114 (2515) | A,B>C,D |
Note: METH = Methamphetamine; HIV = Human Immunodeficiency Virus
Statistics are based on observed data
Pairwise group comparisons were performed with Benjamini-Hochberg correction with a significance alpha level of .05. Group labels: A = METH−/HIV−, B = METH−/HIV+, C = METH+/HIV−, D = METH+/HIV+.
Long-standing patterns of change in affective distress: analysis of slopes
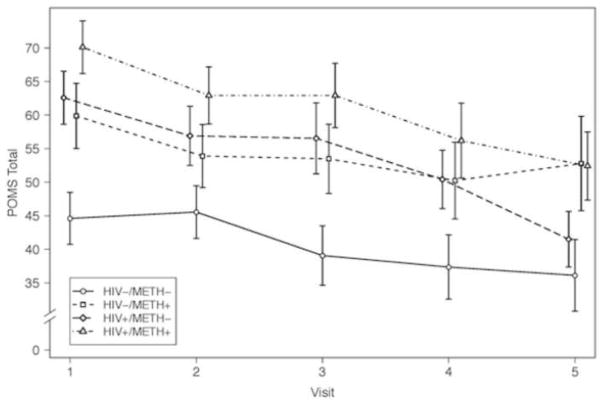
The mean POMS Total Mood Disturbance scores by visit for each group are illustrated in Figure 1. To evaluate the temporal effect of METH and HIV on affective distress, subject-specific slopes (i.e., the long-standing patterns of change) were regressed on METH dependency status and HIV serostatus and then subsequently regressed on T-I predictors. Results are displayed in Table 3. In general, the T-I predictors had weak associations with long-standing patterns of change in POMS scores over time. The models accounted for 1 to 8% of the variance of the slopes for POMS Total Mood Disturbance and subscale scores (p’s<.05). Neither METH dependency status nor HIV serostatus were significant predictors of change in POMS Total Mood Disturbance or subscale scores (p’s>.05).
Figure 1.
Mean POMS Total Mood Disturbance Scores by Visit for Each Group. The figure represents the trajectory of affective distress across the first five study visits (individual group cells contained n>15 at each visit depicted in the figure).
Table 3.
Associations of Long-standing Patterns of Change in Affective Distress and Time-independent Variables
| Models for all observations | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|---|---|---|---|---|
| POMS Total Mood Disturbance | .021 | .034 | ||
| Education | −4.1e-05 | .034* | ||
|
| ||||
| POMS Tension-Anxiety | .075 | <.001 | ||
| Education | −1.7e-05 | .004** | ||
| Cumulative METH quantity (log10) | 2.3e-05 | .035* | ||
|
| ||||
| POMS Depression-Dejection | .023 | .011 | ||
| Education | −1.7e-05 | .053 | ||
| Lifetime MDD | −9.3e-05 | .029* | ||
|
| ||||
| POMS Vigor-Activation | .038 | .026 | ||
| ASPD | −.00052 | .118 | ||
| Cumulative METH quantity (log10) | −.00015 | .112 | ||
| Lifetime alcohol abuse | .00046 | .051 | ||
|
| ||||
| POMS Fatigue-Inertia | .020 | .019 | ||
| Gender | −7.9e-05 | .025* | ||
| Lifetime MDD | −4.9e-05 | .086 | ||
|
| ||||
| POMS Confusion-Bewilderment | .010 | .107 | ||
| Lifetime MDD | −7.2e-05 | .107 | ||
| Age 1st cannabis use (log10) | −.00035 | .078 | ||
|
| ||||
| Model for HIV+ observations only | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|
| ||||
| POMS Confusion-Bewilderment | .094 | .013 | ||
| Lifetime MDD | −9.0e-05 | .066 | ||
| ARV status | ||||
| Naïve (ref) | ||||
| Past | 3.0e-05 | .640 | ||
| Current | .00013 | .017* | ||
Note: ARV = antiretroviral; ASPD = antisocial personality disorder; HCV = Hepatitis C Virus; HIV = Human Immunodeficiency Virus; METH = Methamphetamine; MDD = major depressive disorder; POMS = Profile of Mood States; e = exponential function. Time-independent variables are those that do not vary with time (i.e., demographic covariates and variables sampled at baseline).
The HIV disease indicators were only available for the HIV+ participants and therefore a separate set of regressions was performed on the HIV+ subset of the study sample. There was only a statistically significant model for the POMS Confusion-Bewilderment subscale, which accounted for 9% of the variance of the slopes (p<.05).
Transient changes in affective distress: analysis of residuals
The residuals (i.e., transient changes) of each observation were regressed on METH and HIV statuses and related variables to examine associations with transient changes in affective distress. Residuals may be negative or positive, where larger absolute values indicate greater fluctuations in POMS scores.
The models accounted for 4 to 13% of the variance of the residuals for POMS Total Mood Disturbance and subscale scores (p’s<.001). Results are displayed in Table 4. A diagnosis of current MDD at the various study visits was positively associated with residuals for POMS Total Mood Disturbance scores. This association indicated that for the subset of persons with a current MDD diagnosis, on average the residuals fell significantly above the regression line, indicating higher scores on POMS Total Mood Disturbance than estimated by the regression model if there was no effect of current MDD. Correspondingly, for the residuals of the POMS subscales scores, the same positive association with current MDD was found, except for the POMS Vigor-Activation, which had a negative association. The number of days since last METH use was negatively associated with the POMS Depression-Dejection subscale. Thus, for participants with fewer days since last METH use, the residuals fell above the regression line, indicating that participants with more recent use tended to have higher scores on the POMS Depression-Dejection subscale. Interval METH quantity (i.e., reported quantity of METH consumed between study visits) was positively associated with residuals for the POMS Confusion-Bewilderment model. This positive association indicated that for the subset of participants with greater quantities of interval METH use, the residuals fell above the regression line, indicating that scores on the Confusion-Bewilderment subscale were higher for participants consuming more METH during the study intervals.
Table 4.
Associations of Transient Changes in Affective Distress and Time-dependent Variables
| Models for all observations | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|---|---|---|---|---|
| POMS Total Mood Disturbance | .128 | <.001 | ||
| Current MDD | 1.365 | <.001** | ||
| Interval METH quantity (log10) | .064 | .158 | ||
|
| ||||
| POMS Tension-Anxiety | .104 | <.001 | ||
| Current MDD | .644 | <.001** | ||
| Interval METH quantity (log10) | .044 | .075 | ||
|
| ||||
| POMS Depression-Dejection | .116 | <.001 | ||
| METH dependence | −.200 | .068 | ||
| Current MDD | .907 | <.001** | ||
| Days since last METH use (log10) | −.200 | <.001** | ||
|
| ||||
| POMS Anger-Hostility | ||||
| METH dependence | −.190 | .107 | ||
| Current MDD | .718 | <.001** | ||
| Days since last METH use (log10) | −.098 | .056 | ||
|
| ||||
| POMS Vigor-Activation | .040 | <.001 | ||
| Current MDD | −2.500 | <.001** | ||
|
| ||||
| POMS Fatigue-Inertia | .065 | <.001 | ||
| Current MDD | .590 | <.001** | ||
| Interval METH quantity (log10) | .042 | .139 | ||
|
| ||||
| POMS Confusion-Bewilderment | .103 | <.001 | ||
| METH dependence | −.084 | .153 | ||
| Current MDD | .502 | <.001** | ||
| Interval METH quantity (log10) | .044 | .039* | ||
|
| ||||
| Models for HIV+ observations only | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|
| ||||
| POMS Total Mood Disturbance | .148 | <.001 | ||
| METH dependence | .218 | .339 | ||
| Current MDD | 2.476 | <.001** | ||
| METH-MDD interaction | −1.500 | .010** | ||
|
| ||||
| POMS Tension-Anxiety | .115 | <.001 | ||
| Current MDD | .639 | <.001** | ||
| Interval METH quantity (log10) | .062 | .083 | ||
| HIV Plasma Viral Load | .073 | .059 | ||
|
| ||||
| POMS Depression-Dejection | .101 | <.001 | ||
| Current MDD | .844 | <.001** | ||
| Days since last METH use (log10) | −.140 | .026* | ||
|
| ||||
| POMS Anger-Hostility | .069 | <.001 | ||
| Current MDD | .719 | .001** | ||
| HIV CSF Viral Load | .138 | .071 | ||
|
| ||||
| POMS Vigor-Activation | .067 | <.001 | ||
| Current MDD | −3.000 | <.001** | ||
|
| ||||
| POMS Fatigue-Inertia | .074 | <.001 | ||
| Current MDD | .646 | <.001** | ||
|
| ||||
| POMS Confusion-Bewilderment | .093 | <.001 | ||
| METH dependence | −.025 | .764 | ||
| Current MDD | .887 | <.001** | ||
| METH-MDD interaction | .−590 | .012* | ||
Note: CSF = Cerebrospinal Fluid; HIV = Human Immunodeficiency Virus; METH = Methamphetamine; MDD = major depressive disorder; POMS = Profile of Mood States. Time-dependent variables are those that vary with time.
As with the models involving the slopes, the residual analyses involved a separate set of regressions that were performed on the HIV+ subset of the study sample given that HIV disease indicators were only available for the HIV+ persons. The models of the residuals for HIV+ participants only at all time points accounted for 7 to 15% of the variance of the residuals for POMS Total Mood Disturbance and subscale scores (p’s<.001) (Table 4). As with the model that included all observations, the model with only HIV+ individuals revealed a positive association between a diagnosis of current MDD and residuals for POMS Total Mood Disturbance scores. Furthermore, the model for POMS Total Mood Disturbance scores included a statistically significant interaction between METH dependency status and current MDD (p=.010). The interaction indicated higher POMS Total Mood Disturbance scores for individuals with a current MDD diagnosis compared to those without a current MDD diagnosis. Individuals with a current MDD diagnosis and without a METH dependency diagnosis tended to have higher scores on these subscales than individuals with both current MDD and METH dependency diagnoses. The models with only HIV+ individuals also revealed a positive association between a current MDD diagnosis and residuals for POMS subscale scores, except for the Vigor-Activation scale for which a statistically significant negative association was found. The model for Confusion-Bewilderment scores included a statistically significant interaction between METH dependency status and current MDD (p=.012), exhibiting a pattern similar to the model for POMS Total Mood Disturbance scores. As with the model for all observations, the model involving only HIV+ participants revealed a negative association between the number of days since last METH use and the Depression-Dejection subscale.
Changes in affective distress without consideration of MDD
Given the intuitive finding that MDD absorbed much of the variance across the POMS outcomes, secondary analyses were performed to evaluate changes in affective distress without consideration of MDD as a covariate to allow for detection of other relevant predictors. In correspondence with the primary analyses, subject-specific slopes were regressed on METH dependency status and HIV serostatus and then subsequently regressed on T-I predictors. There were statistically significant models for the POMS Depression-Dejection and POMS Fatigue-Inertia scales, with 1 to 2% of the variance of the slopes accounted for (p’s<.05). The HIV disease indicators were only available for the HIV+ people and therefore a separate set of regressions was performed on the HIV+ subset of the study sample. There was only a statistically significant model for the POMS Confusion-Bewilderment subscale, which accounted for 7% of the variance of the slope, and included age of first cannabis use and ARV status as predictors (p<.05). Results are displayed in Table 5.
Table 5.
Associations of Changes in Affective Distress without Consideration of MDD as a Covariate
| Models of Long-standing Patterns of Change for all observations | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|---|---|---|---|---|
| POMS Depression-Dejection | .011 | .041 | ||
| Education | −1.8e-05 | .041* | ||
|
| ||||
| POMS Fatigue-Inertia | .020 | .021 | ||
| Gender | −6.3e-05 | .080 | ||
| HIV status | −4.9e-05 | .094 | ||
|
| ||||
| Model of Long-standing Patterns of Change for HIV+ observations only | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|
| ||||
| POMS Confusion-Bewilderment | .069 | .026 | ||
| Age 1st cannabis use (log10) | −.00032 | .111 | ||
| ARV status | ||||
| Naïve (ref) | .032* | |||
| Past | 4.3e-05 | .504 | ||
| Current | .00014 | .010* | ||
|
| ||||
| Models of Transient Changes for all observations | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|
| ||||
| POMS Total Mood Disturbance | .010 | .019 | ||
| Days since last METH use (log10) | −.14 | .019* | ||
|
| ||||
| POMS Tension-Anxiety | .008 | .030 | ||
| Days since last METH use (log10) | −.068 | .030* | ||
|
| ||||
| POMS Depression-Dejection | .020 | .005 | ||
| Days since last METH use (log10) | −.190 | .001** | ||
| METH dependence | −.230 | .094 | ||
|
| ||||
| POMS Anger-Hostility | .012 | .026 | ||
| Days since last METH use (log10) | −.140 | .008** | ||
| METH dependence | −.220 | .071 | ||
|
| ||||
| POMS Confusion-Bewilderment | .014 | .028 | ||
| Days since last METH use (log10) | −.051 | .125 | ||
| Interval METH quantity (log10) | .036 | .153 | ||
| METH dependence | −.130 | .057 | ||
|
| ||||
| Model of Transient Changes for HIV+ observations only | Coefficient | Coefficient P-value | Model Adjusted R2 | Model P-value |
|
| ||||
| POMS Tension-Anxiety | .013 | .041 | ||
| HIV Plasma Viral Load | .082 | .041* | ||
Note: ARV = antiretroviral; CD4 = Cluster of Differentiation 4; CSF = Cerebrospinal Fluid; HIV = Human Immunodeficiency Virus; METH = Methamphetamine; POMS = Profile of Mood States; e = exponential function.
The residuals were then regressed on METH and HIV statuses and related characteristic variables to examine associations with transient changes in self-reported affective distress without consideration of current MDD as a covariate. The models accounted for 1 to 2% of the variance of the residuals for POMS Total Mood Disturbance and subscale scores (p’s<.05). For POMS Total Mood Disturbance scores, days since last METH use at the various study visits was negatively associated with residuals. Correspondingly, for the residuals of the POMS Tension-Anxiety, Depression-Dejection, Anger-Hostility, and Confusion-Bewilderment, the same negative association with days since last METH use was found. Thus, for participants with fewer days since last METH use, the residuals fell above the regression line, indicating that participants with more recent use tended to have higher scores on these POMS subscales. For the POMS Depression-Dejection, Anger-Hostility, and Confusion-Bewilderment models, METH dependence status was negatively associated with residuals. This negative association indicated that for the subset of participants with METH dependence, the residuals fell above the regression line, indicating that their scores were higher than predicted by the models. For the POMS Confusion-Bewilderment model, interval METH quantity (i.e., reported quantity of METH consumed between study visits) was positively associated with residuals. This positive association indicated that for the subset of participants with greater quantities of interval METH use, the residuals fell above the regression line, indicating that scores on the Confusion-Bewilderment subscales were higher for participants consuming more METH during the study intervals. An additional set of regressions were performed on the HIV+ subset of the study sample given that HIV disease indicators were only available for the HIV+ persons. The only statistically significant model of the residuals for HIV+ participants was for POMS Tension-Anxiety, which accounted for 1% of the variance (p<.05). In this model, HIV Plasma Viral load was positively associated with the residuals, indicating that for the subset of participants with higher plasma viral load, their residuals fell above the regression line, indicating that scores on the Tension-Anxiety subscale were higher for participants with higher plasma viral loads during the study intervals. Results from the analyses of residuals are displayed in Table 5.
Discussion
Our longitudinal analysis found that persons with current METH use had higher levels of affective distress and, furthermore, greater instability of affect over time. Improved mood was observed over time for all groups; however, the dual and single risk groups have greater levels of affective distress relative to the control group. Static covariates had relatively weak associations with changes in affective distress, while more fine-grained dynamic indices of METH use and HIV disease characteristics were associated with instability of affective distress over time.
Despite many dynamic factors (e.g., HIV disease indicators and alcohol intake), the METH+/HIV+ group remained relatively high in affective distress over time. The persistence of elevated affective distress may point to a neurobiological abnormality underlying substance use and MDD, which may then be worsened by HIV. For example, greater affective distress among METH users is associated with dysfunction in brain regions (e.g., limbic and paralimbic regions) linked to mood disorders (London et al., 2004). Additionally, METH+/HIV+ persons may experience greater social burden, which may contribute to the relatively elevated levels of affective distress. In this regard additive effects of METH and HIV have been observed for measures of functional dependence, such that HIV infection confers an increased concurrent risk of METH− associated everyday living difficulties (Blackstone et al., 2013). Correspondingly, METH− dependent persons are documented to have higher rates of full-time unemployment status (Weber et al., 2012).
Our robust longitudinal analyses afforded the documentation of the temporal association of dynamic METH use indices and instability in affect. Specifically, individuals consuming greater quantities of METH between study visits had greater unpredictable fluctuation in affective distress. Similarly, less abstinent individuals (i.e., individuals with fewer days since last METH use) exhibited less predictability for Depression-Dejection. When current MDD was not considered as a covariate, the association of recent use and less predictability in affective distress was seen across the different subscales of the POMS. These results are consistent with a prior investigation that reported a significant improvement in self-reported affective symptoms for abstinent users relative to individuals who continued to use METH (Iudicello et al., 2010). Furthermore, improvement in affective distress on the POMS for the abstinent group reported in this prior study was found to be unrelated to infectious disease status (i.e., HIV and HCV infection). These findings are congruent with research suggesting reduction of mood symptoms following several days of abstinence (Newton et al., 2004) and decreases in depressive symptoms following abstinence during treatment and for up to one year post-treatment (Peck et al., 2005).
Although HIV serostatus in itself failed to significantly predict acute fluctuations in affective distress, both plasma and CSF viral loads were included in the statistically significant models as predictors, although neither of these predictors met statistical significance. Specifically, individuals with higher HIV CSF viral loads exhibited greater instability in their Anger-Hostility scores. Similarly, individuals with higher HIV plasma viral loads experienced greater instability in their Tension-Anxiety scores. Moreover, when current MDD was removed as a covariate in secondary analyses, plasma viral load significantly predicted fluctuations in Tension-Anxiety. Thus, viral control seems to be associated with transient fluctuations in mood states. These results provide further evidence that instability in affect is synchronized and concurrent with poorer management of disease progression among persons with HIV infection.
One particularly consistent finding in this current study was that individuals meeting criteria for current MDD demonstrated greater transient and unpredictable fluctuations in affective distress. Among HIV+ persons, METH dependency status and current MDD were found to interact such that persons with current MDD had higher affective distress compared to those without a current MDD diagnosis. For persons without current MDD, those with METH dependence tended to have slightly higher affective distress than persons without METH dependence. Furthermore, those with current MDD but without METH dependence tended to have higher affective distress than those with both current MDD and METH dependent diagnoses. Although these results may appear counterintuitive, they suggest that METH+ persons may be self-medicating and reporting less affective distress, or METH+ persons may simply have less insight or perception of affective distress. Several investigations indicate that difficulty with awareness, or tolerance, of internal experiences may serve as a trigger for substance abuse (Carrico et al., 2007; Gifford et al., 2006; Hayes et al., 1996; Morgenstern et al., 1997; O’Cleirigh et al., 2007), which is consistent with the self-medication hypothesis (Khantzian, 1997) that posits affect regulation as a key determinant of substance abuse. Although substance use may allow individuals to avoid negative mood states in the short term, elevation in depressive symptoms is commonly observed over time (Baker et al., 2004; Kosten et al., 1998). Elevated depressive symptoms, in turn, predict continued substance use (Baker et al., 2006; Baker et al., 2004). The cyclic pattern of impaired ability to regulate negative affect without continued substance use is clinically relevant, as this pattern may result in suboptimal management of HIV disease (Levine et al., 2005).
Several factors limit the generalizability of our findings. First, our study lacked data pertaining to the participants’ involvement in METH treatment and thus we were unable to delineate associated patterns of change in affective distress due to the intervention of treatment. Our investigation does not address the issue of route of administration, which may be relevant given that METH injection is associated with poorer mental health (Domier et al., 2000). Our investigation is also limited by the reliance on respondent-driven sampling rather than random sampling to obtain our sample. However, random sampling is not always feasible with populations such as illicit substance users who may not necessarily be willing to participate in research studies. Another notable limitation is that healthier participants may be more likely to attend more study visits, which may thus contribute to the finding that affective distress decreases with time. Finally, our longitudinal investigation involved repeated comprehensive assessments that may have acted as unmeasured interventions. Despite these limitations, our study offers a new dynamic perspective on the temporal relationship among mood states, METH dependence, and HIV infection.
The limitations of the study may be balanced by several strengths, including the novelty of statistically separating longitudinal data of affective distress into long-standing and transient patterns of change. This study does not offer a static examination of mood disruption. Rather, it elucidates the nuances of change in affective distress over a five-year period. Using a longitudinal design, we were able to observe that current METH use characteristics are associated with the transient changes in affective distress.
Conclusion
In summary, this five-year longitudinal investigation involving 296 persons found that transient changes of affective distress over time were associated with recency and quantity of METH use. These results are consistent with prior research indicating that individuals are likely to achieve the greatest relief of symptoms (e.g., alleviation of depressed mood and reduction in sexual risk behaviors) by focusing on sustaining abstinence from METH use (Jaffe et al., 2007). Because greater affective distress is associated with both poorer engagement in medical care and treatment outcomes, the results of this research indicate the desirability of comprehensive monitoring of recent METH use.
Acknowledgments
Role of the Funding Source
The National Institutes of Health’s (NIH) revised policy requires that NIH-funded authors submit to PubMed Central (PMC), or have submitted on their behalf, their peer-reviewed author manuscripts, to appear on PMC no later than 12 months after final publication.
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D., Virawudh Soontornniyomkij, M.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Chief), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Footnotes
Conflict of Interest
The authors have no actual or potential conflict of interest, including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
Contributors
J.L.M., A.U., I.A., J.H.A, and D.J.M. conceived and designed the study; J.B. gathered data, prepared data, and conducted statistical analysis; A.U. and I.A. statistically analyzed the data; J.L.M, A.U., I.A., S.P.W., J.H.A., I.G., D.J.M. interpreted results; A.U. prepared figures; J.L.M. wrote the first draft of the manuscript; J.L.M, A.U., I.A., J.B., S.P.W., J.H.A., I.G., D.J.M. edited and revised manuscript; all co-authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson I, Wolfson T. A Likelihood Approximation and Shrinkage for Unbalanced Repeated Measures. SankhyƒÅ: The Indian Journal of Statistics, Series B. 2002:301–321. [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, Summers J, Sciolla A, Gutierrez R, Ellis RJ, Abramson I, Hesselink JR, McCutchan JA, Grant I. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108:225–234. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Current Directions in Psychological Science. 2006;15:232–236. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, Ellis RJ, Grant I, Woods SP. Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med. 2013 doi: 10.1097/ADM.0b013e318293653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Ake C, Letendre S, Atkinson JH, Patterson TL, Grant I, Everall IP. Negative mood and sexual behavior among non-monogamous men who have sex with men in the context of methamphetamine and HIV. J Affect Disord. 2009;119:84–91. doi: 10.1016/j.jad.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, Steward WT, Remien RH, Wong FL, Rotheram-Borus MJ, Lightfoot MA, Chesney MA. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007;69:785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- Domier CP, Simon SL, Rawson RA, Huber A, Ling W. A comparison of injecting and noninjecting methamphetamine users. J Psychoactive Drugs. 2000;32:229–232. doi: 10.1080/02791072.2000.10400233. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- Gifford EV, Ritsher JB, McKellar JD, Moos RH. Acceptance and relationship context: a model of substance use disorder treatment outcome. Addiction. 2006;101:1167–1177. doi: 10.1111/j.1360-0443.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Green KA, Carragher DJ. Methamphetamine use, sexual behavior, and HIV seroconversion. Journal of Gay & Lesbian Psychotherapy. 2006;10:95–109. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experimental avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. J Consult Clin Psychol. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, Schneiderman N, Solomon G. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, Atkinson JH, Grant I. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A, Shoptaw S, Stein J, Reback CJ, Rotheram-Fuller E. Depression ratings, reported sexual risk behaviors, and methamphetamine use: latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Exp Clin Psychopharmacol. 2007;15:301–307. doi: 10.1037/1064-1297.15.3.301. [DOI] [PubMed] [Google Scholar]
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and stimulant dependence: neurobiology and pharmacotherapy. J Nerv Ment Dis. 1998;186:737–745. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hinkin CH, Castellon SA, Mason KI, Lam MN, Perkins A, Robinet M, Longshore D, Newton T, Myers H, Durvasula RS, Hardy DJ. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS Behav. 2005;9:355–362. doi: 10.1007/s10461-005-9009-y. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1981. [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, Grant I. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012;24:1504–1513. doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Labouvie E, McCrady BS, Kahler CW, Frey RM. Affiliation with Alcoholics Anonymous after treatment: a study of its therapeutic effects and mechanisms of action. J Consult Clin Psychol. 1997;65:768–777. doi: 10.1037//0022-006x.65.5.768. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Semple SJ, Strathdee SA, Patterson TL. HIV risk profiles among HIV-positive, methamphetamine-using men who have sex with both men and women. Arch Sex Behav. 2011;40:793–801. doi: 10.1007/s10508-010-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Ironson G, Smits JA. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behav Ther. 2007;38:314–323. doi: 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JA, Reback CJ, Yang X, Rotheram-Fuller E, Shoptaw S. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health. 2005;82:i100–i108. doi: 10.1093/jurban/jti029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Status of methamphetamine users 2–5 years after outpatient treatment. J Addict Dis. 2002;21:107–119. doi: 10.1300/j069v21n01_09. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, Woods SP. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125:146–153. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JB, Singer JD, Martin NC. The design and analysis of longitudinal studies of development and psychopathology in context: statistical models and methodological recommendations. Dev Psychopathol. 1998;10:395–426. doi: 10.1017/s0954579498001667. [DOI] [PubMed] [Google Scholar]