Abstract
The concept that the gut and the brain are closely connected, and that this interaction plays an important part not only in gastrointestinal function but also in certain feeling states and in intuitive decision making, is deeply rooted in our language. Recent neurobiological insights into this gut–brain crosstalk have revealed a complex, bidirectional communication system that not only ensures the proper maintenance of gastrointestinal homeostasis and digestion but is likely to have multiple effects on affect, motivation and higher cognitive functions, including intuitive decision making. Moreover, disturbances of this system have been implicated in a wide range of disorders, including functional and inflammatory gastrointestinal disorders, obesity and eating disorders.
A major scientific breakthrough in understanding the interaction of the nervous system with the digestive system occurred with the discovery of the so-called enteric nervous system (ENS) in the middle of the nineteenth century1–4 (BOX 1). Even though it is now considered the third branch of the autonomic nervous system, the ENS has been referred to as the ‘second brain’, based on its size, complexity and similarity — in neurotransmitters and signalling molecules — with the brain5. Even before the discovery of the ENS, the importance of interactions between the brain and the digestive system in health and disease has been recognized for many centuries, and has been studied by prominent psychologists, psychiatrists and physiologists during the later part of the nineteenth and earlier part of the twentienth centuries (reviewed in REF. 6). Both top-down modulation of gastrointestinal function by stress and emotions7, and bottom-up signalling from visceral afferents to the brain in abdominal pain syndromes, as well as possible emotion regulation8 have been reported by early investigators (BOX 2). This topic has received increased attention during the past two decades, largely owing to a series of independent but converging scientific discoveries from various fields of research, including enteric neuroscience (reviewed in REF. 1), neuroimaging (reviewed in REF. 9), intestinal microbiology and host microbial interactions (reviewed in REFS 10,11), and most recently, microbial gut–brain signalling (reviewed in REFS 12–14). This Review discusses the emerging role of integrated bidirectional signalling between the brain and the gut in homeostasis and disease, as well as possible consequences for higher-level executive functions and emotional states, with a primary focus on gut to brain signalling.
Box 1. Evolutionary and developmental aspects of the enteric nervous system.
From an evolutionary standpoint, it is clear that the enteric nervous system (ENS) is not uniquely human or even mammalian: homologues of an ENS are found throughout the animal kingdom, including in insects, snails and marine polyps143–145. It has been suggested that the ganglia that form the primitive brains of helminths, and eventually the brains of higher mammals, were derived from the more primitive but homologous enteric nerve circuits146. Thus, neural circuitries and transmitter systems that have evolved to assure optimal responses (approach and withdrawal responses) to the challenges presented by our internal — for example, luminal — environment may have been incorporated into the CNS during evolution. Developmentally, the ENS arises from precursor cells that migrate from the neural crest along the vagus to settle and differentiate in the gut147. Based on its close bidirectional connections with limbic and autonomic regions of the brain148, the ENS can be viewed as a peripheral extension of the limbic system into the gut, where it is exposed closely to our complex internal environment, including powerful mechanical, chemical and microbial influences. Alternatively, parts of the CNS (in particular, pontine, autonomic and limbic circuits) can be viewed as an encephalized portion of the ENS.
Box 2. Influential theories of brain–viscera interactions.
The first comprehensive scientific theory of brain–viscera interactions was formulated in the 1880s by William James and Carl Lange and was based on the central concept that stimuli that induce emotions such as fear, anger or love initially induce changes in visceral function through autonomic nervous system output, and that the afferent feedback of these peripheral changes to the brain is essential in the generation of specific emotional feelings8. According to this theory, we feel anxious because we perceive our heart beating faster, because we become aware of our respiration becoming more frequent and shallower, or because we feel ‘butterflies’ in our stomach. In the late 1920s, Walter Cannon challenged the James–Lange theory, postulating that emotional feelings are generated directly by subcortical brain regions rather than from the feedback of situational bodily changes. He proposed that such bodily changes that are associated with emotional states are simply by-products of these brain changes, and that the visceral responses are too slow to play any part in the subjective experience of emotional feelings7.
Modern theories of emotion and consciousness that were proposed in the form of the ‘somatic marker’ hypothesis by Antonio Damasio81, and the ‘homeostatic emotion’ hypothesis by A. D. Craig78 have re-emphasized the importance of interoceptive feedback in emotional states and cognitive processes, and have largely overcome the long lasting controversy about the directionality of brain–viscera interactions in the generation of emotions.
For example, Damasio eloquently proposed that somatic markers (for example, memories of body states associated with previous feeling states) arise from positive or negative emotional feeling states being associated with visceral and other bodily responses (body loops) to certain contextual situations. According to this theory, these body loops, or their meta-representations in the orbitofrontal cortex (OFC), may play a part not only in how somebody feels at a given moment but may also influence future planning and intuitive decision making. For example, according to Damasio, somatic markers may covertly result in “undeliberated inhibition of a response learned previously … [or] the introduction of a bias in the selection of an aversive or appetitive mode of behavior”81,149.
Craig's homeostatic emotion hypothesis postulates that, in humans and anthropoid primates, an image of the homeostatic state of the body is created in the primary interoceptive cortex (dorsal posterior insula) through lamina I spinothalamic and vagal afferent tract projection to a specific thalamocortical relay nucleus in the posterolateral thalamus77. This interoceptive cortex contains modality-selective representations of all afferent activity from lamina I (that is, sympathetic afferent input) and from the nucleus tractus solitarius (parasympathetic input). According to Craig, re-representation of the image from the posterior insula to the mid and anterior insula, and modulation by inputs from affective, cognitive and reward-related brain circuits creates a feeling about the homeostatic state of the body in the anterior insula78,80. Together with the parallel processed signal in the anterior cingulate cortex (ACC), a homeostatic emotion arises, made up of a feeling dimension represented in the anterior insula and a motivation dimension represented in the ACC78.
Brain to gut signalling during homeostasis
The brain communicates to the viscera, including the gastrointestinal tract, through multiple parallel pathways, including the two branches of the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal (HPA) axis and the sympatho–adrenal axis (modulating the gut-associated lymphoid tissue), and descending monoaminergic pathways (modulating gain of spinal reflexes and dorsal horn excitability). Two key subcortical structures that generate these outputs are the hypothalamus and the amygdala. They receive inputs from a network of cortical regions (the medial prefrontal cortex (PFC) network15,16), including subregions of the medial PFC and anterior cingulate cortex (ACC). Whereas ventral ACC regions (for example, subgenual cingulate cortex and Brodmann area 25 (BA25)) project primarily to the medullary vagal complex, pregenual ACC regions (for example, BA24) project to the periaqueductal grey (PAG)17. The medial PFC network receives input from a lateral PFC and orbitofrontal cortex (OFC) network that provides integrated multisensory information about the representation of complex homeostatic body states, including those related to gut homeostasis, food intake and visceral pain. Outputs from subregions of the medial network, amygdala and hypothalamus are integrated into distinct motor patterns within the mesencephalic PAG18. The most caudal component of the central autonomic networks is represented by pontine and medullary nuclei, including the serotonergic raphe nuclei, the locus coeruleus complex (including Barrington's nucleus) and the dorsal vagal complex. This system of parallel outflows from cortico–limbic–pontine networks, which is engaged by distinct homeostatic states, has been referred to as the emotional motor system (EMS) and consists of integrated motor autonomic, neuroendocrine and pain modulatory components19,20. The medial component of the EMS (including the midline raphe nuclei) is thought to have a role primarily in the tonic modulation through serotonergic, noradrenergic and opioidergic descending spinal pathways of the gain of various spinal reflexes involved in the regulation of gastrointestinal functions, as well as in the modulation of dorsal horn neuronal excitability, to regulate pain sensitivity. An example of the gut-related engagement of the medial component of the EMS is the observation that food consumption inhibits pain-related behaviours in the rat, an analgesia mechanism related to the engagement of descending serotonergic pain modulation pathways21. Descending opioid-dependent pain modulation pathways are also engaged by other behavioural and motivational states, including vigilance and fear22. By contrast, the lateral system (including subregions of the PAG, the central nucleus of the amygdala, bed nucleus of the striae terminalis, lateral hypothalamus and pontine locus coeruleus complex) may play a part in executing distinct regional motor patterns of the viscera mediated by the engagement of function-specific subsets of sympathetic and parasympathetic pathways.
ANS output can be triggered reflexively by ascending interoceptive signals from the gut, by descending cognitive or emotional influences or in response to external or internal demands (BOX 3). For example, top-down modulation can override local (for example, ENS-based) reflex function in the context of threats to body homeostasis (haemorrhagic shock), severe environmental stressors23 or during strong emotions such as fear, anger and sadness24,25.
Box 3. The brain–gut axis is a system of hierarchical homeostatic reflexes.
The brain–gut axis can be conceptualized as a hierarchy of reflexes, from the reflex circuits contained within the enteric nervous system to the highest reflex loop involving the insular cortex and anterior cingulate cortex (AC C) (see the figure). Whereas many local physiological responses of the intestine to stretch or chemical stimulation (peristaltic, secreto-motor reflex) only involve enteric reflexes (for example, the entire reflex loop is contained within the bowel wall), most gastric reflexes and reflexes involving spatially distinct regions of the gastrointestinal tract (for example, duodeno–gastric feedback pathways in the regulation of gastric emptying) involve mesenteric reflexes (the reflex loop involves mesenteric ganglia) or vago-vagal reflexes (the reflex loop involves the dorsal vagal nucleus). Gut responses to nociceptive stimuli typically involve spinal and supraspinal reflexes, engaging strong emotional and autonomic responses. The engagement of circuits outside of the gut wall integrates interoceptive and exteroceptive information to optimize homeostatic regulation of intestinal function. Pontomedullary nuclei (including the midline raphe nuclei and the locus coeruleus complex) exert a tonic inhibitory influence on the gain of these reflexes, whereas top-down influences that originate in prefrontal regions can regulate sympathetic and vagal outputs as well as modulate the dorsal horn through descending influences. Top-down influences are engaged in responses to environmental stimuli (threat and safety) and in the recall of memories, including interoceptive memories. The conscious awareness of interoceptive images that is generated either by signals from the gut or by the recall of interoceptive memories of such signals is associated with the perception of emotional feelings, including pain, disgust or well being. The nucleus tractus solitarius (NTS) is the vagal relay nucleus in the medulla that receives input from function-specific vagal afferents and signals through interneurons to the vagal premotor neurons in the dorsal motor nucleus of vagus (DMNV). The NTS and DMNV make up the dorsal vagal complex (DVC). A1, A2 and A5 are medullary catecholaminergic nuclei to autonomic effector regions in the rostro–ventro–lateral medulla (RVLM) and ventromedial medulla (VMM). The periaqueductal grey (PAG) receives inputs from the parabrachial nucleus and also from forebrain regions, including the hypothalamus, amygdala and anterior cingulate cortex, and the prefrontal cortex (PFC). It is organized into function-specific columns. The PFC provides the highest cortical modulatory input to the insula (INS) and ACC. ANS, autonomic nervous system.
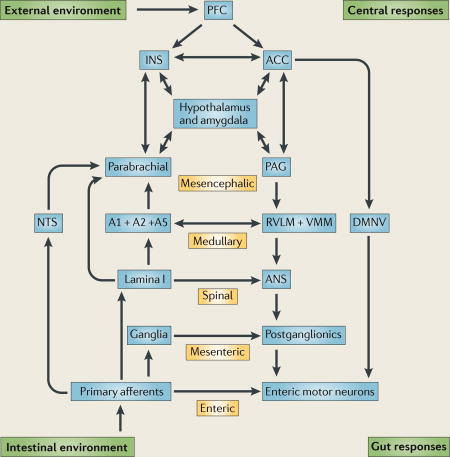
Outputs from medullary pontine nuclei project to function-specific sympathetic preganglionics located in the intermediolateral column of the spinal cord, and projections to the medullary vagal complex and the pontine Barrington's nucleus modulate function-specific vagal and sacral parasympathetic outflows, respectively26. Preganglionic parasympathetic neurons are viscerotopically organized in the dorsal motor nucleus of the vagus and are modulated by a variety of hormonal and humoral substances27,28.
Effects on the gut
The sympathetic innervation of the gastrointestinal tract and its role in the modulation of gastrointestinal function has been extensively reviewed1,28,29. It has been divided into subclasses of postganglionic vasoconstrictor neurons, secretion inhibiting neurons and motility inhibiting neurons. The overall effect of sympathetic outflow to the gut is inhibitory, slowing gastrointestinal transit and secretion. This inhibitory effect is largely accomplished by inhibitory modulation of cholinergic transmission and by a stimulatory effect on smooth muscle in sphincteric regions. Another subset of sympathetic postganglionics is involved in mucosal immune modulation30 and possibly in the modulation of interactions between the micro-flora and the mucosa (reviewed in REFS 12,31). Although the best experimental evidence for such communication between the sympathetic nervous system, lymphocytes and macrophages comes from the spleen32, evidence for sympathetically mediated immune modulation in the gut has been shown both in Peyer's patches and in the non-follicular mucosa in close proximity to different classes of immune cells, including dendritic cells, mast cells and B lymphocytes (reviewed in REF. 31). In addition, evidence of a noradrenaline-mediated reduction in the expression of toll-like receptors (TLRs) by intestinal epithelial cells has been reported33. Finally, noradrenaline-mediated modulation of microbial virulence has been reported for a number of pathogens31, even though the mechanisms by which intraluminal bacteria are exposed to noradrenaline that is released from sympathetic nerve terminals is unclear.
The parasympathetic innervation of the gastrointestinal tract has been studied intensively (for reviews see REFS 28,34). It is comprised of the vagal and sacral parasympathetic divisions, which innervate foregut and hindgut structures, respectively. Function-specific vagal motor neurons provide input to the stomach, small intestine and proximal portion of the colon. Excitatory vagal input occurs to ganglia within the ENS to mediate vago–vagal motor reflexes and the cephalic phase of gastric acid secretion, to gastrin- and somatostatin-containing enteroendocrine cells and histamine releasing enterochromaffin cells, and to enterochromaffin cells to mediate 5-hydroxytryptamine (5-HT) release35. While vago–vagal reflexes are the primary neural mechanism to regulate gastric function, such extra intestinal reflexes play a lesser part (compared to intrinsic reflexes) in the modulation of intestinal function. Vagal modulation of macrophage activation through nicotinic acetylcholine receptors has been reported as part of a vago–vagal anti-inflammatory reflex36.
Top-down engagement of subsets of function-specific postganglionic sympathetic and parasympathetic neurons are likely to mediate reported emotion-related patterns of regional changes in motor, secretory and possibly immune activity in the gastrointestinal tract37,38, which may be viewed as analogous to distinct emotion-related facial expressions and body postures (mediated by the somatic branch of the EMS). These emotion-related changes in peripheral target cells will directly or indirectly (for example, through changes in smooth muscle activity) influence the interoceptive feedback to the brain, possibly contributing to the characteristic prolonged duration of many emotional states, which can outlast the initiating event by hours. Prolonged alterations in ANS output to the gut is likely to induce changes in peripheral target cells — such as downregulation of adrenergic receptors on immune cells39, reduced expression of TLRs33 on epithelial cells or changes in primary afferent neurons40 — changing the gain of gut to brain signalling chronically and possibly resulting in the remodelling of brain regions that receive this enhanced input41. Such lasting changes in brain–gut signalling may be associated with tonic ANS dysfunction, which is in turn associated with altered emotional states such as anxiety disorders or depression.
Gut to brain signalling during homeostasis
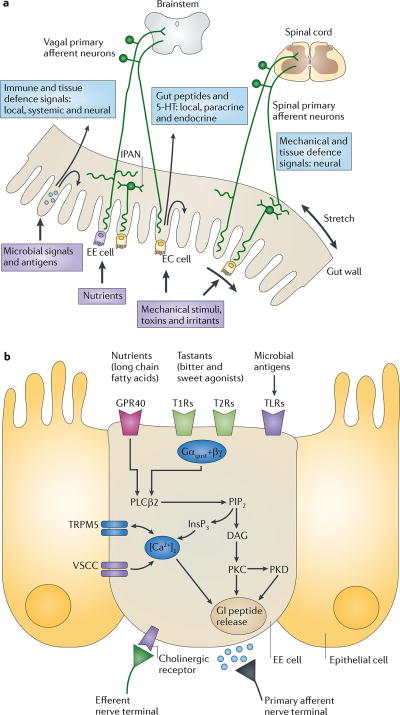
There are between 200 and 600 million neurons in the human ENS, which is equal to the number of neurons in the spinal cord1. They have been classified on the basis of their morphology, electrophysiological properties and chemical coding into distinct classes of functional specific neurons, including several classes of afferents1,42. The size and complexity of the ENS is not surprising when considering the challenges posed by the interface of the organism with its luminal environment: it interfaces closely with our largest body surface (the intestinal surface area, which is approximately 100 times larger than the surface area of the skin), with the largest population of commensal microorganisms of all body surfaces (100 trillion microorganisms from 40,000 species with 100 times the number of genes in the human genome43), with the gut-associated immune system (containing two-thirds of the body's immune cells) and with thousands of enteroendocrine cells (containing more than 20 identified hormones). These unparalleled relationships between the gastrointestinal tract and the brain, with multiple bidirectional and often interacting interoceptive communication systems, emphasize the importance of this system in the maintenance of homeostasis. There are three basic mechanisms by which sensory information is encoded in the gut: by primary afferent neurons, by immune cells and by enteroendocrine cells (FIG. 1a).
Figure 1. Gut to brain communication.
a | Endocrine, immune and neuronal afferent signalling from the gut to the CNS. Information about luminal factors and conditions of the gut are signalled through extrinsic vagal and spinal afferents to the brain stem and spinal cord, respectively. Mechanical stimuli (stretch, pressure, distortion and shearing forces) can activate spinal, vagal and intrinsic primary afferents (IPANs) directly, without intermediary cells such as the enteroendocrine (EE) cells. Although no synaptic connections have been found between IPANs and extrinsic afferents, the latter form networks around myenteric ganglia (intraganglionic laminar endings27), many of which receive synaptic input from IPANs. Signalling molecules (including proteases, histamine, serotonin and cytokines) that are produced by immune cells in Peyer's patches and within the gut epithelium can activate their respective receptors on vagal and spinal afferents. Similarly, neuropeptides and hormones (gut peptides) that are released from EE cells in response to other luminal factors, such as nutrients, toxins or antigens, can act both in an endocrine fashion, reaching targets in the brain (area postrema, dorsal vagal complex and hypothalamus), and through receptor activation on spinal and vagal afferents, in a paracrine fashion. Enterochromaffin (EC) cells signal to both IPANs and vagal afferents. b | Encoding of multiple luminal signals by EE cells. Different classes of EE cells are interspersed between gut epithelial cells throughout the gastrointestinal tract. Upon luminal stimulation (or upon activation by postganglionic sympathetic or vagal nerves), these cells can release up to 20 different gut peptides from their basolateral (and possibly luminal) surface. Released peptides can activate closely adjacent vagal afferent nerve terminals in a paracrine fashion, or when released into the circulation they can exert an endocrine effect, signalling to various sites in the brain and other parts of the gastrointestinal tract. Different types of receptors have been identified on the luminal side of EE cells, including G protein-coupled taste receptors (GPCRs) for sweet and bitter tastants, GPCRs that are responsive to fatty acids and toll-like receptors (TLRs). The intestinal taste receptors that are shown are coupled to a specific Gα protein subunit, gustducin (Gαgust), and receptor-induced increases in intracellular calcium result in peptide release from the basolateral membrane. [Ca2+]i, intracellular calcium concentration; DAG, diacylglycerol; GI peptide, gastrointestinal peptide; GPR40, G protein-coupled receptor 40; InsP3, Inositol-1,4,5-trisphosphate;. PIP2, aquaporin PIP2 member; PKC, protein kinase C; PLCβ2, phospholipase Cβ; T1R, taste receptor type 1 member; TRPM5, transient receptor potential cation channel subfamily M member 5 (specifically linked to taste receptor signalling); VSCC, voltage-sensitive Ca2+ channel.
Neuronal signaling
Based on the location of their cell body, the afferent neurons that innervate the gut are divided into extrinsic (spinal and vagal afferents) as well as several classes of intrinsic, primary afferents (IPANs)44 (FIG. 1a). IPANs greatly outnumber the extrinsic connections, with an estimated 100 million IPANs versus 50,000 extrinsic afferent nerve processes in the human gut1. Both intrinsic and extrinsic primary afferents show mechano- and chemosensitivity to both physiological and noxious mechanical stimuli45. Luminal signals do not influence afferent nerve terminals directly, but act through intermediate cells in the lamina propria (including enteroendocrine cells)46. Both extrinsic and intrinsic primary afferents provide input to multiple reflex loops to optimize gut function and maintain gastrointestinal homeostasis during internal perturbations (BOX 3). Subpopulations of modality-specific extrinsic primary afferents synapse with dorsal horn neurons in lamina I of the spinal dorsal horn or with cell bodies in the vagal nucleus tractus solitarius (NTS), providing the brain with a wide range of gut-related information. There are differences between the stomach and the intestine in the relative importance of intrinsic and extrinsic reflex regulation: whereas gastric function is predominantly regulated by vago–vagal reflexes, which are modulated within the dorsal vagal complex by cortical, humoral and endocrine signals, intestinal function relies primarily on intrinsic reflex regulation, mediated by IPANs and enteric motorneurons1.
IPANs are connected with each other through excitatory synapses, forming extensive self-reinforcing net-works47. Such networks are likely to amplify local signals that arise through direct activation of IPANs by an afferent stimulus. Some IPANs are polymodal, for example, responding to both chemical stimuli arising from entero-chromaffin cells as well as to mechanical stimuli that result from gut contractions and distensions. Whereas IPANs feed this information into a variety of local intramural reflexes that regulate and integrate gut functions such as propulsion, secretion and blood flow (but do not communicate directly with the CNS), extrinsic afferents provide afferent information to mesenteric, spinal and supraspinal reflex loops involving larger distances of the bowel and different regions of the gastrointestinal tract.
The fibres of the mechanosensitive extrinsic afferents that innervate the gastrointestinal tract end within the mesentery and longitudinal and circular muscle sheets, within the enteric ganglia48–50, or in close proximity to interstitial cells of Cajal51. These afferent terminals are sensitive to distension, contractions or movement of the gut through activation of a variety of mechano-sensitive ion channels, including members of the transient receptor potential (TRP) family (TRPV1, TRPV2, TRPV3 and TRPV4, and TRPA1) and acid-sensing channels (ASICs)52. Subclasses of extrinsic afferents have been identified based on their mechanical and electro-physiological response properties, including low- and high-threshold mechanosensitive vagal and spinal afferents53–55. A large number of such afferents do not respond to mechanical stimuli under normal conditions but develop mechanosensitivity following inflammation56,57.
Different subpopulations of primarily vagal afferents terminate in close proximity (but without specialized synaptic connections) to enteroendocrine cells58 Their terminals are chemosensitive, responding to neuropeptides released from enteroendocrine cells in response to luminal chemical and mechanical events (FIG. 1). Different classes of vagal afferents express receptors for both orexigenic (hunger generating) as well as anorexigenic (satiety generating) gut peptides contained in specialized enteroendocrine cells distributed throughout the gastrointestinal tract, from the oral cavity to the colon. Recent evidence suggests that the expression of these receptors on vagal afferents can be altered by the nutritional state of the animal and possibly by diet59, and that the cholecystokinin 1 receptor (CCK1R; also known as CCKAR) has an important role in this process60,61. The expression of receptors for orexigenic peptides was found to be increased by fasting (increasing the signal to eat), whereas that for anorexigenic peptides was decreased. These findings demonstrate the plasticity of the vagal afferent phenotype depending on the homeostatic state of the animal, analogous to the inflammation-induced phenotype switching of mechanosensitive afferents discussed above. Terminals of some vagal afferents end in close proximity to mucosal immune cells62 and have been shown to respond to a variety of signalling molecules that are released by mast cells and lymphocytes, including tryptase, histamine, 5-HT and various cytokines46.
Endocrine and paracrine signaling
Although enteroendocrine cells make up less than 1% of all gut epithelial cells, they constitute the largest endocrine organ of the body. Based on their content, over 20 different types of enteroendocrine cells have been described63,64. Enteroendocrine cells provide the first level of integration for all chemical and certain mechanical stimuli that are related to intraluminal gut contents. Their output is involved both in the regulation of digestive functions through ENS circuits and in the regulation of CNS processes through endocrine and paracrine signalling to vagal afferents. The relative importance of paracrine (for example, vagal) versus endocrine signalling to the hypothalamus and other brain regions in the regulation of food intake remains to be determined. Some enteroendocrine cells (so-called open cells) respond to luminal contents through multiple receptors (FIG. 1b). Mechanisms of enteroendocrine and enterochromaffin cell activation include depolarization by activation of mechanosensitive cation channels located on microvilli in response to the shearing forces of moving intestinal contents64, activation by fatty acids through an acyl chain length-specific Ca2+-dependent mechanism65, presumably a G protein-coupled receptor (GPCR)66, activation by vagal efferents35 as well as depolarization through G protein-coupled taste receptors67. In addition, enter-oendocrine cells respond to the presence and activity of intraluminal microbial organisms through pattern recognition receptors (including TLRs), (reviewed in REFS 12,68). One example for this multimodal sensitivity is illustrated by the observation of increased release of cholecystokinin from enteroendocrine cells in response to intestinal pathogens69,70. There has been a recent surge of interest in the sensors that detect sweet and bitter sensations and amino acids in the gut, which were originally described as taste receptors in the tongue67. The taste receptor type 1 (T1R) family comprises three members that heterodimerize to form sweet taste receptors (T1R2 and T1R3) and amino acid receptors (T1R1 and T1R3), and the T2R family includes GPCRs that are involved in bitter taste reception. As in the tongue, taste receptor signalling involves a specific Gα subunit, α-gustducin, a taste-specific signalling protein with a prominent role in bitter taste71. Each of the channels described above triggers different signal transduction mechanisms, resulting in intracellular calcium increases and peptide release, or TLR-mediated activation of NF-κb, activator protein 1 (AP1; also known as transcription factor AP1) and interferon regulatory factors (IRFs); which leads to cytokine release12. Crosstalk among these signalling channels might enable signals from pathogenic and commensal microorganisms to alter the response of certain enteroendocrine cells to mechanical and/or chemical stimulation59.
A particularly well-studied type of enteroendocrine cell is the 5-HT-containing enterochromaffin cell of the small and large intestine64. 95% of the organism's 5-HT is located in the gut, primarily in enterochromaffins. It is released on the basolateral side of these cells in response to shearing forces that are produced by moving luminal contents64 that activate mechanosensitive cation channels located on microvilli on the apical side of enterochromaffin cells. Although mechanically released 5-HT is essential for triggering normal peristalsis and secretomotor reflexes, it is unknown if signalling to the CNS occurs during such physiological sensing and if tonic, vagally medidated 5-HT signalling to the brain is involved in modulating affective brain circuits.
Immune-related signaling
70–80% of the body's immune cells are contained within the gut-associated lymphoid tissue, reflecting the unique challenge for this part of the immune system to maintain a homeostatic balance between tolerance and immunity in the intestine. A single layer of columnar intestinal epithelial cells (IECs) forms the barrier between the host and one of the largest and most complex microbial habitats. The fact that mucosal immune cells remain immunologically hyporesponsive to commensal bacteria but also maintain their responsiveness to pathogenic organisms, suggests that under physiological conditions, the intestinal immune system recognizes commensal bacteria and elicits basal or tonic signals in the absence of full activation of the innate and adaptive immune response72. The physical constraints that the undisturbed epithelial layer imparts on sampling the luminal space are overcome by specialized lymphoid structures (including small intestinal Peyer's patches) that are embedded in the lamina propria throughout the intestine72. Two epithelial transduction mechanisms have been identified: specialized microfold cells that can sample antigens and microorganisms, and deliver them through transcytosis to antigen processing cells within Peyer's patches, and lamina propria dendritic cells, which extend their dendrites between epithelial tight junctions, to sample the luminal environment. Similarly to enteroendocrine cells, both EICs and dendritic cells express a wide range of pattern recognition receptors, including TLRs73, emphasizing the central role of the gut in interactions with microorganisms and in discriminating between pathogens and commensals. Subsets of vagal afferent nerve terminals are in close proximity to mucosal immune cells and contain receptors for signalling molecules released from these cells, including mast cell products (proteases, histamine, serotonin and corticotropin releasing factor (CRF))62 and cytokines that are released from macrophages. Immune cell products can indirectly influence the functional properties of enteroendocrine cells, as illustrated by the increased cholecystokinin secretion in a mouse model of gut inflammation owing to the release of interleukin-4 (IL-4) and IL-13 from CD4+ T cells70. Similarly, changes in the properties of the enterochromaffin cell-based 5-HT signalling system in the context of gut inflammation have been described74.
The effect on the brain
Despite the continuous stream of interoceptive signals from the gut to the brain, only a small fraction of this information is consciously perceived, usually that which requires a conscious behavioural response (for example, ingestive behaviours and defecation). However, recent evidence suggests that various forms of subliminal interoceptive inputs from the gut, including those generated by intestinal microbes, may influence memory formation, emotional arousal and affective behaviours75.
Primary vagal and spinal afferents from the gastrointestinal tract project to the NTS and the lamina I of the dorsal horn, respectively (FIG. 2). In addition, laminae V, VII and X receive additional nociceptive spinal inputs. In mammals, activity of vagal projections from the NTS and from lamina I are integrated in the parabrachial nucleus, which then provides an integrated signal to behavioural control regions of the forebrain, including the hypothalamus and amygdala76. Collaterals from these ascending vagal and spinal projections to medullary serotonergic (raphe) and catecholaminergic nuclei (including the locus coeruleus complex) provide input to ascending monoaminergic projections that can modulate cortical arousal. In non-human and human primates, there is a direct projection from lamina I and from the NTS to ventromedial thalamic nuclei. Parallel projections of lamina I afferents provide a direct thalamo–cortical pathway that activates the ACC. Based on electrophysiological and neuroanatomical data obtained in animals, Craig proposed the concept of a lamina I spino–thalamo–cortical pathway, visible in primates and well-developed only in humans, that provides a direct cortical image of the homeostatic state of the body (in the posterior and mid insular cortex) that is modulated by input from emotional and cognitive brain circuits in the ventral and dorsal portions of the anterior insula (aINS)77–80 (BOX 2). Even though a close reciprocal relationship between interoceptive information (including different aspects of gut-related information) and emotion has previously been proposed8,81, the human INS — and its related brain networks (including the ACC, OFC and amygdala) — has emerged as the most plausible brain region supporting this integration. Several recent studies, including two quantitative meta-analyses82,83, have largely defined the general functional subregions of the INS, with substantial homology to the functional neuroanatomy of non-human primates. In one of these meta-analyses82, evidence for both differentiation into function-specific subregions as well as integration of multiple functions within a single subregion has been demonstrated. For example, visceral and sensory input were represented in the mid posterior aspects of the INS, and olfactory and gustatory stimuli were represented in the aINS and mid INS respectively, suggesting that distinct but closely adjacent INS subregions are responsible for the primary representation of different aspects of ingestion-related interoceptive inputs. A conjunction analysis across all of the domains revealed that the majority of all tested functions overlapped in the dorsal aINS (with the exception of basic somatosensory processes), suggesting that this brain region may be concerned with integration of food-related (olfaction and taste), interoceptive, emotional and cognitive functions.
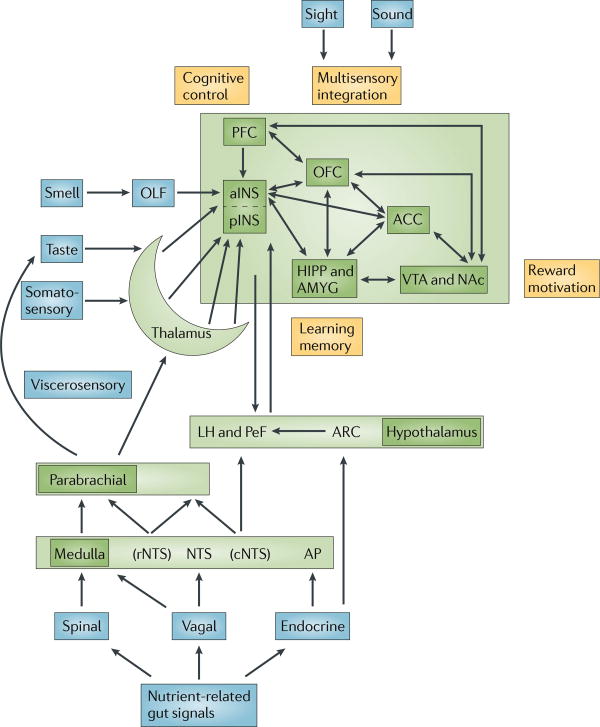
Figure 2. Gut–brain signalling related to food intake.
Nutrient-related signals reach the CNS through spinal, vagal and endocrine signalling pathways. Endocrine signalling of gut peptides that are released into the systemic circulation reach the dorsal vagal complex through the area postrema where they modulate the transmission of afferent vagal signals to the dorsal motor nucleus. These gut peptides also reach specialized neurons within the hypothalamus. Paracrine signals activate function-specific vagal afferent fibres that ultimately signal to subregions of the anterior insula (aINS). The sensory aspect of taste is primarily encoded in the aINS, but the multimodal integration of satiety signals with the sensory properties of food (including its flavour, palatability and reward value) as well as the context of food intake (including food related visual and auditory signals) occurs in the orbitofrontal cortex (OFC). Further integration with inputs from the reward system and with interoceptive memories of previous food ingestion generates a multidimensional food-related experience that ultimately determines ingestive behaviour. Prefrontal regions exert cognitive control over ingestive behaviours. Learning about food-related experiences and the formation of interoceptive memories is an important aspect of the cortical circuitry that is involved in this process. ACC, anterior cingulate cortex; AP, area postrema; ARC, arcuate nucleus; cNTS, caudal NTS; HIPP, hippocampus; LH, lateral hypothalamus; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PeF, pernifornical hypothalamus; OLF, olfaction; PFC, prefrontal cortex; rNTS, rostral NTS; VTA, ventral tegmental area.
Earlier intra-operative electrical stimulation studies84 had shown that stimulation of the aINS was associated with gustatory and olfactory sensations, whereas stimulation of more posterior subregions resulted in interoceptive and somatosensory sensations. The fact that changes in gastric motility were also observed in these experiments is consistent with the homeostatic reflex concept discussed in BOX 3. In addition to the mapping of different nutrient-related functions onto the aINS, activation of a dorsal aINS subregion was seen in correlation with cognitive functions79,80, and activation in a ventral aINS subregion was seen in correlation with social–emotional functions, consistent with homologous findings in non-human primates85. A recent connectivity study based on spatiotemporal oscillations in the resting brain confirmed the existence of a ventral aINS-centered network86. Based on the close structural connectivity of different INS subregions in non-human primates85, it can be assumed that the close spatial association of function-specific INS subregions found in human brain imaging studies allows for the necessary integration of parallel inputs related to the homeostatic state of ingestion-related functions with cognitive and emotional influences. In the case of signals related to the olfactory87, gustatory88 and viscerosensory dimensions of food intake, this integration is accomplished through close connections of the aINS both to a ventral circuit (including the amygdala, ACC and OFC, and the ventral striatum) and a dorsal circuit involving the dorsolateral PFC and the dorsal striatum89.
Emotional and executive function
Effects of gut to brain signalling on emotional function
Although it is intriguing to speculate about the effects of enteroendocrine and enterochromaffin cell–vagal signalling to the brain as an important mechanism of emotion modulation in health and disease, the experimental literature mostly supports the hedonic effects of food intake (taste and satiation) and the aversive effects of acute mucosal inflammation or irritation by luminal toxins and pathogens. Ingesting foods that are beneficial for the organism is associated with a feeling of pleasure, whereas ingestion of potentially harmful foods elicits negative emotions, such as nausea. Gut-based neuroendocrine signalling systems (involving signalling molecules located in different classes of enteroendocrine cells) can signal satiety (including cholecystokinin, peptide YY, glucagon-like peptide 1 (GLP1)), hunger (ghrelin) and nausea (5-HT), whereas those located in mucosal immune cells (including CRF, 5-HT, proteases and cytokines) can signal pain, discomfort, nausea and fatigue. Little is known about the possible role of enterochromaffin cell–vagal signalling or the role of intestinal taste receptor activation in background emotions. However, a small number of studies in animal models show a possible role of the gut microbiota in emotional behaviour in rodents13,14,90–92, even though the mediating signalling mechanisms are poorly understood. Intraluminal infusion of nutrients has been shown to modulate flavour preferences and acceptance in rodents93. Intragastric infusion of fatty acids in human subjects resulted in a reduced brain response to experimentally induced sad emotions94
Meta-representation of gut-related feeling states
A growing literature on the topic of different sensory modalities supports the concept that actual viscerosensory (and somatosensory) stimulation, as well as expected or imagined sensory experiences, engage different regions of the INS. For example, although actual distension of the gut in healthy subjects is associated with activation of the mid INS and aINS, consistent with the reported findings about insular viscerosensory representation82, the expectation of visceral pain has been shown to be associated with activation of the aINS only95, consistent with the role of the aINS in the processing of interoceptive memories. Similar findings had previously been reported for expected somatic pain96.
Activations of aINS (and ACC) have also been reported when subjects experienced disgust, or viewed images of others experiencing disgust97, in the absence of a vagally mediated signal from the gastrointestinal tract signalling nausea. This suggests that homeostatic reflexes at the level of the aINS and ACC can be triggered in the absence of interoceptive input, presumably based on the recall of interoceptive memories while viewing an interoceptive feeling expressed by another individual or by expecting or imaging a particular feeling state.
The mechanisms and functional neuroanatomy underlying the formation of interoceptive memories of complex gut-related homeostatic states with affective and motivational dimensions (such as pain, nausea, hunger and satiation) are incompletely understood but are likely to include the encoding of such memories during development, possibly beginning with the earliest experience of pain (hunger and abdominal discomfort) and pleasure related to food intake of the newborn. Gut to brain signalling in the newborn underlies early undifferentiated emotions and stereotypic behavioural responses (suckling, vomiting, defecation and crying). It represents the primary mechanism that regulates positive and negative feeling states, providing the child with the first value-based map of the world, founded on positive and negative experiences related to food intake, satiation and associated gut responses. For example, the response to sweet taste receptor stimulation of the tongue in the newborn is a stereotypically organized reflex response also seen in decerebrate rats98 and in anencephalic infants99. The concept of a body-based value map is closely related to the ‘somatic marker’ hypothesis or to the concept of interoceptive memories (BOX 2). For example, the close relationship between sucrose ingestion and the resulting activation of sweet taste receptors on the tongue and central release of opioids (important mediators of homeostasis and well being), has been shown both in rodents100 and in children101. This basic gut-based neurochemical encoding of feeling states is often maintained in the adult but complemented by learned meta-representation of such sensations (FIG. 3). For example, the close relationship of the gastric-based ghrelin signalling system, which plays a prominent part in the generation of the homeostatic emotion of hunger, and the associated craving for food, with the dopamine-based reward system is maintained throughout development, as peripherally released ghrelin can stimulate central dopamine release through vagal and endocrine mecha-nisms102. These initially gut-based responses to appetitive and aversive stimuli may also form the basis for interoceptive brain-based memories of basic emotional states (FIG. 3) that are relevant for complex social emotions, including predictions about the future and intuitive decision making. The fact that predictions and prediction errors are associated with activations of sub-regions of the aINS103,104, and that patients with lesion of the OFC, a brain region closely connected to the aINS, are greatly compromised in their ability to make adaptive decisions about the future105, provides indirect evidence for such a hypothesis. It has been speculated that a system of specialized cells (von Economo (VEN) cells) that are located in the aINS and share certain receptors and peptides that are rare in other brain regions but abundant in the ENS (including bombesin-like peptides and 5-HT receptor 2B (5HT2B)), may be related in evolutionary terms to gut–brain signals106. The proposed close relationship between brain and gut systems is supported by evolutionary, developmental and neurobiological evidence. For example, there is a close relationship between pleasant gut-related emotions (associated with food intake) and social interactions in societies and cultures of human and non-human primates, as well as in lay language. Conversely, there is a close relationship between negative gut-related feeling states (including nausea and abdominal discomfort) and social withdrawal.
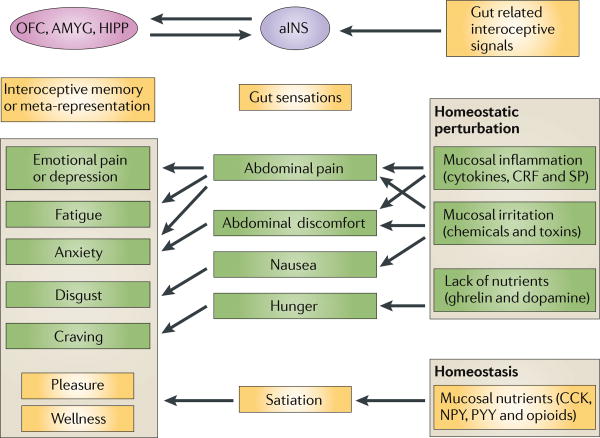
Figure 3. Gut signalling systems, gut sensations and meta-representations of such sensations.
Gastrointestinal interoceptive signals to the brain play a key part in the maintenance of homeostasis under resting conditions. Whereas signalling under homeostatic conditions is generally associated with pleasant sensations (satiety), signalling under non-homeostatic conditions is typically associated with aversive sensations (pain and nausea). Perception of these signals as gut sensations is associated with activation of the anterior insula (aINS) and the orbitofrontal cortex (OFC), where gustatory, olfactory and viscerosensory signals are integrated and where they are modulated by affective and cognitive input. The generation of such gut sensations can lead to the formation of interoceptive memories of the multidimensional experience associated with the sensation (such as the feeling of nausea associated with a disgusting experience or a particular taste with a pleasant social experience). The interoceptive memory is encoded within a distributed network involving the aINS, OFC, the amygdala (AMYG) and hippocampus (HIPP). Often, environmental stimuli that are unrelated to the gut, such as an image, sound or smell, may trigger the recall of these feeling states as meta-representations of the original gut-triggered sensation. The experience of such memories can result in the engagement of similar brain regions (aINS, anterior cingulate cortex (ACC), OFC and amygdala) in the absence of gut signalling. This recall may be the basis for feelings such as disgust, craving or emotional pain, in the absence of any interoceptive input from the gut, and may occur when watching another person in pain, when hearing about a disgusting experience or when viewing images of rich foods. Recall of such memories without conscious awareness may bias behavioural responses149, and such behavioural biases may be a reflection of an internal value map based on ‘gut feelings’. 5-HT, 5-hydroxytryptamine (serotonin); CKK, cholecystokinin; CRF, corticotropin releasing factor; NPY, neuropeptide Y; PY, peptide YY; SP, substance P.
The close relationship between gut signalling systems, basic feeling states and meta-representations of these feeling states in basic emotions and behaviours is not limited to nutrient-related signals. Gut-based signalling systems that are related to an insult of gut homeostasis (by chemicals, toxins and inflammation) can easily fit into this general model. This includes the 5HT–5HT3 receptor–vagal signalling system related to toxin-induced nausea107, the cytokine–vagal signalling system108 that is engaged in the generation of fatigue and sickness behaviour, and the CRF signalling system that is related to the threat of mucosal homeostasis by gut inflammation109. The concept of a primordial stress response system that is related to immune activation, which is co-opted into a generalized stress response system for both interoceptive and exteroceptive stressors has previously been suggested110. Given the magnitude and complexity of gut-based signalling systems, compared to those of other organs, brain–gut interactions have to be considered an important, but largely ignored, component of the neuroscience of emotions.
Decisions based on gut feelings
As discussed above, the popular statement that somebody has made a decision based on their gut feelings may have an actual neurobiological basis related to brain–gut interactions, and to interoceptive memories related to such interactions. Specifically, evidence from neuroimaging studies in humans has implicated the fronto-insular cortical regions, in particular subregions of the aINS, in intuitive decision making. Intuitive decision making can be defined as the rapid assessment of the probability of a favourable or unfavourable outcome of a planned behaviour in a situation of uncertain outcomes, which is dependent on previous experiences rather than on serial processes of inductive and or deductive reasoning103,111. It has been suggested that large spindle shaped neurons that are primarily located in the fronto-insular region of the brain, and present in humans and other mammals with complex social interactions, may be involved in such unique cognitive processes106. It remains to be determined if such intuitive decision making is based on an interoceptive map of gut responses, acquired during infancy and refined during development.
Perturbation of homeostasis
Considerable experimental evidence demonstrates the plasticity of the brain–gut axis during acute and chronic perturbation of homeostasis, including changes in gut to brain signalling that are associated with increased consumption of fat-rich diets59 and neuroplastic changes in visceral afferent pathways associated with gut inflammation74,112.
Alterations in signalling systems
Extensive evidence supports the involvement of different peripheral signalling systems in perturbations of gut to brain signalling. For example, when intestinal enterochromaffin cells are activated by potentially noxious stimuli, such as food-related or bacterial toxins (or certain chemotherapeutic drugs), increased 5-HT release activates 5-HT3 receptors on myenteric IPANs as well as on vagal afferents, resulting not only in exaggerated intestinal motor and secretory reflexes but also in the activation of brain regions receiving vagal input. Activation of this enterochromaf-fin cell–vagal afferent pathway by harmful substances, including certain pathogens, is associated with the subjective sensations of discomfort and nausea, and may be accompanied by vomiting. Together, the peripheral and central responses initiate a complex adaptive response aimed at expelling the offending agent.
Another well-characterized example of a brain–gut signalling system that is engaged during perturbation of homeostasis is the CRF signalling system. Central release of CRF during experimental stress, or central injection of this peptide in unstressed animals produces a distinct, stereotypic pattern of stress-induced gastrointestinal motor and secretory responses that are associated with anxiety-like behaviours23. As the stress-induced gut response can be prevented by either peripheral or central CRF1 receptor antagonism, both peripheral and central CRF–CRF receptor 1 (CRFR1) signalling systems seem to be involved. The slowing or reversal of gastric motility and the acceleration of distal bowel motility and secretion result in the reduced exposure of the gastrointestinal tract to luminal factors, reducing the metabolic demand generated by gut-related motor, secretory and absorptive function, and shifting blood flow to the cardiovascular and skeletomotor system for the fight and flight response.
Inflammatory changes in the viscera, including the liver and the gut, can result in cytokine-mediated vagal activation of the hypothalamus and related limbic brain regions, resulting in hyperalgesia and social withdrawal referred to as ‘sickness behaviour’113. Additional non-vagal mechanisms by which peripheral cytokines can reach the CNS have been reported114,115. Mucosal release of cytokines and other inflammatory mediators including bradykinin, histamine and proteases can also result in sensitization and activation of spinal afferent terminals, resulting in visceral hyperalgesia. Multiple effects of such inflammation or toxin-induced changes in afferent terminals have been reported, including release of neuropeptides such as substance P and calcitonin gene-related peptide from afferent nerve terminals116, development of mechanosensitivity of previously unresponsive afferents and development of peripheral and central sensitization, resulting in greatly increased signalling of visceral afferent information to the CNS56.
Brain–gut interactions in chronic disease
Even though dysregulations of the brain–gut axis have been implicated in several chronic disorders, convincing evidence to support such a pathophysiological role is limited to several chronic gastrointestinal disorders (including so-called functional gastrointestinal disorders (FGIDs) and inflammatory bowel diseases (IBDs)) and to eating disorders, particularly obesity117 and anorexia nervosa89.
FGIDs comprise a heterogeneous group of disorders characterized by chronically recurring pain and discomfort that is conveyed to different regions of the gastrointestinal tract. They are often associated with alterations in gastrointestinal function, and characterized by the absence of any agreed upon biological, physiological or anatomical abnormalities that would explain the symptoms118. In the majority of patients, increased levels of anxiety are present, and co-morbid diagnoses of anxiety and depression are seen in up to 60% of patients. Based on symptom criteria, but in the absence of any syndrome-specific pathophysiological findings, up to 40 different functional gastrointestinal syndromes have been postulated, including functional chest pain of presumed oesophageal origin, functional dyspepsia and irritable bowel syndrome (IBS)118,119. A characteristic feature of all of these disorders is the frequent overlap of symptoms belonging to one syndrome with one or several others in the same patient, concurrently or at different life periods. One of the most common and best-studied syndromes is IBS120,121. Despite intense clinical and preclinical research efforts during the past 20 years, no single biological abnormality has been identified that would explain the symptoms of chronically recurring pain and discomfort in all of the patients meeting current symptom-based criteria. Based on animal models and on studies performed in human patient populations, peripheral mechanisms that have been implicated include long lasting sensitization of primary afferent pathways innervating the intestine, altered microflora– epithelial–immune interactions (including alterations in mast cell regulation, increased epithelial permeability and altered gut microflora)122. Perceptual hypersensitivity to experimental and physiological gut signals is common among patients with IBS123, but it remains unclear if this reflects an increased interoceptive signal from the gut, a central amplification mechanism related to altered cognitive and affective modulation of the perception of normal or even reduced gut signals. Based on a growing number of brain imaging studies in patients with IBS, alterations in brain responses to controlled gut distension as well as to expectation of such gut discomfort have been reported9. In a recent meta-analysis of 18 studies comparing brain responses to rectal distension in patients with IBS and healthy control subjects, patients with IBS showed greater activation of brain regions that are involved in the processing of visceral afferent information, in emotional arousal and in cortical modulation. Recent evidence suggests the presence of regional cortical thickness changes in corresponding brain regions41,124. It remains to be determined whether the observed functional and structural brain changes are secondary to chronically increased gut to brain signalling and/or whether they are primary brain-based abnormalities that play a part in pain amplification and altered autonomic regulation of gut function. An important role of central dysregulation in IBS pathophysiology is suggested by the common co-morbidity with other functional gastrointestinal and non-gastrointestinal syndromes (including, but not limited to, interstitial cystitis or painful bladder syndrome, fibromyalgia and temporomandibular disorder), by the common affective co-morbidity, the prominent role of stress in symptom onset or exacerbation, the responsiveness of these syndromes to centrally targeted therapies (including tricyclic antidepressants and cognitive behavioural therapies) and the fact that the first onset of abdominal pain symptoms in children is often preceded by increased anxiety125.
Inflammatory gastrointestinal disorders
Despite a wealth of preclinical data on the acute and persistent effects of gut inflammation on intrinsic and extrinsic gut innervation (reviewed in REFS 56,74,112,126), the role of chronic intestinal inflammation on symptoms and brain responses in human patients with inflammatory bowel diseases (IBDs), such as ulcerative colitis and Crohn's disease, or gut infections are poorly understood. Surprisingly, abdominal pain or persistent visceral hyperalgesia to experimental stimuli is not a prominent feature in most patients with an uncomplicated IBD, and only a small percentage of patients with IBD or with infectious gastroenteritis, experience persistent pain once the inflammation has resolved127. The effective engagement of peripheral128 as well as central129 pain inhibition systems have been proposed as plausible mechanisms for this paucity of pain symptoms in the majority of patients with IBD. Similarly to IBS, it remains to be determined whether commonly reported co-morbidity with anxiety and depression130,131 is a consequence of the primary disease, plays a part in the chronicity of symptoms or is a vulnerability factor. Evidence from animal models supports a modulating role of the brain on gut inflammation132.
Eating disorders
Disturbances in ingestive behaviour are common in industrialized societies and both obesity and anorexia nervosa are major health problems in terms of morbidity, mortality and healthcare costs133,134. Although obese subjects eat beyond their caloric needs, with food intake driven primarily by the craving for the hedonic value of food, despite the known negative health consequences117, individuals with anorexia nervosa have an ego-syntonic resistance to eating and an obsession with weight loss, despite a preoccupation with food and eating rituals89.
Despite the extensive progress in the understanding of peripheral and central contributions of altered brain– gut interactions in obesity (reviewed in REFS 117,135), attempts at pharmacological treatments have been disappointing and various gastric surgical procedures are currently the most effective treatment136. Even though the current obesity epidemic is likely to be multifactorial, a shift in the balance between hedonic and homeostatic regulation of food intake, a compromised effectiveness of cortical control mechanisms and changes in peripheral encoding mechanisms in the gut seem to play an important part. For example, diet-induced down-regulation of satiety-inducing gut to brain signaling mechanisms have been reported, including leptin and insulin resistance and alterations in the normal chole-cystokinin-dependent molecular switching mechanisms in vagal afferents during food intake from an orexigenic to an anorexigenic phenotype137. At the same time, considerable evidence for alterations in the regulation of the hedonic aspects of food intake have been reported, consistent with the concept of food addiction135. Brain imaging findings in humans who are obsese are consistent with an enhanced sensitivity of the reward circuitry to food cues (for example, viewing images of high caloric food) that predict reward, but a decreased sensitivity to the rewarding effects of actual food ingestion (interoceptive stimuli) in dopaminergic pathways. As several of the gut peptides are able to stimulate the central dopamine system (including ghrelin, leptin and insulin), the observed changes in the central reward circuits may in part be due to diet-related neuroplastic alterations in peripheral encoding mechanisms and the reduced responsiveness of interoceptive signalling mechanisms from the gut.
The alterations in the central regulation of food intake in anorexia nervosa have recently been reviewed89. Despite the behavioural abnormalities, and more recently the brain abnormalities, that have been reported in patients, little is known about possible alterations in gut to brain signalling mechanisms and in particular about possible alterations in vagally mediated neu-ropeptide signalling to the brain. However, the fact that patients with anorexia nervosa showed reduced responses in homeostatic afferent brain regions (the aINS and ACC) in response to oral sucrose administration and that no correlation between activity in these regions and self-ratings of the pleasantness of the administered sucrose was observed, suggest that the influence of interoceptive signals from the gastrointestinal tract on ingestive behaviour is reduced.
Proposed disease model for altered brain–gut interactions
Analogous to cognitive models of anxiety138–140 and based on the central role of ineffective coping strategies (such as catastrophizing or hypervigilance) and anxiety in determining symptom severity in several functional gastrointestinal disorders, a central role of altered appraisal and prediction error has been proposed125 (FIG. 4). This model posits a mismatch between the actual interoceptive image of the digestive system represented in the INS and the predicted state, based on a negatively valenced interoceptive memory of such experiences. The mismatch results in hypervigilance towards gut-related sensations and emotional arousal, engagement of ANS reponses and possibly enhanced sensory perception. In the case of obesity and food addiction, the proposed model postulates a mismatch between the expected reward and the actual delivery of reward. This mismatch will promote compulsive overeating as an attempt to achieve the expected level of reward135. The mechanisms that underlie the failure to correct the mismatch by updating the predicted state are unclear but have been discussed in the context of learning theory and reinforcement learning141,142.
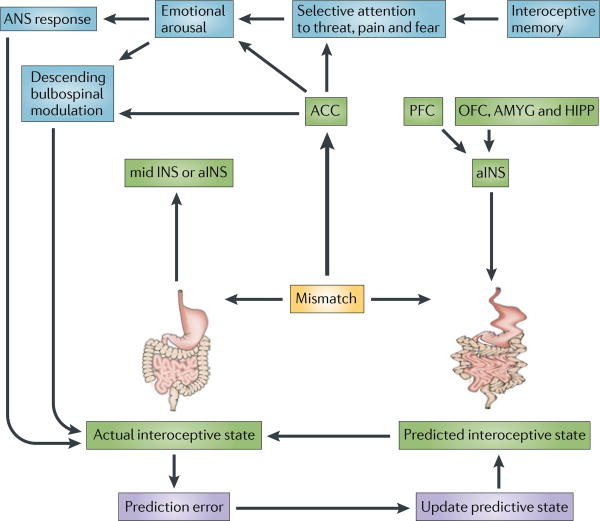
Figure 4. Interoceptive memory and prediction error in chronic disease.
According to a theory proposed by Paulus and Stein138, the mismatch of actual interoceptive input reaching the anterior insula (aINS) through body to brain signalling, with a falsely predicted interoceptive state (from interoceptive memory and/or influences on the aINS from prefrontal and limbic influences) results in the engagement of anterior cingulate cortex (ACC). Mismatch-related ACC activation is associated with emotional arousal, increased sympathetic and sacral parasympathetic activity and engagement of descending bulbospinal sensory facilitation systems, and may be associated with a conscious feeling of anxiety and worry. When adapted to functional gastrointestinal disorders125, the autonomic response to the mismatch is likely to change the state of the gut through modulation of multiple target cells (for example, activation of motor and secretory activity in the distal colon that is associated with stress), which in turn is likely to produce altered interoceptive feedback to the INS. The failure to correct the prediction error by updating the predictive state (as would be expected in a healthy individual) results in chronicity of symptoms through chronic functional and structural dysregulation of the brain–gut axis. Mismatches between actual interoceptive input from nutrient related signals from the gut and interoceptive memories of hedonic experiences of food intake have been implicated in obesity135. ACC, anterior cingulate cortex; AMYG amygdala; ANS, autonomic nervous system; HIPP, hippocampus; OFC orbitofrontal cortex; PFC prefrontal cortex.
Conclusions and future directions
Tremendous progress has been made in our understanding of the bidirectional crosstalk between the brain and the digestive system. This includes the remarkable success in mapping the functional neuroanatomy of the ENS, in our understanding of how the brain modulates these ENS circuits and gut functions, and in unravelling the complexity of gut to brain signalling through multiple parallel but interacting communication channels. Alterations in brain–gut interactions that are associated with gut inflammation, chronic abdominal pain syndromes, eating disorders and psychosocial stressors have been characterized, even though the development of effective therapies for any of these conditions has been disappointing. By contrast, many aspects of gut to brain signalling that are addressed in this article, in particular the role of this signalling in emotional and cognitive function, remain speculative at this point but may guide future research. Several important questions remain to be addressed. What role does gut to brain signalling have in brain development during early life and what part does it play in adults? For example, does gut to brain signalling early in life influence the development of adult ingestive behaviour, visceral pain sensitivity, mood and affect, interoceptive memory and intuitive decision making? What roles do taste receptors and the microflora play in this interoceptive signalling? Does mucosally driven vagal input to the brain play a part in brain development? Is there a role for alterations in microbial signalling to the gut and in gut signalling to the brain in the pathophysiology of anxiety and depression, as suggested by the common overlap of several brain–gut disorders with disorders of mood and affect? And what part do interoceptive memories and prediction errors play in human disease states? One of the great advantages in addressing these questions is the fact that most of these hypotheses can be studied in human patients, from whom stool samples for metagenomic sequencing of the microflora and gut tissue for analysis of signalling mechanisms can be obtained relatively easily by endoscopic biopsies, and brain structure, function and signalling can be studied with non-invasive neuroimaging techniques.
Acknowledgments
Supported by grants DK048351, DK064539, DK082370 and AT002681 from the National Institutes of Health.
Abbreviations
- Enteric nervous system
Ganglionated plexus of neurons that is located between the layers of the gut. These neurons, which are equal in number to those in the spinal cord, are able to regulate basic gut functions, such as the peristaltic reflex.
- Emotional motor system
System of parallel outflows from cortico–limbic–pontine networks that is engaged during distinct homeostatic states. A medial component provides tonic modulation of spinal reflexes and a lateral component plays a part in executing distinct regional motor patterns of the viscera through autonomic pathways
- Enteroendocrine cell
Specialized epithelial cell that releases secretory granules, containing one or several gut peptides, on the basolateral side (and possibly luminal side) in response to luminal chemical, mechanical and possibly neural stimuli.
- Enterochromaffin cell
Specialized epithelial cell that releases secretory granules, containing primarily serotonin, on the basolateral side (and possibly luminal side) in response to luminal chemical, mechanical and possibly neural stimuli.
- Intrinsic reflex
Also known as an intramural reflex. A reflex of the enteric nervous system in which the afferents, interneuron and efferent neurons that are involved are all contained within the gut wall.
- Intrinsic, primary afferent
Afferent neuron with its cell body contained within the enteric nervous system, that encodes mechanical and paracrine signals.
- Commensal bacteria
Refers to the 100 trillion bacteria that live in symbiosis with the gut and make up the intestinal microflora.
- Myenteric
Subplexus of the enteric nervous system, which is localized between the circular and longitudinal muscle layer.
- Ego-syntonic
Psychological term referring to behaviours, values and feelings that are in harmony with, or acceptable to, the needs and goal of a person or that are consistent with a person's ideal self image. This trait is typically seen in patients with anorexia nervosa.
Footnotes
Competing interests statement: The author declares no competing financial interests.
Further Information: Emeran A. Mayer's homepage: http://www.uclacns.org
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Furness JB. The Enteric Nervous System. Blackwell; Oxford: 2006. A comprehensive overview of all aspects of the enteric nervous system. [Google Scholar]
- 2.Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 3.Costa M, Furness JB, Llewellyn-Smith IJ, Johnson LR. In: Physiology of the Gastrointestinal Tract. Johnson LR, et al., editors. Raven; New York: 1987. pp. 1–40. [Google Scholar]
- 4.Gershon MD, et al. In: Physiology of the Gastrointestinal Tract. Johnson LR, et al., editors. Raven Press; New York: 1994. pp. 381–422. [Google Scholar]
- 5.Gershon MD. The Second Brain. Harper Collins; New York: 1998. [Google Scholar]
- 6.Mayer EA, Brunnhuber S. Handbook of Clinical Neurology. 3. in the press. [DOI] [PubMed] [Google Scholar]
- 7.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 8.James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- 9.Mayer EA, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252–264. doi: 10.1038/mi.2008.21. [DOI] [PubMed] [Google Scholar]
- 12.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. A review of emerging concepts and preclinical evidence that support the idea of intestinal microbiota to brain signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 14.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 15.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex: a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 16.Ongur D, Price JL. The organization of networks within the orbital and medical prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 17.Vogt BA. In: The Cerebral Cortex. Jones EG, Peters A, editors. Plenum Press; New York: 1985. pp. 89–149. [Google Scholar]
- 18.Bandler R, Keay KA. In: The Emotional Motor System. Progress in Brain Research. Holstege G, Bandler R, Saper CB, editors. Elsevier; Amsterdam: 1996. pp. 285–300. [DOI] [PubMed] [Google Scholar]
- 19.Holstege G, et al. In: The Emotional Motor System. Jones EG, Peters A, editors. Elsevier; Amsterdam: 1996. pp. 3–6. [Google Scholar]
- 20.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason P. From descending pain modulation to obesity via the medullary raphe. Pain. 2010;152:S20–S24. doi: 10.1016/j.pain.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields H. State-dependent opioid control of pain. Nature Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 23.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 24.Almy TP, Kern F, Tulin M. Alterations in colonic function in man under stress. I: experimental production of sigmoid spasm in healthy persons. Gastroenterology. 1947;8:616–626. [PubMed] [Google Scholar]
- 25.Welgan P, Meshkinpour H, Ma L. Role of anger in antral motor activity in irritable bowel syndrome. Dig Dis Sci. 2000;45:248–251. doi: 10.1023/a:1005487821063. [DOI] [PubMed] [Google Scholar]
- 26.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunction. Trends in Pharmacol Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- 27.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13. doi: 10.1016/j.autneu.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaenig W. In: The Integrative Action of the Autonomic Nervous System. Jaenig W, editor. Cambridge Univ. Press; New York: 2006. pp. 317–318. [Google Scholar]
- 29.Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol. 1974;69:1–51. [PubMed] [Google Scholar]
- 30.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:585–638. [PubMed] [Google Scholar]
- 31.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2010;343:23–32. doi: 10.1007/s00441-010-1050-0. A comprehensive overview of evidence for interactions between peripheral stress mediators and bidirectional interactions of the mucosa and intestinal microbiota. [DOI] [PubMed] [Google Scholar]
- 32.Hori T, Katafuchi T, Take S, Shimizu N, Niijima A. The autonomic nervous system as a communication channel between the brain and the immune system. Neuroimmunomodulation. 1995;2:203–215. doi: 10.1159/000097198. [DOI] [PubMed] [Google Scholar]
- 33.Gopal R, Birdsell D, Monroy FP. Regulation of toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 2008;30:563–576. doi: 10.1111/j.1365-3024.2008.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powley TL, et al. In: Brain–Gut Interactions. Tache Y, Wingate D, editors. CRC Press; Boston: 1991. pp. 73–82. [Google Scholar]
- 35.Stephens RL, Tache Y. Intracisternal injection of a TRH analogue stimulates gastric luminal serotonin release in rats. Am J Physiol. 1989;256:G377–G383. doi: 10.1152/ajpgi.1989.256.2.G377. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Almy TP, Kern F, Tulin M. Alterations in colonic function in man under stress. II: experimental production of sigmoid spasm in healthy persons. Gastroenterology. 1949;12:425–436. [PubMed] [Google Scholar]
- 38.Welgan P, Meshkinpour H, Beeler P. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology. 1988;94:1150–1156. doi: 10.1016/0016-5085(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 39.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann NY Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 40.Khasar SG, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. The first evidence that psychosocial stressors can modulate the phenotype of afferent neurons, providing the possible mechanisms for stress induced hyperalgesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seminowicz DA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood JD. In: Physiology of the Gastrointestinal Tract. Johnson LR, editor. Raven; New York: 1987. pp. 67–110. [Google Scholar]
- 43.Kurokawa K, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clerc N, Furness JB. Intrinsic primary afferent neurones of the digestive tract. Neurogastroenterol Motil. 2004;16:24–27. doi: 10.1111/j.1743-3150.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta JN, Gebhart GF. In: Physiology of the Gastrointestinal Tract. Johnson LR, editor. Raven; New York: 1994. pp. 483–519. [Google Scholar]
- 46.Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 47.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu |Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 48.Grundy D, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 49.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 50.Lynn P, Zagorodnyuk V, Hennig G, Costa M, Brookes S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J Physiol. 2005;564:589–601. doi: 10.1113/jphysiol.2004.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders KM. Interstitial cells in smooth muscles. Review series. J Cell Mol Med. 2010;14:1197–1198. doi: 10.1111/j.1582-4934.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci. 2009;153:58–68. doi: 10.1016/j.autneu.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci. 2011;5:16. doi: 10.3389/fnins.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80. doi: 10.1016/j.autneu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Brierley SM, Jones RC, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nature Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol. 2010;300:G170–G180. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 59.de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011 Mar 2; doi: 10.1016/j.physbeh.2011.02.040. A review of interactions between intestinal microflora, mucosal inflammation and altered cholecystokinin–vagal interactions in high fat induced obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept. 2009;155:6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 61.de Lartigue G, et al. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2009;138:1479–1490. doi: 10.1053/j.gastro.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barbara G, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2009;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. A comprehensive review of the role of the gut-based serotonin signalling system in health and its possible role in gastrointestinal disease. [DOI] [PubMed] [Google Scholar]
- 65.McLaughlin JT, et al. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol. 1998;513:11–18. doi: 10.1111/j.1469-7793.1998.011by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liou AP, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007;7:557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nature Rev Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leslie FC, et al. Plasma cholecystokinin concentrations are elevated in acute upper gastrointestinal infections. QJM. 2003;96:870–871. doi: 10.1093/qjmed/hcg140. [DOI] [PubMed] [Google Scholar]
- 70.McDermott JR, et al. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut. 2006;55:492–497. doi: 10.1136/gut.2005.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and α-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 72.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 73.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 74.Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009;21:481–491. doi: 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- 76.Craig AD. An ascending general homeostatic afferent pathway originating in lamina I. Prog Brain Res. 1996;107:225–242. doi: 10.1016/s0079-6123(08)61867-1. [DOI] [PubMed] [Google Scholar]
- 77.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 78.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 79.Craig A. In: Handbook of Emotions. 3. Lewis M, Haviland-Jones JM, editors. Guilford Publications; New York: 2008. pp. 272–288. [Google Scholar]
- 80.Craig AD. How do you feel-now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 81.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; New York, New York: 1999. [Google Scholar]
- 82.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. A comprehensive quantitative meta-analysis of published neuroimaging studies involving insula activation, providing the best evidence to date for function-specific subregions of the human insula. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mutschler I, et al. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 84.Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- 85.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 86.Cauda F, et al. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. A detailed characterization of resting state connectivity of insula subregions, supporting the concept of function-specific networks related to ventral and dorsal subregions of the anterior insular cortex. [DOI] [PubMed] [Google Scholar]
- 87.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 88.Small DM. Central gustatory processing in humans. Adv Otorhinolaryngol. 2006;63:191–220. doi: 10.1159/000093761. [DOI] [PubMed] [Google Scholar]
- 89.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. A comprehensive review of current biological concepts related to eating disorders, with an emphasis on altered processing and modulation of intereoceptive signals. [DOI] [PubMed] [Google Scholar]
- 90.Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288–289. doi: 10.1136/gut.2010.226779. [DOI] [PubMed] [Google Scholar]
- 91.Bercik P, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. The first demonstration of an important role of intestinal inflammation in brain signalling systems and associated behavioural changes in rodents. [DOI] [PubMed] [Google Scholar]
- 92.Heijtz RD, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. The first demonstration of an important role of intestinal microbiota early in life in brain development and adult anxiety-like behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 94.Van Oudenhove L, et al. Emotional modulation of fatty acid induced gut-brain signalling in brainstem, subcortical and cortical regions: an fMRI study. Gastroenterology. 2010;138:S-45. The first demonstration in humans of an interaction between subliminal gut stimuli and experimentally induced emotional states. [Google Scholar]
- 95.Berman SM, et al. Abnormal CNS response to anticipation of visceral distension in female patients with irritable bowel syndrome (IBS) an FMRI study. Gastroenterology. 2006;130:A-78. [Google Scholar]
- 96.Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 97.Wicker B, et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 98.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 99.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973:254–278. [PubMed] [Google Scholar]
- 100.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119:210–218. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- 103.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. An elegant demonstration of the involvement of ventral and dorsal anterior insula subregions in risk prediction and error correction, supporting the concept of function-specific insula subregions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ploghaus A, et al. Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci USA. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 106.Allman JM, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- 107.Gershon MD. Review article: serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 108.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 109.la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci USA. 2005;102:7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maier SF, Watkins LR. Cytokines for psychologists: implications and bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 111.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20:73–80. doi: 10.1111/j.1365-2982.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 113.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 114.Dunn AJ. Mechanisms by which cytokines signal the brain. Int Rev Neurobiol. 2002;52:43–65. doi: 10.1016/s0074-7742(02)52005-5. [DOI] [PubMed] [Google Scholar]
- 115.D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factorα signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 118.Drossman DA, et al., editors. ROME III: The Functional Gastrointestinal Disorders. Degnon Associates; McLean, Virginia: 2006. [Google Scholar]
- 119.Longstreth GF, et al. In: ROME III: The Functional Gastrointestinal Disorders. Drossman DA, et al., editors. Degnon Associates; McLean, Virginia: 2006. pp. 487–556. [Google Scholar]
- 120.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358:1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tillisch K, Labus JS. Advances in imaging the brain-gut axis: functional gastrointestinal disorders. Gastroenterology. 2011;140:407–411e1. doi: 10.1053/j.gastro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayer EA, et al. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 125.Mayer EA, Bushnell MC. In: Functional Pain Syndromes: Presentation and Pathophysiology. Mayer EA, Bushnell MC, editors. IASP Press; Seattle: 2009. pp. 531–565. [Google Scholar]
- 126.Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;194:31–74. doi: 10.1007/978-3-540-79090-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 128.Verma-Gandhu M, et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358–364. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang L, et al. Differences in brain responses to rectal distension between patients with inflammatory and functional GI disorders. Gastroenterology. 2004;126:A-106. [Google Scholar]
- 130.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 131.Tache Y, Bernstein CN. Evidence for the role of the brain-gut axis in inflammatory bowel disease: depression as cause and effect? Gastroenterology. 2009;136:2058–2061. doi: 10.1053/j.gastro.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghia JE, et al. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288.e1–4. doi: 10.1053/j.gastro.2009.02.069. An interesting study demonstrating the interaction of stress, emotional states and gut inflammation. [DOI] [PubMed] [Google Scholar]
- 133.Fairburn CJ, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- 134.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. An excellent review of data and concepts related to food addiction and obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Padwal R, et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev. 2011 Mar 28; doi: 10.1111/j.1467-789X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 137.Paulino G, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:e898–e903. doi: 10.1152/ajpendo.90796.2008. An interesting study that demonstrates the molecular mechanisms underlying fat induced phenotypic changes in vagal afferents, which may play a part in the decreased influences of interoceptive mechanisms in diet-induced obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 139.Grupe DW, Nitschke JB. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 2011;11:413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sarinopoulos I, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sutton RS, Barton AG. Reinforcement Learning: An Introduction. MIT press; Cambridge, USA: 1998. [Google Scholar]
- 142.Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nature Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. An elegant study demonstrating how human subjects assess uncertainty and adjust their decision making accordingly. The study indentifies a key role of brain responses of the anterior cingulate cortex in this process. [DOI] [PubMed] [Google Scholar]
- 143.Ganfornina MD, Sanchez D, Bastiani MJ. Embryonic development of the enteric nervous system of the grasshopper Schistocerca americana. J Comp Neurol. 1996;372:581–596. doi: 10.1002/(SICI)1096-9861(19960902)372:4<581::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 144.Campbell G, Burnstock G. In: Handbook of Physiology: the Gastrointestinal System. Code CF, editor. American Physiological Society; Washington D. C.: 1968. pp. 2213–2266. [Google Scholar]
- 145.Shimizu H, Koizumi O, Fujisawa T. Three digestive movements in Hydra regulated by the diffuse nerve net in the body column. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:623–630. doi: 10.1007/s00359-004-0518-3. [DOI] [PubMed] [Google Scholar]
- 146.Bullock TH, Horridge GA. Structure and function in the nervous system of invertebrates. W. H. Freeman & Co.; San Francisco and London: 1965. [Google Scholar]
- 147.Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- 148.MacLean PD, Vogt BA, Gabriel M. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhäuser; Boston: 1993. pp. 1–15. [Google Scholar]
- 149.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Phil Trans R Soc Lond. 1996;B 351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]