Abstract
BACKGROUND
Combined oral contraceptives (COCs) reduce levels of androgen, especially testosterone (T), by inhibiting ovarian and adrenal androgen synthesis and by increasing levels of sex hormone-binding globulin (SHBG). Although this suppressive effect has been investigated by numerous studies over many years, to our knowledge no systematic review concerning this issue had been performed. This systematic review and meta-analysis was performed to evaluate the effect of COCs on concentrations of total T, free T and SHBG in healthy women and to evaluate differences between the various types of COCs (e.g. estrogen dose, type of progestin) and the assays used to assess total T and free T.
METHODS
A review of the literature was performed using database searches (MEDLINE, EMBASE and the Cochrane Central Register of Clinical Trials) and all publications (from inception date until July 2012) investigating the effect of COCs on androgen levels in healthy women were considered eligible for selection. Three reviewers were involved in study selection, data extraction and critical appraisal. For the meta-analysis, data on total T, free T and SHBG were extracted and combined using random effects analysis. Additional subgroup analyses were performed to evaluate differences between the various types of COCs (e.g. estrogen dose, type of progestin) and the assays used to assess total T or free T.
RESULTS
A total of 151 records were identified by systematic review and 42 studies with a total of 1495 healthy young women (age range: 18–40 years) were included in the meta-analysis. All included studies were experimental studies and 21 were non-comparative. Pooling of the results derived from all the included papers showed that total T levels significantly decreased during COC use [mean difference (MD) (95% confidence interval, CI) −0.49 nmol/l (−0.55, −0.42); P < 0.001]. Significantly lower levels of free T were also found [relative change (95% CI) 0.39 (0.35, 0.43); P < 0.001], with a mean decrease of 61%. On the contrary, SHBG concentrations significantly increased during all types of COC use [MD (95% CI) 99.08 nmol/l (86.43, 111.73); P < 0.001]. Subgroup analyses revealed that COCs containing 20–25 µg EE had similar effects on total and free T compared with COCs with 30–35 µg EE. In addition, suppressive effects on T levels were not different when comparing different types of progestins. However, subgroup analyses for the estrogen dose and the progestin type in relation to changes in SHBG levels did show significant differences: COCs containing second generation progestins and/or the lower estrogen doses (20–25 µg EE) were found to have less impact on SHBG concentrations.
CONCLUSIONS
The current literature review and meta-analysis demonstrates that COCs decrease circulating levels of total T and free T and increase SBHG concentrations. Due to the SHBG increase, free T levels decrease twice as much as total T. The estrogen dose and progestin type of the COC do not influence the decline of total and free T, but both affect SHBG. The clinical implications of suppressed androgen levels during COC use remain to be elucidated.
Keywords: combined oral contraception, androgens, testosterone, SHBG, systematic review
Introduction
In normo-ovulatory women, testosterone (T) levels (Burger, 2002; Speroff and Fritz, 2005; Haring et al., 2012; Pesant et al., 2012) are reported to vary between 0.42 and 2.94 nmol/l (Haring et al., 2012; Pesant et al., 2012). T arises from three sources in the female. Approximately 50–60% is derived from the peripheral conversion of the pro-hormones androstenedione (AD) and dehydroepiandrosterone (DHEA) and its sulphate (DHEA-S), whereas 25% is secreted by the ovary and 25% by the adrenal gland (Longcope, 1986; Bachmann et al., 2002; Burger, 2002). Around 65–70% of circulating T is bound and inactivated by sex-hormone-binding globulin (SHBG). Most of the remaining 30–35% is bound by albumin and only 0.5–3% represents freely circulating T (free T). Since the binding of T to albumin is rather weak, the free- and albumin-bound T together are defined as the bioavailable T (Dunn et al., 1981; Speroff and Fritz, 2005).
There is much debate regarding adequate methods for measuring total T as well as free T concentrations (Vesper et al., 2008; Legro et al., 2010; Rosner and Vesper, 2010; Vesper and Botelho, 2010; Haring et al., 2012). The most accurate method to assess total T is liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Rosner et al., 2007; Vesper et al., 2009), although even with LC-MS/MS assays variation in precision exists (Legro et al., 2010). The ‘Princeton Consensus Statement’ (Bachmann et al., 2002) recommends equilibrium dialysis as the ‘gold standard’ method to measure free T concentrations. These methods, however, are very expensive, labour intensive and not available to the great majority of the laboratories. Because of their ease and low costs, immunoassays [radioimmunoassay (RIA) and electrochemiluminescence immunoassays (ECLIA)] are most commonly used in clinical practice; however, they are known for their inaccuracy and variability (Taieb et al., 2003; Miller et al., 2004; Groenestege et al., 2012). In particular, immunoassays are affected by cross-reactivity with other steroids (Middle, 2007; Benton et al., 2011) and by high levels of SHBG (Masters and Hahnel, 1989; Wierman et al., 2006). The accuracy of RIA can be improved by the addition of an extraction step or chromatography (Rosner et al., 2007; Groenestege et al., 2012), but these procedures are time consuming. The very low concentrations of free T in female blood (35–700 pmol/l; Speroff and Fritz, 2005) are an additional difficulty in obtaining accurate measurements (Taieb et al., 2003; Groenestege et al., 2012). Therefore, free T is often calculated based on total T, SHBG and albumin concentrations (Södergård et al., 1982; Vermeulen et al., 1999). This method has been demonstrated to be a reliable measure for free T concentrations (Rinaldi et al., 2002; Miller et al., 2004).
Combined oral contraceptives (COCs) reduce androgen levels (Fern et al., 1978; Aden et al., 1998; Wiegratz et al., 2003; Ågren et al., 2011a). In particular, blood levels of T, the most potent circulating androgen in women, decrease by up to 50% (Van der Vange et al., 1990; Coenen et al., 1996; Greco et al., 2007). Three possible underlying mechanisms may be held responsible for this effect: (i) Suppression of ovarian androgen synthesis; (ii) increased SHBG levels and (iii) suppression of adrenal androgen synthesis.
To exert their contraceptive effect, COCs suppress the gonadotrophins (Gaspard et al., 1984; Aden et al., 1998). Low levels of LH result in an inhibition of the LH-dependent synthesis of T by the ovarian theca cells (Speroff and Fritz, 2005; Davison and Bell, 2006). As the suppression of T already occurs before the LH levels drops, it is suggested that the contraceptive steroids may have a direct inhibitory effect on ovarian androgen synthesis (Kuhl et al., 1985; Jung-Hoffman et al., 1988a; Aden et al., 1998).
SHBG is a carrier protein synthesized by the liver. Its synthesis is stimulated by estrogens and inhibited by androgens (Granger et al., 1982; Speroff and Fritz, 2005). The estrogenic component of COCs, ethinyl estradiol (EE), induces hepatic SHBG production in a dose-dependent manner, resulting in elevated circulating blood levels of SHBG (Jung-Hoffmann and Kuhl, 1987; Van der Vange et al., 1990; Hammond et al., 2008; Ågren et al., 2011a). Consequently, more T is bound and inactivated, resulting in lower levels of free T and the free androgen index (which is a measure of bioavailable T, i.e. free and albumin-bound T) (Van der Vange et al., 1990; Coenen et al., 1996; Boyd et al., 2001; Wiegratz et al., 2003; Ågren et al., 2011a). This EE-induced increase of SHBG can be counteracted by the progestin-component of COC, as a result of the androgenicity of the progestin. COCs containing a progestin with androgenic activity [e.g. levonorgestrel (LNG)] will induce a less pronounced increase in SHBG, whereas progestins with concomitant anti-androgenic activity [e.g. cyproterone acetate (CPA)] will lead to higher SHBG levels (Van Kammen et al., 1975; Bergink et al., 1981; Hammond et al., 1984; Van der Vange et al., 1990; Odlind et al., 2002; Ågren et al., 2011a, b).
The third mechanism of action causing lower levels of androgens is the inhibitory effect of COCs on adrenal androgen synthesis (Fern et al., 1978; Madden et al., 1978; Carlström et al., 2002). Several studies have shown that COCs decrease both DHEA and DHEA-S concentrations in blood (Gaspard et al., 1984; Falsetti et al., 1987; Wiegratz et al., 1995; Coenen et al., 1996, Wiegratz et al., 2003; Ågren et al., 2011a). The underlying mechanism is not yet established, but it has been suggested that a reduced release of adrenocorticotrophic hormone (ACTH) and increased levels of cortisol during COC use may account for the reduced concentrations of adrenal androgens (Carr et al., 1979; Klove et al., 1984; Carlström et al., 2002). Consistent with this, ACTH is known to stimulate adrenal androgen secretion (Rosenfield et al., 1972; Longcope, 1986).
Overall, little attention has been paid to the suppressive effect of COCs on androgens along with its potential clinical implications. Awareness of the significance of androgens for women is increasing, however, and T deficiency is thought to be associated with a broad range of undesired effects including diminished well-being and quality of life, mood changes (depression, irritation, moodiness), loss of energy, cognitive disturbances, interference with optimal sexual functioning, declining muscle mass and strength and lowering of bone density (Bachmann et al., 2002; Traish et al., 2007).
Although the effect of COCs on T has been extensively investigated, to our knowledge no systematic review or meta-analysis has been performed previously. This analysis aims to evaluate the effect of COC on levels of total T, free T and SHBG in healthy women. Additional subgroup analyses have been performed to evaluate differences between the various types of COCs (e.g. estrogen dose and type of progestin) and to examine the influence of different assessment methods of total T and free T.
Methods
This systematic review and meta-analysis has been conducted and reported according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Liberati et al., 2009; Moher et al., 2009). Before the start of the study, the review question and inclusion/exclusion criteria were defined. The strategy of this review was discussed and agreed upon, although an official protocol was not published or registered. The review question was: What is the effect of COC on levels of total T, free T and SHBG in healthy women?
Search strategy
Studies were identified by searches of the following electronic databases with no restrictions in date of publication (i.e. from inception date until July 2012): MEDLINE, EMBASE and the Cochrane Central Register of Clinical Trials. The search strategy was based on synonyms in title and abstract using the following terms: (‘hormonal contracept*’ OR ‘contracept*’ AND ‘steroids’ OR ‘contracept*’ AND ‘pill’) AND (‘androgen*’ OR ‘testost*’). In all searches, limits were set for MeSH (humans and female), publication type (clinical trial) and language (English, German or Dutch).
In addition, a ‘pearl growing’ strategy was employed, whereby, after obtaining the full text articles, the reference lists of all included studies were reviewed for additional publications that could be used in this review.
Selection criteria
All studies investigating the effect of COCs on androgen levels in healthy women were eligible for selection. At least two of the following three outcome parameters had to be reported: total T, free T and SHBG. Exclusion criteria were studies in men or animals, women suffering from acne, polycystic ovary syndrome, hirsutism or endometriosis, (post)menopausal women, no or other types of hormonal contraception (e.g. progestin-only contraceptives, implants, patches, vaginal rings, intrauterine device), COC with a continuous regimen, emergency contraception, treatment duration of less than one cycle (i.e. one cycle is 28 days), other outcome parameters (e.g. cervical mucus, acne, endometriosis) and bioequivalence/bioavailable studies. Reviews, case reports, letters to the editor and conference papers were also excluded.
The results of the searches were screened to meet the pre-defined eligibility criteria. A first selection was performed based on their titles, followed by a second selection performed by two independent reviewers (Y.Z. and W.W.), who reviewed the abstracts of all remaining records. Any disagreement in the selection of abstracts was resolved by consensus or by a third reviewer (B.F.). Full text articles for review and data processing were obtained for all selected abstracts.
Data extraction
Data were extracted from full text articles into a specially designed data extraction form by one reviewer (Y.Z.) in close consultation with another reviewer (M.E.). Any obscurities were discussed and decisions were taken by consensus. During the process of full text review and data extraction, studies were excluded if they did not fulfil the eligibility criteria or if the reported results were in a format that could not be used or were judged to be of insufficient quality. No investigators were contacted in the case of missing or obscure data.
The following data were extracted: authors, year of publication, title, study design, characteristics of the study population (e.g. study criteria, age, body weight/height/BMI, use of previous hormonal contraceptives), number of randomized, early discontinued and completed subjects, details on intervention (e.g. pill composition and regimen, multi- or monophasic, treatment duration and number of treatment groups), study objective, outcome parameters with special attention to the androgens and SHBG, sampling details (e.g. timing in treatment period and cycle day), analytical methods and statistical methods.
The study results [mean and standard deviation (SD)] for total T, free T and SHBG concentrations in International System (SI) units were extracted in a separate standardized form. In a few cases, study results were only presented in figures and data were obtained by measuring directly from these figures that were printed on a large scale. T concentrations reported in conventional units were converted to SI units using the following formula: conversion factor (CF) × conventional unit (e.g. gram) = SI (e.g. mol), where CF for T was 3.467. For the conversion of SHBG concentrations, the molecular weight of SHBG (43 780 g/mol) was used.
The standard error (SE) or SE of the mean (SEM) were converted to SD using the formula: SD = SEM × √n, where n is the sample size. In a few cases, neither the SD nor the SEM was reported. The SD was calculated if the SD (or SEM) was reported for the other time-points in the study using the following formula: SD2 = SD1 × (mean2/mean1), where SD2 is the SD which is unknown, SD1 is the SD reported for another time point in the study, mean2 is the mean that belongs to SD2 and mean1 the mean that belongs to SD1. A 95% confidence interval (CI) was converted to SD using the following formula: (CIhi − CIlo)/((2 × 1.96) × √n), where CIhi is the upper limit, CIlo is the lower limit and n is the sample size. In cases where the change from baseline was reported, the end of treatment value was calculated from the baseline value minus the change from baseline. Finally, when a median plus range of variability was reported instead of a mean plus SD, the mean and SD were calculated by assuming the data had a log-normal distribution.
To prevent extraction errors, a quality control check between the final data used in the meta-analysis and the original publications was performed.
Critical appraisal
The risk of bias for the individual studies included in the meta-analysis was assessed based on guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011). All studies were critically appraised for selection bias (sequence generation, allocation concealment), performance bias, detection bias, attrition bias, reporting bias and other bias by two reviewers (Y.Z. and M.E.), in relation to the outcome parameters used in the meta-analysis. Studies were not excluded from the meta-analysis based on a high risk of bias, but their risk of bias was taken into consideration during the interpretation of the results.
Statistical analysis
Only a few papers reported data from observational studies comparing women using COC with women not using COC (control group) and no specific type of COC was investigated in these studies. The majority of the selected studies were experimental studies investigating the effects of one or more COCs on different outcome parameters. Because an identical control group in these studies could not be identified, the experimental studies which compared the pretreatment study results (i.e. total T, free T and/or SHBG levels) with the end of treatment results were used in the meta-analysis. By using these studies, differences between the various types of COC could also be evaluated. In this context, an important additional inclusion criterion was used; study participants should not have taken a hormonal contraceptive prior to starting the study medication (including a wash-out period).
For the meta-analysis, the inverse variance method with random effects as analysis model was applied. As effect measures, the mean difference (MD) and associated 95% CIs were calculated based on the means of the pretreatment and the end of treatment levels of total T, free T and SHBG. Data of each treatment group were entered separately. For the end of treatment time point, the assessment (mean/SD) after a 6-cycle treatment duration was used for reasons of standardization. In cases where this time point was not reported, the three cycle or the nearest available reported time point was used.
In cases where the data showed large variation (e.g. larger SD in studies with larger mean values, plus identification of a significant heterogeneity in the meta-analysis), logarithms of these values were used to reduce the wide range of values. For the pooled concentrations in logarithmic scale, the generic inverse variance method with random effects was used. As effect measure, the relative change and associated 95% CI from pretreatment to end of treatment was calculated. Heterogeneity between the results of the different studies was examined using the I2 value. Heterogeneity was found to be significant when I2 > 65%.
Subgroup analyses were performed for different estrogen doses (20–25 µg EE versus 30–35 µg EE) and for the type of progestin (first generation (estranes) versus second generation (gonanes) versus third generation (gonanes) versus fourth or unclassified generation) on all outcome parameters. The classification of the progestins was based on their chemical structure combined with the time of introduction.
As the methods to assess total T and free T concentrations could influence the outcome of the meta-analysis, subgroup analyses for the type of assay (RIA/ECLIA/enzyme-linked immunosorbent assay (ELISA) versus RIA with extraction/chromatography) and for the method to determine free T concentrations (direct immunoassay versus calculation or equilibrium dialysis) were performed. In cases where more than two subgroups were included and a significant difference was identified, it was pre-determined which subgroups would undergo separate statistical testing. For the progestin type, an additional comparison was made between second versus third generation or between second versus third generation, only including monophasic COCs containing 30–35 µg EE.
All analyses were performed with Review Manager (RevMan), version 5.2 (2012).
Results
Study selection
A flow chart of the included/excluded studies is shown in Fig. 1. Database searches yielded 904 records from MEDLINE, 929 from EMBASE and 538 from the Cochrane Central Register of Clinical Trials. Following the first review based on the title, 193 records remained (122 from MEDLINE, 39 from EMBASE and 32 from the Cochrane Central Register of Clinical Trials). After removal of duplicate records, 151 records remained and the abstracts were reviewed based on the pre-defined eligibility criteria. A total of 66 records were selected for full text review and data processing. During this phase, 27 papers were excluded, 3 studies were included by hand search and 42 studies were finally included in the meta-analysis. Table I shows the reasons for exclusion. Not all studies reported all three outcome parameters, therefore 39 studies were included in the meta-analysis for COC effect on total T and SHBG and 29 studies were included for the effect on free T.
Figure 1.
Flowchart of the study selection process.
Table I.
Reasons for excluding articles.
| First author, year | Reason |
|---|---|
| Bancroft et al. (1980) | Wrong study population (women with impaired sexual function); baseline comparison problem group versus non-problem group, but only problem group takes part in study phase with prescribed study medication |
| Bergink et al. (1981) | Study results are reported as geometric mean, which could not be converted to mean; no SD or SE reported; unit of Free T (%) could not be converted |
| Spona et al. (1986) | Treatment duration was unclear (i.e. from Day 5 to Day 25 and unclear if 21:7 regimen); not specified if results are reported in mean/SD; not clear if study participants had been using previous steroids prior to starting the study medication; not able to convert the unit of SHBG to SI units |
| Jung-Hoffmann and Kuhl (1987) | Assumption same study as reported by Kuhl et al. (1985); the latter has been used in the meta-analysis, but publication of Jung-Hoffmann and Kuhl (1987) was also used to obtain additional information |
| Jung-Hoffmann et al. (1988b) | The same study was published in an English journal by the same first author (Jung-Hoffmann et al., 1988a). The latter has been used in the meta-analysis, but this publication (Jung-Hoffmann et al., 1988b) was also used to obtain additional information |
| Alexander et al. (1990) | Observational study (COC users versus non-COC users); substudy of Bancroft et al. (1991a, b); various types of COCs were used |
| Van der Vange et al. (1990) | Study results are reported as geometric mean, which could not be converted to mean and SD is not reported. Results are only presented in figures with a low quality for direct measurement |
| Bancroft et al. (1991a) | Observational study (non-COC users versus users); various types of COCs were used; study results reported in two publications Bancroft et al. (1991a, b) |
| Bancroft et al. (1991b) | Observational study (non-COC users versus users); various types of COCs were used; study results reported in two publications Bancroft et al. (1991a, b) |
| Sobbrio et al. (1991) | Not able to obtain a copy of the full text |
| Janaud et al. (1993) | Not able to obtain a copy of the full text; assumption same study as reported by Janaud et al. (1992) |
| Pasinetti and Falsetti (1993) | Not able to obtain a copy of the full text |
| Erdmann et al. (1994) | Wrong study population (women with acne, seborrhoea, hirsutism and alopecia) |
| Coenen et al. (1995) | Not able to obtain a copy of the full text; assumption same study as reported by Coenen et al. (1996) |
| Oettel et al. (1997) | Not able to obtain a copy of the full text |
| Sulak et al. (1999) | Wrong study population (a part of the study population is post-partum [given birth <60 days of screening] and results are not separately reported); units for the lab parameters are not specified |
| Stanczyk et al. (2000) | Not able to obtain a copy of the full text |
| Aliyeva (2002) | Not able to obtain a copy of the full text |
| Endrikat et al. (2002) | Pretreatment values for total T and free T were measured on Day 1 of treatment Cycle 1, which was directly after a 7-day pill-free period. This pill-free period followed the single dose administration part of the study (one tablet on Day 21 of menstrual cycle). This measurement is not a real pretreatment value. In addition, no SD or SEM reported for total T concentrations at pretreatment |
| Oranratanaphan and Taneepanichskul (2006) | Not able to obtain a copy of the full text |
| Graham et al. (2007) | Publication combines results of two studies; results of one of these studies are also reported by Greco et al. (2007). The latter has been used in the meta-analysis, but publication of Graham et al. (2007) was also used to obtain additional information |
| Winkler and Sudik (2009) | Results were judged to be of insufficient quality; SHBG converted, but after conversion values do not seem to be correct; all measurements for free T values during treatment are 2.08 pmol/l, therefore no SD can be reported (assumed that LOQ of assay was 2.08 pmol/l, therefore free T concentrations could not be measured and the LOQ has been reported. |
| Schaffir et al. (2010) | Observational study and wrong study population (women are using a COC at pretreatment) |
| Heiman et al. (2011) | Observational study; no study medication; comparison between women with a normal sexual function and women with Hypoactive Sexual Desire Disorder of which half of the group was using hormonal contraception |
| Sanam and Ziba (2011) | Results were found to be of insufficient quality (e.g. not reported if results are expressed as mean/SD; seems that reported SD is sometimes the mean and vice versa [see body weight] and wrong numbers reported (e.g. baseline acne). |
| Elaut et al. (2012) | Study results are reported in boxplots from which no mean could be retrieved; no pretreatment assessment for COC-only group reported |
| Haring et al. (2012) | Observational study; no study medication; study was performed to establish age-specific reference ranges for serum sex hormone concentrations in women (without and with hormonal contraception) using mass spectrometry (LC-MS/MS method) |
Characteristics of included studies
Characteristics of included studies are reported in Table II. All 42 studies were experimental with one or more treatment groups. Only three studies used a double-blind or single-blind design (Wiegratz et al., 2003; Greco et al., 2007; Legro et al., 2008). There were 21 studies which were non-comparative, whereas 16 studies compared two COCs and 5 studies compared 3 or more different types of COCs. Various types of COCs were investigated including both monophasic and multiphasic regimens. Almost all included studies investigated the effects of a COC with a 21:7 regimen, except for two studies that both included a COC with a 24:4 regimen (Duijkers et al., 2010; Ågren et al., 2011a). Most of the studies included COCs containing EE as the estrogenic component, but four studies (Wiegratz et al., 2003; Duijkers et al., 2010; Ågren et al., 2011a; Caruso et al., 2011) also investigated the effects of a COC with estradiol (E2) or E2 valerate. These treatments often did not qualify for the subgroup analyses. The duration of treatment varied from 1 treatment cycle (Kuhnz et al., 1991) up to 12 treatment cycles (Jung-Hoffman et al., 1988a; Åkerlund et al., 1994; Wiegratz et al., 1995; Sänger et al., 2008). Treatment was assessed for 6 cycles (the effect measure used for the meta-analysis) in 16 studies, 3 cycles in 14 studies and for 12 studies another time point had to be taken for the analyses.
Table II.
Details of studies included in meta-analysis.
| First author, year | Study design | Study population | Characteristics per treatment group |
Study treatment (duration, composition and regimen) | End-points used in meta-analysis | Details hormone assessment (type of assay or calculation) |
Domains with low risk of bias (n)1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Total testosterone | Free testosterone | ||||||||
| Cullberg et al. (1982) | Randomized, comparative, parallel group | Healthy women 18–36 years regular menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 10 Mean age: NR Weight/BMI: NR |
3 cycles 30 EE/150DSG 21:7 |
Control cycle (day NR) Tr. cycle 3 (Day 21) |
Extraction/chromatography RIA Intra-assay CV: <6% Inter-assay CV: <10% LLQ: NR |
Not applicable | 5 |
| 2 |
n = 10 Mean age: NR Weight/BMI: NR |
3 cycles 30 EE/150 LNG 21:7 |
|||||||
| Granger et al. (1982)2 | Comparative, parallel group | Healthy women 20–30 years normal menstrual cycle No OC: not specified, but only normally cycling women were included |
1 |
n = 5 Mean age: NR Weight/BMI: NR |
2 cycles 30 EE/300NG 21:7 |
Control cycle (early proliferative phase and late luteal phase) Tr. Cycle 2 (Days 18–21) |
Extraction/chromatography RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Not applicable | 4 |
| 2 |
n = 5 Mean age: NR |
2 cycles 35 EE/400NET 21:7 |
|||||||
| Vermeulen and Thiery (1982) | Non-comparative | Healthy women 20–23 years Normal menstrual cycle No OC: ≥2 months |
1 |
n = 12 Mean age: NR Weight/BMI: NR |
6 cycles 30-40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
Control cycle (NR) Tr. cycle 6 (Days 18–21) |
RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Equilibrium dialysis (measuring free fraction which is used to calculate the AFTC (Vermeulen et al., 1971) | 6 |
| Gaspard et al. (1983) | Comparative, parallel group | Healthy women No OC: ≥8 weeks |
1 |
n = 13 Mean age: 22.75 years Weight: NR (normal body weight) |
6 cycles 30-40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
Control cycle (7 days before presumed menses) Tr. cycle 6 (Days 19–21) |
Extraction/chromatography RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
AFTC; calculated based on total T, SHBG and albumin (Vermeulen et al., 1971) | 5 |
| 2 |
n = 13 Mean age: 22.75 years Weight: NR (normal body weight) |
6 cycles 50–50 EE/0-125DSG (7–14), 21:7 |
|||||||
| 3 |
n = 13 Mean age: 22.75 years Weight: NR (normal body weight) |
6 cycles 30 EE/150DSG 21:7 |
|||||||
| Gaspard et al. (1984) | Comparative, parallel group | Healthy women No OC: ≥8 weeks |
1 |
n = 22 Mean (SE) age: 24.4 ± 1.6 years Mean (SE) weight: 57.6 ± 1.6 kg |
9 cycles 50 EE/250 LNG 21:7 |
Control cycle (7 days before presumed menses) Tr. cycle 6 (Days 19–21) |
Extraction/chromatography RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
AFTC; calculated based on total T, SHBG and albumin (Vermeulen et al., 1971) | 5 |
| 2 |
n = 18 Mean (SE) age: 20.9 ± 1.8 years Mean (SE) weight: 57.1 ± 1.8 kg |
9 cycles 30-40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
|||||||
| Hammond et al. (1984) | Comparative, parallel group | Healthy women 20–36 years Regular menstrual cycle No OC: ≥3 months |
1 |
n = 10 Mean age: NR Weight/BMI: NR |
3 cycles 30 EE/150DSG 21:7 |
Control cycle (last few days) Tr. cycle 3 (Days 18–21) |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Not applicable | 5 |
| 2 |
n = 10 Mean age: NR Weight/BMI: NR |
3 cycles 30 EE/150 LNG 21:7 |
|||||||
| Siegberg et al. (1984)2 | Open-label, non-comparative | Adolescent girls Regular menstrual cycle 15–20 years No hormonal contraceptives: ≥12 months |
1 |
n = 10 Mean (SD) age: 17.0 ± 1.5 years Mean (SD) weight: 54.7 ± 4.6 kg |
6 cycles 50 EE/1 mg lynestrenol OR 35 EE/0.8 mg lynestrenol 21:7 |
Control cycle (>Day 12; 1×/week) Tr. cycle 6 (2 times) |
RIA Intra-assay CV: 6% Inter-assay CV: 12% LLQ: NR |
RIA Intra-assay CV: 6% Inter-assay CV: 12% LLQ: NR |
6 |
| Kuhl et al. (1985)3 | Randomized, crossover, comparative, parallel group | Healthy women 24–35 years No OC: ≥3 months Control cycle: confirmed ovulation |
1 |
n = 22 Mean age: NR Weight/BMI: NR |
9 cycles 30-40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
Control cycle (Day 21) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: 7.2% Inter-assay CV: 8.1% LLQ: 0.87 nmol/l |
Direct RIA Intra-assay CV: 3.8% Inter-assay CV: 4.2% LLQ: 5.2 pmol/l |
5 |
| 2 |
n = 22 Mean age: NR Weight/BMI: NR |
9 cycles 30 EE/150 DSG 21:7 |
|||||||
| Falsetti et al. (1987) | Open-label, non-comparative, non-controlled | Healthy women menstrual cycle 22–38 years No hormonal contraceptives: ≥3 months |
1 |
n = 39 Mean (SD) age: 30.1 ± 4.5 years Mean (SD) weight: 56.4 ± 7.7 kg |
6 cycles 20 EE/150DSG 21:7 |
Control cycle (7 days before presumed menses) Tr. cycle 6 (day NR) |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
7 |
| Jung-Hoffmann et al. (1988a) | Randomized, comparative, parallel group | Healthy women 18–35 years Regular menstrual cycle No OC: ≥3 months Control cycle: ovulation confirmed |
1 |
n = 11 Mean age: NR Weight/BMI: NR |
12 cycles 30 EE/75 GSD 21:7 |
Control cycle (Day 21) Tr. cycle 6 (Day 21) |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
4 |
| 2 |
n = 11 Mean age: NR Weight/BMI: NR |
12 cycles 30 EE/150 DSG 21:7 |
|||||||
| Murphy et al. (1990) | Prospective, non-comparative | Healthy women 18–35 years normal menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 33 Mean age: NR Weight/BMI: NR |
9 cycles 35 EE/1000NET 21:7 |
Control cycle (Days 1–5) Tr. cycle 6 (Days 1–5) |
Direct RIA Intra-assay CV: 1.5% Inter-assay CV: NR LLQ: 11 ng/dl (0.38 nmol/l)4 |
Not applicable | 7 |
| Refn et al. (1990) | Randomized, comparative, parallel group | Healthy women 19–36 years Regular menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 17 Mean age: 24.7 years Weight/BMI: NR |
6 cycles 30–40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
Control cycle (luteal phase, Days 24–26) Tr. cycle 6 (Days 18–20) |
Direct RIA Intra-assay CV: 6% Inter-assay CV: 10% LLQ: NR |
Not applicable | 5 |
| 2 |
n = 16 Mean age: 24.7 years Weight/BMI: NR |
6 cycles 30-40-30 EE/50-70-100 GSD (6-5-10), 21:7 |
|||||||
| De Leo et al. (1991) | Open-label, non-comparative, non-controlled | Healthy women 17–25 years No hormonal contraceptives: not specified, but pretreatment samples were collected during follicular phase, so assumption no OC prior IP |
1 |
n = 10 Mean age: NR Weight/BMI: NR |
6 cycles 20 EE/150 DSG 21:7 |
Control cycle (Days 8–10) Tr. cycle 6 (Days 16–19) |
Not applicable | RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
7 |
| Kuhnz et al. (1991) | Open-label, non-comparative | Healthy women 19–34 years normal menstrual cycle No hormonal contraceptives: ≥2 months |
1 |
n = 10 Mean (SD) age: 31.7 ± 6.0 years Mean (SD) weight: 59.9 ± 4.5 kg |
1 cycle 30-40-30 EE/50-70-100 GSD (6-5-10), 21:7 |
Control cycle (Day 21) Tr. cycle 1 (Day 21) |
Direct RIA Intra-assay CV: NR Inter-assay CV: <10% LLQ: 0.1 ng/mL (0.3 nmol/l) |
Direct RIA Intra-assay CV: NR Inter-assay CV: <10% LLQ: 0.2 pg/ml (0.7 pmol/l) |
7 |
| Janaud et al. (1992) | Randomized, comparative, parallel group | Healthy women 18–38 years Regular menstrual cycle No hormonal contraceptives: not specified, but only women with regular menstrual cycle were included |
1 |
n = 34 Mean (SD) age: 24.7 ± 4.6 years Mean (SD) weight: 55.6 ± 6.5 kg |
6 cycles 35 EE/180-215-250 NGM 21:7 |
Control cycle (follicular phase) Tr. cycle 6 (Days 1–6 and Days 14–28) |
Not reported Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Equilibrium dialysis Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
5 |
| 2 |
n = 32 Mean (SD) age: 26.1 ± 6.0 years Mean (SD) weight: 55.67 ± 6.1 kg |
6 cycles 30-40-30 EE/50-75-125 LNG 21:7 |
|||||||
| Song et al. (1992)5 | Randomized, comparative, parallel group, crossover | Healthy women Regular menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 12 Mean (SD) age: 32.2 ± 2.4 years Mean (SD) weight: 52.8 ± 4.8 kg |
3 cycles 30 EE/150 DSG 21:7 |
Control cycle (follicular phase, Days 6–9; luteal phase, Days 21–24) Tr. cycle 3 (Day 21) |
RIA Intra-assay CV: <10% Inter-assay CV: <10% LLQ: NR |
Not applicable | 4 |
| 2 |
n = 12 Mean (SD) age: 32.2 ± 2.4 years Mean (SD) weight: 52.8 ± 4.8 kg |
3 cycles 20 EE/150 DSG 21:7 |
|||||||
| 3 |
n = 12 Mean (SD) age: 32.2 ± 2.4 years Mean (SD) weight: 52.8 ± 4.8 kg |
3 cycles 30 EE/150 LNG 21:7 |
|||||||
| Kuhl et al. (1993) | Non-comparative | Healthy women 20–29 years Regular menstrual cycle No hormonal contraceptives: ≥3 months Control cycle: ovulation confirmed |
1 |
n = 19 Mean age: NR Weight/BMI: NR |
6 cycles 40–30 EE/25–125 DSG (7–15), 21:7 |
Control cycle (Days 21–24) Tr. cycle 6 (Days 18–22) |
Direct RIA Intra-assay CV: 7.6% Inter-assay CV: 8.2% LLQ: NR |
Direct RIA Intra-assay CV: 3.8% Inter-assay CV: 4.2% LLQ: NR |
7 |
| Kuhnz et al. (1993a) | Open-label, non-comparative |
Healthy women 18–32 years No OC: ≥2 months |
1 |
n = 14 Mean (SD) age: 27 ± 5 years Mean (SD) weight: 66 ± 6 kg |
3 cycle 30-40-30 EE/50-70-100 GSD (6-5-10), 21:7 |
Control cycle (day NR) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: 8% Inter-assay CV: 8% LLQ: NR |
Direct RIA Intra-assay CV: 4% Inter-assay CV: 8–14% LLQ: NR |
7 |
| Kuhnz et al. (1993b) | Open-label, non-comparative | Healthy women normal menstrual cycle No hormonal contraceptives: ≥2 months |
1 |
n = 15 Mean (SD) age: 26 ± 6 years Mean (SD) weight: 63 ± 7 kg |
3 cycles 35 EE/2 mg CPA 21:7 |
Control cycle (day NR) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: NR Inter-assay CV: 8–12% LLQ: NR |
Direct RIA Intra-assay CV: NR Inter-assay CV: 5–9% LLQ: NR |
7 |
| Åkerlund et al. (1994) | Randomized, double-blind, comparative, parallel group | Healthy women 18–40 years No OC: ≥2 months |
1 |
n = 25 Mean age: 25.0 years Weight/BMI: NR |
12 cycles 20 EE/150 DSG 21:7 |
Control cycle (Days 18–21) Tr. cycle 6 (Days 18–21) |
Direct RIA CV: 6.5–8.9% at 3 nmol/l Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Calculated based on total T, SHBG and albumin (Levell et al., 1987) CV: 8.5–12.6% at 50 pmol/l |
6 |
| 2 |
n = 26 Mean age: 21.8 years Weight/BMI: NR |
12 cycles 30 EE/150 DSG 21:7 |
|||||||
| Kuhnz et al. (1994) | Open-label, non-comparative | Healthy women No OC: ≥2 months |
1 |
n = 14 Mean (SD) age: 23 ± 3 years Mean (SD) weight: 61 ± 8 kg |
3 cycles 30–40-30 EE/50-75-125 LNG (6-5-10), 21:7 |
Control cycle (day NR) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: <10% Inter-assay CV: 4–8% LLQ: NR |
Direct RIA Intra-assay CV: <10% Inter-assay CV: 7–8% LLQ: NR |
7 |
| Volpe et al. (1994)2 | Open-label, non-comparative |
Healthy women 17–35 years normal menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 30 Mean (SD) age: 23.2 ± 5.21 years Weight/BMI: NR |
9 cycles 40-25 EE/30-125 DSG (7–15), 21:7 |
Control cycle (Day 22) Tr. cycle 6 (Day 22) |
Direct RIA Intra-assay CV: 6.5% Inter-assay CV: 8.4% LLQ: 0.1 ng/mL (0.3 nmol/l) |
Direct RIA Intra-assay CV: 3.5% Inter-assay CV: 4.5% LLQ: 0.15 pg/ml (0.5 pmol/l) |
7 |
| Heuner et al. (1995) | Open-label, non-comparative | Healthy women No OC: ≥2 months |
1 |
n = 14 Mean (SD) age: 27.9 ± 3.9 years Mean (SD) weight: 65.5 ± 11.8 kg |
3 cycles 20 EE/75 GSD 21:7 |
Control cycle (Day 21) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: <10% Inter-assay CV: 4–8% LLQ: NR |
Direct RIA Intra-assay CV: <10% Inter-assay CV: 7–8% LLQ: NR |
7 |
| Moutos et al. (1995) | Randomized, comparative, parallel group | Healthy women 18–35 years Regular menses (every 25–35 days) No OC: ≥12 months |
1 |
n = 20 Mean age: 25.8 years BMI: 21.7 kg/m2 |
6 cycles 50 EE/1 mg NET 21:7 |
Control cycle (Day 21) Tr. cycle 6 (Day 21) |
Direct RIA Intra-assay CV: 5% Inter-assay CV: NR LLQ: 4 ng/dl (0.1 nmol/l) |
Not applicable | 7 |
| 2 |
n = 20 Mean age: 23.5 years BMI: 21.5 kg/m2 |
6 cycles 35 EE/1 mg NET 21:7 |
|||||||
| 3 |
n = 20 Mean age: 24.3 years BMI: 21.4 kg/m2 |
6 cycles 35 EE/0.5 mg NET 21:7 |
|||||||
| Wiegratz et al. (1995) | Comparative, parallel group | Healthy women 18–36 years Regular menstrual cycle No hormonal contraceptives: ≥3 months Control cycle: ovulation confirmed |
1 |
n = 26 Mean age: NR Weight/BMI: NR |
12 cycles 30-40-30 EE/50-70-100 GSD (6-5-10), 21:7 |
Control cycle (Day 21) Tr. cycle 6 (Day 21) |
Direct RIA Intra-assay CV: 7.6% Inter-assay CV: 8.2% LLQ: 0.08 ng/mL (0.3 nmol/l) |
Direct RIA Intra-assay CV: 3.8% Inter-assay CV: 4.2% LLQ: 0.15 pg/ml (0.5 pmol/l) |
5 |
| 2 |
n = 26 Mean age: NR Weight/BMI: NR |
12 cycles 35 EE/250 NGM 21:7 |
|||||||
| Coenen et al. (1996) | Randomized, open-label, comparative, parallel group | Healthy women 18–38 years Regular menstrual cycle No hormonal contraceptives: ≥2 months |
1 |
n = 25 Mean (SD) age: 26.9 ± 4.2 years Mean BMI: 22.2 ± 2.3 kg/m2 |
6 cycles 35 EE/250 NGM 21:7 |
Control cycle (luteal phase, Days 24–27) Tr. cycle 6 (Days 18–21) |
Extraction/chromatography RIA Intra-assay CV: 5.6% Inter-assay CV: 6.9% LLQ: NR |
Direct RIA Intra-assay CV: 6.4% Inter-assay CV: 12.1% LLQ: NR |
5 |
| 2 |
n = 25 Mean (SD) age: 26.3 ± 4.9 years Mean (SD) weight: 22.2 ± 2.4 kg |
6 cycles 30 EE/75 GSD 21:7 |
|||||||
| 3 |
n = 25 Mean (SD) age: 27.1 ± 5.1 years Mean (SD) weight: 22.2 ± 2.4 kg |
6 cycles 30 EE/150 DSG 21:7 |
|||||||
| 4 |
n = 25 Mean (SD) age: 27.3 ± 5.3 years Mean (SD) weight: 22.4 ± 2.2 kg |
6 cycles 20 EE/150 DSG 21:7 |
|||||||
| Spona et al. (1996) | Non-comparative | Healthy women 20–34 years normal menstrual cycle No hormonal contraceptives: not specified, but women had to have a normal menstrual cycle |
1 |
n = 24 Mean (SD) age: 27.5 ± 4.3 years Weight/BMI: NR |
3 cycles 20 EE/100 LNG 21:7 |
Control cycle (day 20) Tr. cycle 3 (Day 20) |
Direct RIA Intra-assay CV: 5–9% Inter-assay CV: 5–9% LLQ: NR |
Direct RIA Intra-assay CV: 5–9% Inter-assay CV: 5–9% LLQ: NR |
7 |
| Aden et al. (1998) | Randomizsed, crossover, comparative | Healthy women 21–32 years Regular menstrual cycle No OC: ≥2 months control cycle: ovulation confirmed |
1 |
n = 29 Mean age: NR Weight/BMI: NR |
3 cycles 30-40-30 EE/50-75-150 LNG (6-6-9), 21:7 |
Control cycle (Day 21) Tr. cycle 3 (Day 21) |
Direct RIA Intra-assay CV: 5.1% Inter-assay CV: 10.4% LLQ: 0.2 ng/ml (0.7 nmol/l) |
Direct RIA Intra-assay CV: 3.8% Inter-assay CV: 4.2% LLQ: 0.6 pg/ml (2.1 pmol/l) |
7 |
| 2 |
n = 26 Mean age: NR Weight/BMI: NR |
3 cycles 30-40-30 EE/50-75-150 LNG (6-5-10), 21:7 |
|||||||
| Thorneycroft et al. (1999) | Randomized, open-label, comparative, parallel group | Healthy women 18–28 years Regular menstrual cycle No hormonal contraceptives: ≥3 months |
1 |
n = 27 Mean (SD) age: 22.9 ± 2.8 years Mean (SD) weight: 74.9 ± 25.0 kg |
3 cycles 20 EE/100 LNG 21:7 |
Control cycle (day NR) Tr. cycle 3 (Days 17–21) |
Extraction/chromatography RIA Intra-assay CV: 5–10% Inter-assay CV: 10–15% LLQ: NR |
Not applicable | 5 |
| 2 |
n = 25 Mean (SD) age: 22.5 ± 3.0 years Mean (SD) weight: 67.5 ± 16.2 kg |
3 cycles 20 EE/1 mg NETA (plus ferrous fumarate on non-hormonal days, Loestrin®) 21:7 |
|||||||
| Boyd et al. (2001)6 | Non-randomized, open-label, non-comparative | Healthy women 20–39 years Regular menstrual cycle No hormonal contraceptives: ≥6 months (implants/OC) or ≥12 months (injectables) |
1 |
n = 17 Mean age: NR Weight range: 52.6–141 kg |
3 cycles 20-30-35/1 mg NETA (5-7-9), 21:7 |
Control cycle (Days 3, 4, 5, 10, 11, 12, 19, 20 and 21) Tr. cycle 3 (Day 21) |
Extraction/chromatography RIA Intra-assay CV (%RSD): <8.1% Inter-assay CV (%RSD): <13.4% LLQ: 3 ng/dL (0.1 nmol/l) |
Equilibrium dialysis Intra-assay CV (%RSD): <6.86% Inter-assay CV (%RSD): <10.9% LLQ: 0.1% free |
5 |
| Wiegratz et al. (2003) | Randomized, double-blind, comparative, parallel group | Healthy women 18–35 years Regular menstrual cycle No hormonal contraceptives: ≥4 weeks Control cycle: ovulation confirmed |
1 |
n = 25 Mean age: NR Weight/BMI: NR |
6 cycles 30 EE/2 mg DNG 21:7 |
Control cycle (Days 21–26) Tr. cycle 6 (Days 18–21) |
Not applicable | Direct RIA Intra-assay CV: 23.7% Inter-assay CV: 24.1% LLQ: 0.15 pg/ml (0.5 pmol/l) |
5 |
| 2 |
n = 25 Mean age: NR Weight/BMI: NR |
6 cycles 20 EE/2 mg DNG 21:7 |
|||||||
| 3 |
n = 25 Mean age: NR Weight/BMI: NR |
6 cycles 10 EE/E2 V/2 mg DNG, 21:7 |
|||||||
| 4 |
n = 25 Mean age: NR Weight/BMI: NR |
6 cycles 20 EE/100 LNG 21:7 |
|||||||
| Rickenlund et al. (2004)2 | Open-label, non-comparative | Healthy women 16–35 years Regular menstrual cycle No hormonal contraceptives: not specified, but only women with regular menstrual cycles were included |
1 |
n = 12 Mean (SD) age: 20.9 ± 4.2 years BMI: 19.9 ± 1.4 kg/m2 |
10 cycles 30 EE/150 LNG 21:7 |
Control cycle (day NR) Tr. cycle 10 (day NR) |
Direct RIA Intra-assay CV: 6% Inter-assay CV: 10% LLQ: 0.1 nmol/l |
Calculated based on total T, SHBG and fixed albumin [40 g/l] (Södergård et al., 1982) |
7 |
| White et al. (2005)2 | Randomized, comparative, parallel group | Healthy women 18–40 years regular menstrual cycle (25–35 days) No hormonal contraceptives: ≥3 months |
1 |
n = 9 Mean (SD) age: 25.1 ± 2.8 years BMI: 21.8 ± 3.4 kg/m2 |
3 cycles 35 EE/250 NGM 21:7 |
Control cycle (day NR) Tr. cycle 3 (Days 17–21) |
Extraction/chromatography RIA Intra-assay CV: 4–7% Inter-assay CV: 9–12% LLQ: NR |
Calculated based on total T, SHBG and albumin (Vermeulen et al., 1999) |
5 |
| Elkind-Hirsch et al. (2007)2 | Randomized, prospective, comparative, parallel group | Healthy women 18–40 years No hormonal contraceptives: ≥2 months control cycle: ovulation confirmed |
1 |
n = 31 Mean (SD) age: 29.5 ± 4.7 years BMI: 29.5 ± 6.8 kg/m2 |
5 cycles 20 EE/100 LNG 21:7 |
Control cycle (Days 2–5) Tr. cycle 5 (Days 8–21) |
ECLIA Intra-assay CV: <10% Inter-assay CV: <10% LLQ: NR |
Not applicable | 6 |
| Greco et al. (2007) | Randomized, single-blind comparative, parallel group | Healthy women ≥8 years Regular menstrual cycle No OC: ≥3 months |
1 |
n = 24 Mean (SD) age: 19.7 ± 2.2 years Weight/BMI: NR |
3 cycles 35 EE/180-215-250 NGM, 21:7 |
Control cycle (2 days around ovulation) Tr. cycle 2 (Days 12–14) |
Direct RIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Method used is based on centrifugal ultrafiltration dialysis; calculated from a normogram constructed using serum SHBG and %FT (Hammond et al., 1980); free T can then be determined from T (Hammond et al., 2003) |
6 |
| 2 |
n = 24 Mean (SD) age: 19.9 ± 1.3 years Weight/BMI: NR |
3 cycles 25 EE/180-215-250 NGM, 21:7 |
|||||||
| Legro et al. (2008)2 | Randomized, double-blind, comparative, parallel group | Healthy women No OC: ≥3 months normal menstrual cycle (21–35 days) non-smoking |
1 |
n = 31 Mean (SD) age: 27.5 ± 4.7 years Mean (SD) weight: 67.7 ± 13.9 kg |
6 cycles 20 EE/1 mg NET 21:7 |
Control cycle (Days 15–21) Tr. cycle 6 (days 15–21) |
Direct RIA Intra-assay CV: <10% Inter-assay CV: <10% LLQ: NR |
Not applicable | 7 |
| Sänger et al. (2008)2 | Randomized, prospective, comparative, parallel group | Healthy women 18–40 years Regular menstrual cycle No hormonal contraceptives: ≥4 weeks |
1 |
n = 29 Mean (SD) age: 24.6 ± 3.2 years Mean (SD) weight: 63.7 ± 8.0 kg |
12 cycles 30 EE/2 mg DNG 21:7 |
Control cycle (Days 21–26) Tr. cycle 4 (Days 19–21) |
CLIA Intra-assay CV: 4.6% Inter-assay CV: 7.4% LLQ: 0.07 nmol/l |
Direct RIA Intra-assay CV: 12.3% Inter-assay CV: 18.3% LLQ: 0.52 pmol/l |
7 |
| Duijkers et al. (2010) | Randomized, open-label, comparative, parallel group | Healthy women 18–35 years No hormonal contraceptives: not specified, but women using OC and only after return of spontaneous menses control cycle was started (= at least 2 cycles no OC) Control cycle: ovulation confirmed |
1 |
n = 32 Mean (SD) age: 22.8 ± 3.3 years Mean (SD) weight: 68.5 ± 12.3 kg |
6 cycles 1.5 mg E2/2.5 mg NOMAC, 24:4 |
Control cycle (Days 5–7) Tr. cycle 6 (Days 21) |
ECLIA Intra-assay CV: NR Inter-assay CV: 1.9–3.7% LLQ: NR |
Calculated based on total T, SHBG and albumin (Vermeulen et al., 1999) |
7 |
| 2 |
n = 16 Mean (SD) age: 22.9 ± 4.3 years Mean (SD) weight: 66.3 ± 10.4 kg |
6 cycles 30 EE/3 DRSP 21:7 |
|||||||
| Strufaldi et al. (2010) | Randomized, prospective, open-label, comparative, parallel group | Healthy women 18–40 years No hormonal contraceptives: ≥3 months |
1 |
n = 51 Mean (SD) age: 28.7 ± 6.83 years Mean (SD) weight: 60.6 ± 3.0 kg |
6 cycles 30 EE/150 LNG 21:7 |
Control cycle (Days 2–4) Tr. cycle 6 (Days 2–4) |
CMIA Total precision: 3.1–8.0% LLQ: 0.28 nmol/l |
Not applicable | 7 |
| 2 |
n = 50 Mean (SD) age: 26.8 ± 5.89 years Mean (SD) weight: 62.5 ± 12.6 kg |
6 cycles 20 EE/100 LNG 21:7 |
|||||||
| Ågren et al. (2011) | Randomized, open-label, comparative, parallel group | Healthy women 18–50 years No OC: 6 weeks |
1 |
n = 60 Mean (SD) age: 28.2 ± 8.2 years Mean (SD) weight: 62.8 ± 9.7 kg |
6 cycles 1.5 mg E2/2.5 mg NOMAC, 24:4 |
Control cycle (follicular phase, nd half) Tr. cycle 6 (Days 15–21) |
ECLIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Calculated based on total T, SHBG and albumin (Vermeulen et al., 1999) | 7 |
| 2 |
n = 58 Mean (SD) age: 29.1 ± 7.8 years Mean (SD) weight: 61.7 ± 9.0 kg |
6 cycles 30 EE/150 LNG 21:7 |
|||||||
| Caruso et al. (2011) | Prospective, open-label, non- comparative | Healthy women 18–48 years No hormonal contraceptives: duration not specified Control cycle: ovulation confirmed |
1 |
n = 57 Mean (SD) age: 28.7 ± 3.3 years BMI: 21.3 ± 3.7 kg/m2 |
6 cycles 2–3 mg E2 V/3-2-1 mg DNG 21:7 |
Control cycle (follicular phase, Days 5–8) Tr. cycle 6 (Days 5–8) |
ELISA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Not applicable | 7 |
| Battaglia et al. (2012) | Pilot, non-comparative | Young eumenorrheic, healthy women normal menstrual cycle No hormonal contraceptives: at least 6 months |
1 |
n = 22 Mean (SD) age: 25.0 ± 2.8 years BMI: 21.7 ± 3.2 kg/m2 |
3 cycles 30 EE/3 mg DRSP 21:7 |
Control cycle (Days 3–5) Tr. cycle 3 (Days 3–5) |
ECLIA Intra-assay CV: NR Inter-assay CV: NR LLQ: NR |
Not applicable | 7 |
1, risk of bias assessment performed in relation to the effect measures used in the meta-analysis (scoring low, high or unclear risk, seven domains; see Supplementary data, Table SI for assessment per study); 2, study has more treatment or study groups, but only data of group(s) fulfilling the selection criteria for inclusion in the meta-analyses have been used; 3, information derived from Jung-Hoffmann and Kuhl (1987; including free T results); 4, reported unit is µg/dl, but assumed this is ng/dl; 5, characteristics similar for all treatment groups due to crossover design; 6, total T results are not reported.
AFTC, apparent free testosterone concentration; BMI, body mass index; CPA, cyproterone acetate; CV, coefficients of variation; DNG, dienogest; DRSP, drospirenone; DSG, desogestrel; E2, estradiol; E2 V, estradiol valerate; (E)CLIA, (electro)chemiluminescence immunoassay; EE, ethinyl estradiol; ELISA, enzyme-linked immunosorbent assay; GSD, gestodene; LLQ, lower limit of quantification; (L)NG, (levo)norgestrel; OC, oral contraceptive; N, number; NET(A), norethindrone (acetate); NGM, norgestimate; NOMAC, nomegestrol acetate; NR, not reported; RIA, radioimmunoassay; RSD, relative standard deviation; SD, standard deviation; SE, standard error; SHBG, sex hormone-binding globulin; T, testosterone; Tr., treatment.
The total number of women randomized in all studies was 1495 with an average number of 21 (range 5–60) women per treatment group. The study population consisted of healthy women in the age range of 18–40 years. Two studies (Siegberg et al., 1984; De Leo et al., 1991) also enrolled younger women (minimum age of 15 and 17 years, respectively). One study enrolled Chinese women (Song et al., 1992). The period without intake of hormonal contraceptives before the start of the study was more than 2 or 3 months in most studies, ranging from 4 to 6 weeks (Wiegratz et al., 2003; Sänger et al., 2008; Ågren et al., 2011a) up to 6 months (Boyd et al., 2001; Battaglia et al., 2012) and 12 months (Siegberg et al., 1984; Moutos et al., 1995). For those studies that did not specify a pre-study period without hormonal contraceptives, a regular menstrual cycle with or without proven ovulation was an inclusion criterion (Granger et al., 1982; De Leo et al., 1991; Janaud et al., 1992; Spona et al., 1996; Rickenlund et al., 2004; Duijkers et al., 2010; Caruso et al., 2011).
With regard to the methods to determine total T and free T concentrations, 30 studies used a direct immunoassay, 9 studies incorporated an extraction/chromatography step before RIA and no studies used the LC-MS/MS method. One study did not specify the type of assay used to measure total T concentrations (Janaud et al., 1992). The free T concentrations were analysed by a direct immunoassay in 18 studies, determined by calculation (based on total T, SHBG and albumin concentrations) in 8 studies and by equilibrium dialysis in 3 studies. Five studies used the formula of Vermeulen et al. (1971, 1999).
Methodological quality
Outcome parameters used in the meta-analysis and the type of comparison (a within-subject comparison on pretreatment (no COC) versus end of treatment (COC) were assessed for risk of bias. An overview of the risk of bias of the studies included in the meta-analysis is provided in Table II and Supplementary data, Table SI.
To assess selection bias, reported procedures on sequence generation and allocation concealment were judged for all studies. Often randomization and concealment was not applicable for studies, because only one treatment group was investigated. Furthermore, selection bias was expected not to have a major impact on the outcome of the analyses as study participants acted as their own control. Overall, three studies (Granger et al., 1982; Gaspard et al., 1984; Hammond et al., 1984) were judged as having inadequate sequence generation or allocation concealment (high risk of bias). In many studies the procedures were not clearly described and no judgement on risk of bias could be made (Cullberg et al., 1982; Gaspard et al., 1983; Kuhl et al., 1985; Jung-Hoffmann et al., 1988a, b; Refn et al., 1990; Janaud et al., 1992; Song et al., 1992; Wiegratz et al., 1995; Coenen et al., 1996; Thorneycroft et al., 1999; Wiegratz et al., 2003; White et al., 2005). With regard to performance and detection bias, it was assumed that lack of blinding of participants and personnel or lack of blinding of outcome assessment were not likely to have had an influence on the measurement of the biochemical outcome parameters. Therefore, risk of performance and detection bias was judged as not relevant and was considered to be low for all studies. On the other hand, incomplete outcome data (attrition bias) was found to be important, as missing data could potentially have an impact on the observed effect size. Reasons for early discontinuation could be due to being lost to follow-up, or could be related to side effects associated with low androgen levels (e.g. emotionally labile, lack of sexual desire). For the judgement of attrition bias, it was assumed that a completion rate of ≥ 80% per treatment group had not affected the outcome. Ten studies were considered to have a high risk of attrition bias (Åkerlund et al., 1994; Thorneycroft et al., 1999; White et al., 2005; Elkind-Hirsch et al., 2007; Greco et al., 2007) or an unclear risk (Granger et al., 1982; Vermeulen and Thiery, 1982; Siegberg et al., 1984; Jung-Hoffmann et al., 1988a, b; Boyd et al., 2001). The reporting bias (selective outcome reporting) was difficult to assess due to the lack of study protocols. For all studies it was verified whether the reported laboratory parameters were in adherence to the objectives of the study as specified in the paper. This was also done for the reported statistical significances. One study (Boyd et al., 2001) was judged as having a high risk of reporting bias, because total T results were not reported. Finally, all studies were also judged for other possible sources of bias. The only additional source for bias was the inadequate statistics used in a crossover study pooling the data for both treatment groups (Song et al., 1992).
Several other issues were identified that were not considered as a source for bias, but could still have affected the outcome of the analyses. In one study, only Chinese women were enrolled (Song et al., 1992). Some of the procedures performed during data extraction (e.g. obtaining values from figures, converting SEM to SD, converting to SI units were considered not to cause bias, but they could potentially cause some inaccuracy in the data used for the analyses. This is also applicable for calculating missing SDs based on SDs that were available for other time points in the study or based on 95% CI (Granger et al., 1982; Murphy et al., 1990; Kuhnz et al., 1994; Moutos et al., 1995; Legro et al., 2008). Furthermore, for some studies the reported units or measures for variation (SEM) did not seem correct in view of the expected ranges and assumptions were made for correction (Granger et al., 1982; Falsetti et al., 1987; White et al., 2005). Most of the studies did perform their sampling at the end of the pill cycle just prior to the pill-free period (e.g. on Days 19–21); however, in a few studies samples were collected during the first week (Murphy et al., 1990; Strufaldi et al., 2010; Caruso et al., 2011; Battaglia et al., 2012). One study (Moutos et al., 1995) retrospectively collected samples from a larger study. Although steroids are known to be stable when stored for a longer period, it could be possible that there is some in vitro effect during storage, which could result in small deviations of the results. Finally, in one study (Siegberg et al., 1984) two different COCs were used in one treatment group (50 µg EE/1 mg lynestrenol and 35 µg EE/0.8 mg lynestrenol), and this study was excluded from the subgroup analyses between the different EE dosages. These issues are not considered as bias, but could be a source for heterogeneity.
Effect of COC treatment on total testosterone, SHBG and free testosterone concentrations
The results of the analyses including the different subgroup analyses can be found in Table III and Supplementary data, Table SII.
Table III.
Summary of key findings of the meta-analysis on the effect of COCs on total T, free T and SHBG concentrations with the comparison no COC (pretreatment) versus COC (end of treatment).
| Type of analysis | Number of studies | Number of treatment groups | Number of subjects Total (no COC versus COC) | MD [95% CI] | P value | Heterogeneity (I2) (%) |
|---|---|---|---|---|---|---|
| COC effect on total T [nmol/l] | 39 | 64 | 2741 (1405 versus 1336) | −0.49 [−0.55, −0.43] | <0.001 | 55 |
| Subgroup: type of progestin | 39 | 61 | 2452 (1256 versus 1196) | −0.50 [−0.56, −0.43] | 0.20 | 34.7 |
| First generation (estranes) | 8 | 313 (159 versus 154) | −0.33 [−0.50, −0.17] | |||
| Second generation (gonanes) | 21 | 934 (474 versus 460) | −0.54 [−0.63, −0.45] | |||
| Third generation (gonanes) | 28 | 1044 (542 versus 502) | −0.51 [−0.62, −0.40] | |||
| Fourth or unclassified generation | 4 | 161 (81 versus 80) | −0.52 [−0.77, −0.27] | |||
| COC effect on SHBG [nmol/l] | 39 | 67 | 2917 (1487 versus 1430) | 99.10 [86.44, 111.75] | <0.001 | 96 |
| Subgroup: type of progestin | 38 | 64 | 2628 (1338 versus 1290) | 103.09 [89.51, 116.66] | <0.001 | 97.8 |
| First generation (estranes) | 9 | 347 (176 versus 171) | 89.06 [65.94, 112.19] | |||
| Second generation (gonanes) | 20 | 934 (470 versus 464) | 35.84 [26.86, 44.82] | |||
| Third generation (gonanes) | 28 | 1036 (536 versus 500) | 136.76 [120.66, 152.86] | |||
| Fourth or unclassified generation | 7 | 311 (156 versus 155) | 157.39 [111.62, 203.16] | |||
| [95% CI] | ||||||
| COC effect on free T (log scale; relative change) | 29 | 47 | 2043 (997 versus 1046) | 0.39 [0.35, 0.43] | <0.001 | 79 |
| Subgroup: type of progestin | 29 | 45 | 1865 (954 versus 911) | 0.38 [0.35, 0.42] | 0.32 | 12.0 |
| First generation (estranes) | 2 | 54 (27 versus 27) | 0.38 [0.31, 0.48] | |||
| Second generation (gonanes) | 13 | 596 (302 versus 294) | 0.42 [0.36, 0.50] | |||
| Third generation (gonanes) | 24 | 946 (490 versus 456) | 0.37 [0.32, 0.43] | |||
| Fourth or unclassified generation | 6 | 269 (135 versus 134) | 0.34 [0.25, 0.46] |
CI, confidence interval; COC, combined oral contraceptive; MD, mean difference; SHBG, sex hormone-binding globulin; T, testosterone.
Effect of COC treatment on total testosterone concentrations
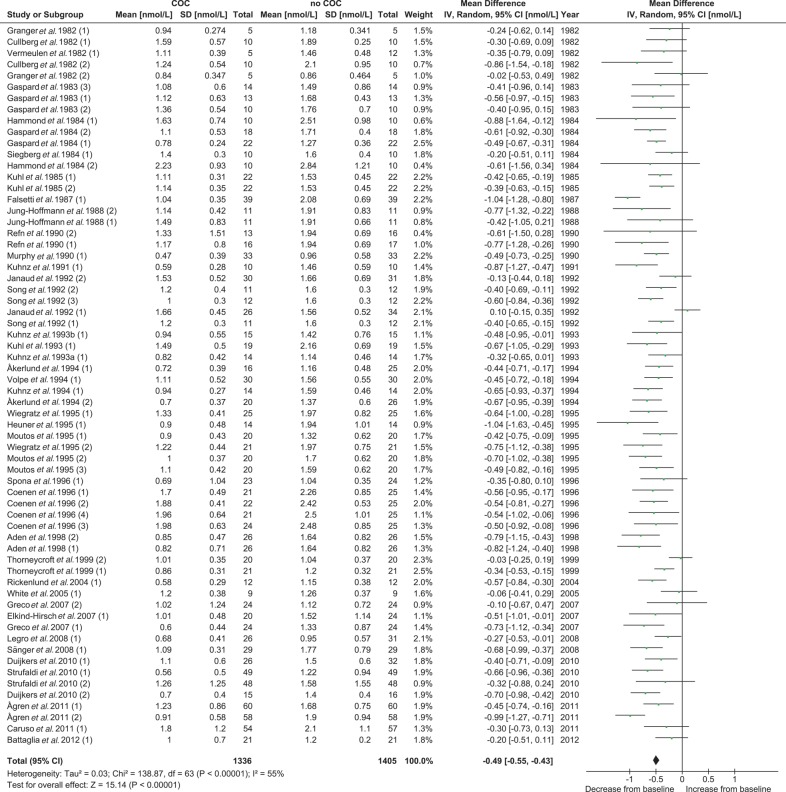
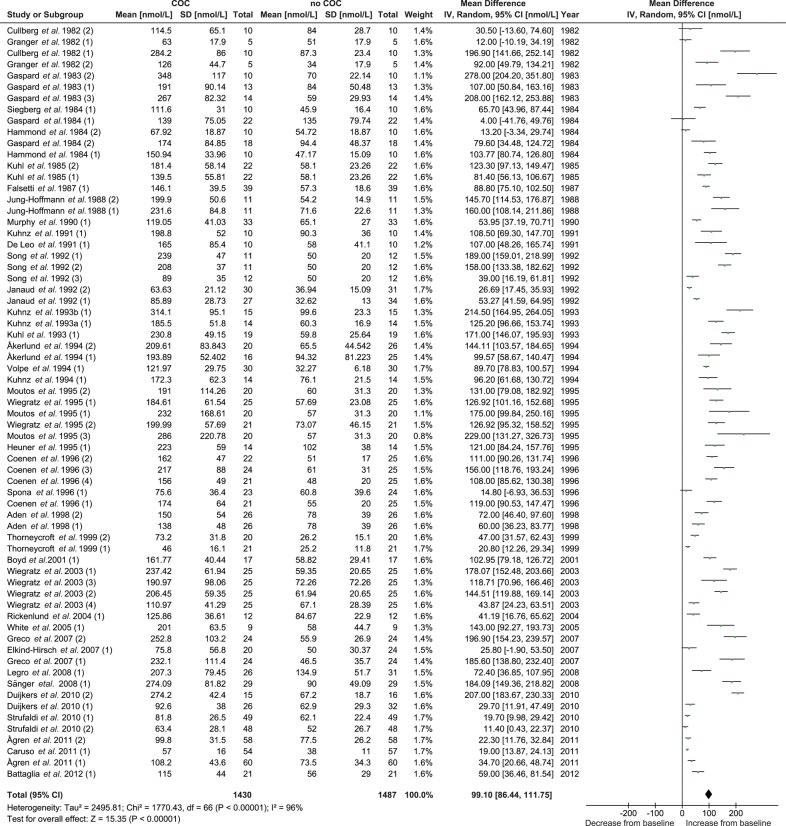
A total of 39 studies and 64 treatment groups were included in the meta-analysis for the effect of COC treatment on total T levels with data from 1405 women (no COC) versus 1336 women (COC). The pooled total T concentration at the end of treatment was significantly (P < 0.001) lower than the pooled total T concentration at pretreatment. No significant between-study heterogeneity was found (Table III and Fig. 2).
Figure 2.
Meta-analysis of 39 studies on the effect of COCs on total T concentrations. COC, combined oral contraceptive; T, testosterone.
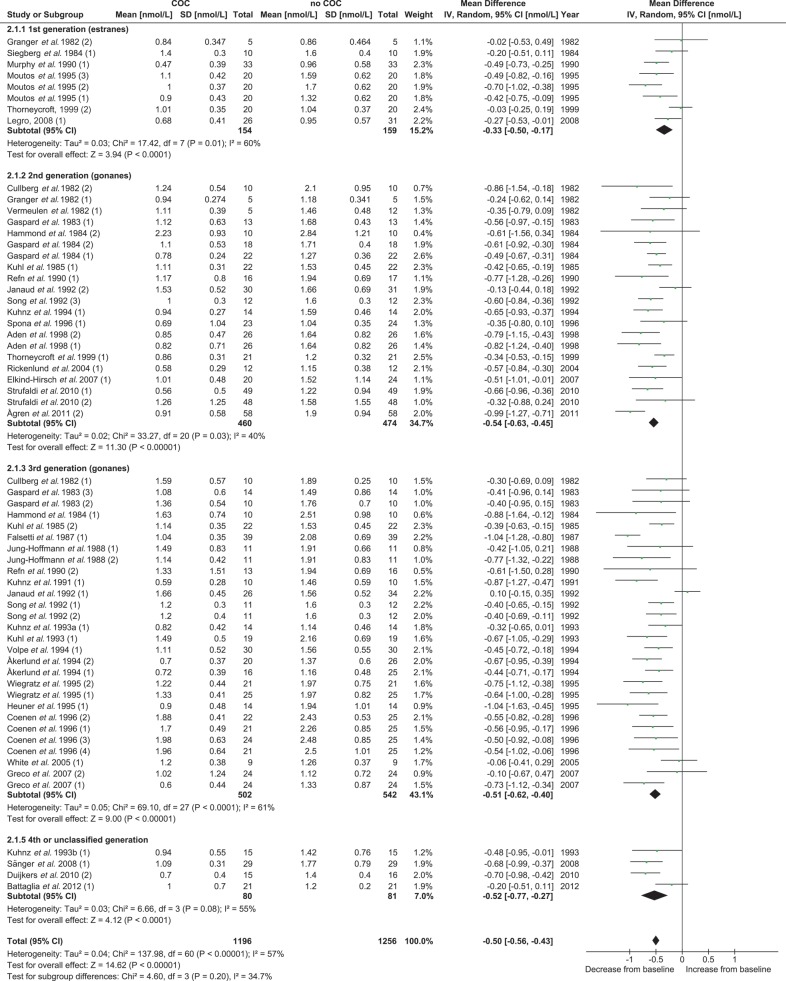
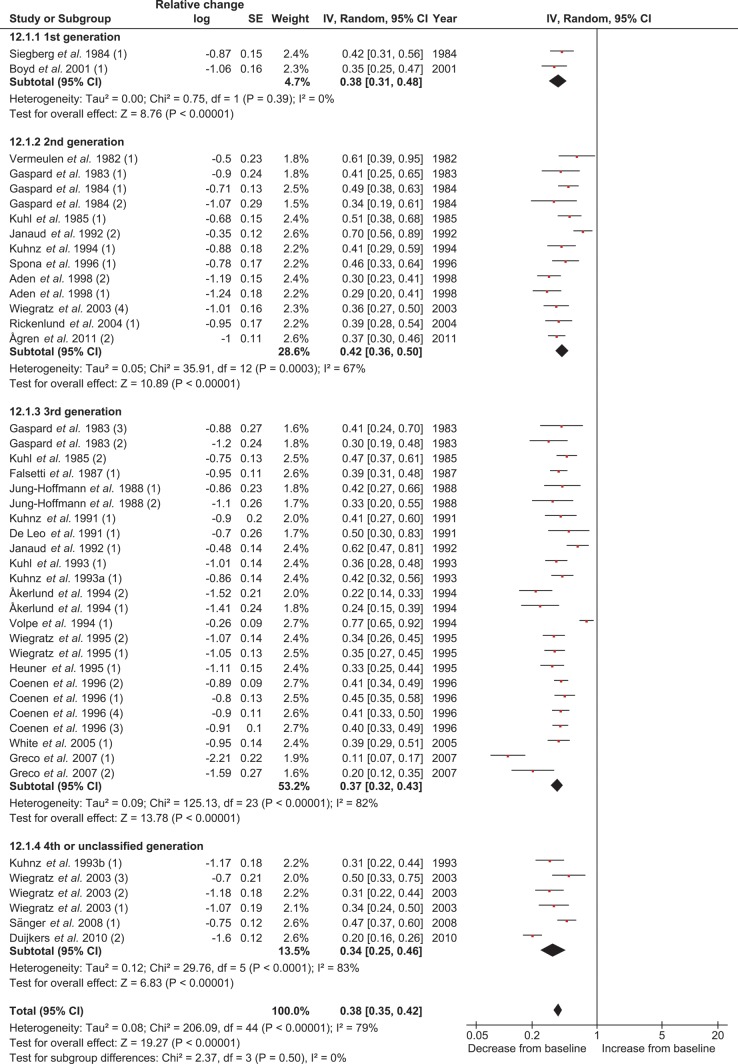
The suppressive effect of COCs on total T did not reveal a significant difference (P = 0.54) between the COCs containing 20–25 µg EE dose and the COCs containing 30–35 µg EE (Supplementary data, Table SII and Supplementary data, Fig. S1). The subgroup analysis between the different types of progestin (first generation versus second generation versus third generation versus fourth or unclassified generation) also revealed no significant difference (P = 0.20) between the groups for the effect on total T (Table III and Fig. 3). When a more detailed comparison was made combining the EE dose and the type of progestin, a significant difference was found between the subgroups (P = 0.02). In particular, the first and second generation COCs containing 20–25 µg EE showed less suppressive effects on total T than did the other subgroups (Supplementary data, Table SII and Supplementary data, Fig. S2). A separate analysis comparing the second and third generation monophasic COCs containing 30–35 µg EE revealed no differences between progestins on total T levels (Fig. 3 Supplementary data, Table SII, Figs S1, S2 and S3). For all these subgroup analyses, no significant heterogeneity was found (Table III and Supplementary data, Table SII).
Figure 3.
Subgroup analysis for the effect of type of progestin on total T concentrations in the meta-analysis. COC, combined oral contraceptive; T, testosterone.
For the comparison between the methods used to measure total T, lower differences in total T concentrations were reported with the RIA incorporating extraction or chromatography compared with the assays without this extra step. This difference was statistically significant (P = 0.003); however, a significant heterogeneity was also identified (Supplementary data, Table SII and Supplementary data, Fig. S4).
Effect of COC treatment on SHBG concentrations
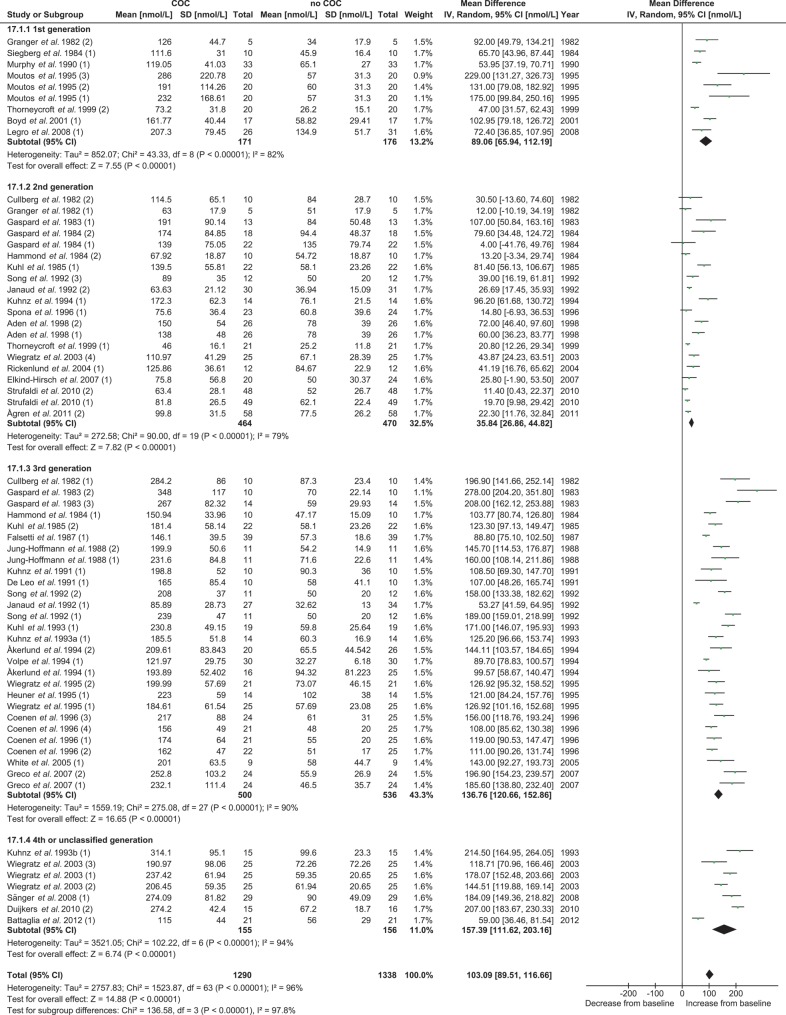
The 39 studies and 67 treatment groups included in the meta-analysis reported data on 1430 women being treated with COC. The pooled SHBG concentration was significantly (P < 0.001) higher at the end of treatment than at pretreatment. The increase in SHBG levels was significantly (P = 0.05) less after treatment with COCs containing 20–25 µg EE compared with COCs containing 30–35 µg EE (Supplementary data, Table SII and Supplementary data, Fig. S5). A more pronounced and significantly different effect was observed with different types of progestins in the subgroup analysis (P < 0.001). Treatment with second generation COCs resulted in the least increase in SHBG, whereas treatment with the third and fourth generation COCs resulted in the highest increase (Table III and Fig. 5). When performing a subgroup analysis combining the estrogen dose with the progestin type, a significant difference (P < 0.001) was found. The COCs with a second generation progestin and containing the lower 20–25 µg EE dose caused the lowest increase of SHBG (Supplementary data, Table SII and Supplementary data, Fig. S6). An additional subgroup analysis comparing the second and third generation monophasic COCs containing 30–35 µg EE confirmed that the second generation progestin had significantly less effect on SHBG (P < 0.001) (Supplementary data, Table SII and Supplementary data, Fig. S7). For all comparisons made with the pooled SHBG concentrations, significant heterogeneity was identified (Table III, Figs 4, 5 and Supplementary data, Table SII, Figs S5, S6, S7).
Figure 5.
Subgroup analysis for the effect of type of progestin on SHBG concentrations in the meta-analysis. COC, combined oral contraceptive; SHBG, sex hormone-binding globulin.
Figure 4.
Meta-analysis of 39 studies on the effect of COCs on SHBG concentrations. COC, combined oral contraceptive; SHBG, sex hormone-binding globulin.
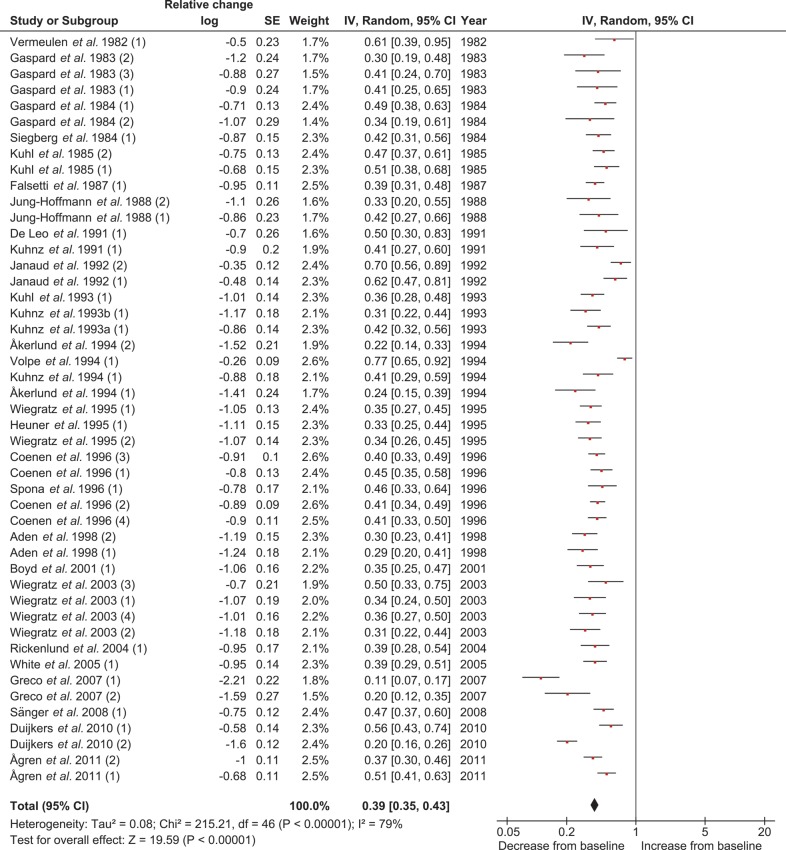
Effect of COC treatment on free testosterone concentrations
The 29 studies and 47 treatment groups used for the meta-analysis for the COC effect on free T concentrations included 1046 women at pretreatment and 997 women at end of treatment. The levels of free T showed a large variation. After inspection of the data, it was decided to use a logarithmic scale for the free T concentrations with the relative change as the effect measure. At the end of COC use, the free T levels were significantly (P < 0.001) decreased. The average relative change was 0.39, which means that the free T levels were 61% lower compared with the levels before starting the COC (Table III and Fig. 6). No significant difference in treatment effect was found after performing subgroup analyses for comparison between the EE dosages and between the type of progestins or when combining the EE dosage with the type of progestin (Table III, Figs 6, 7, Supplementary data, Table SII, Figs S8 and S9). Significant heterogeneity was found for the main analysis, but not for the subgroup analyses (Table III and Supplementary data, Table SII).
Figure 6.
Meta-analysis of 29 studies on the effect of COCs on free T concentrations (log scale; relative change). COC, combined oral contraceptive; T, testosterone.
Figure 7.
Subgroup analysis for the effect of type of progestin on free T concentrations in the meta-analysis on free T (log scale; relative change). T, testosterone.
To judge whether the methods to assess the free T levels had any effect on the size of the observed effect, a subgroup analysis between the different methods was performed. No significant difference (P = 0.27) in pooled free T concentration was found between the results obtained by direct RIA and with the methods reported to be more accurate (calculation or equilibrium dialysis). No significant between-study heterogeneity was found (Supplementary data, Table SII and Supplementary data, Fig. S10).
Discussion
This systematic review and meta-analysis was performed to evaluate the effect of COCs on concentrations of total T, free T and SHBG in healthy women. A total of 42 studies involving 1495 women were included in the current analyses. Both pooled total T and free T concentrations decreased significantly following COC use. All studies reported such an effect, except one (Janaud et al., 1992). The suppression of free T was twice as high compared with total T. The decline in total T and free T levels were both independent from the estrogen dose or progestin type. SHBG levels increased significantly during COC use, as reported by all included studies. The lower dosage of EE had less of an effect on SHBG than the higher dosage, and the greatest effect was with the COCs containing a third or fourth generation progestin. The COCs with a second generation progestin caused the smallest increase in SHBG. The between-study heterogeneity was acceptable for all comparisons with total T and the subgroup comparisons with free T. However, a significant heterogeneity was found for the analyses with SHBG and for the main analysis with free T.
The between-study heterogeneity can be explained by the different types of COCs that were investigated in the included studies. Additionally, the duration without the use of a hormonal contraceptive prior to starting the COC varied from 1 month to 12 months. Another explanation may be the difference in handling of blood samples and assessment methods. Timing of blood sampling was not always consistent among studies. Most of the studies performed their sampling just prior to the start of the pill-free period, whereas a few studies (Murphy et al., 1990; Strufaldi et al., 2010; Caruso et al., 2011; Battaglia et al., 2012) collected samples during the first week of the cycle. Some studies did not provide information on the sampling time points, so it could be that no specific time window was followed. The sample timing could be an important factor when evaluating the effect of COC use on hormonal parameters. The fact that some studies measured the hormone concentrations in plasma and some in serum could also be a confounding factor, although this is mainly overcome by the within-subject comparison used in the current meta-analysis. Furthermore, there is no consensus on adequate methods for measuring total T as well as free T concentrations and its standardization (Vesper et al., 2008; Legro et al., 2010; Rosner and Vesper, 2010; Vesper and Botelho, 2010; Haring et al., 2012), which is apparent from the studies using different types of analytical methods.
The subgroup analysis between the studies which used a direct immunoassay with the studies using RIA with extraction and chromatography revealed a significant difference. When an extraction and chromatography step was included, significantly lower differences in concentrations of total T were measured. Other studies have also reported the overestimation of concentrations by direct immunoassays (Rosner et al., 2007; Groenestege et al., 2012). As data were grouped per assay method, the between-study heterogeneity in the subgroups was lower compared with the main analysis. The subgroup analysis performed for the free T assessment did not identify a significant difference between concentrations obtained using direct immunoassay versus the calculation method or equilibrium dialysis. The latter two methods are regarded to be a more reliable measure of free T concentrations (Bachmann et al., 2002; Rinaldi et al., 2002; Miller et al., 2004), so this is an unexpected finding. This may be attributable to procedural differences, which, in the case of the calculation method or equilibrium dialysis apparently differ more widely between laboratories than the RIA method. Other sources for the variation can be explained by the variations in the study population, e.g. different age and body mass index (BMI) ranges. No adjustment could be made for these confounding factors.
This meta-analysis has limitations which should be considered. The included studies were not strong in design with regard to preventing selection bias and only a few studies used a double-blind or single-blind design. However, due to the within-subject comparison, this bias seems to be of little relevance. Incomplete outcome data (attrition bias) seemed to be one of the most important factors affecting the analyses as some reasons for early discontinuation could be related to low androgen levels. The search was performed using terms (MeSH) in the title or abstracts, and therefore some publications could have been missed. However, this problem was partly covered by performing a hand search on the reference lists of all selected papers. For consistency reasons, only studies investigating COCs with a 21:7 or 24:4 regimen were eligible for inclusion. Only two of the included studies evaluated the effects of a COC with a 24:4 regimen (Duijkers et al., 2010; Ågren et al., 2011a, b). This COC containing E2 and nomegestrol acetate (NOMAC) was not included in the subgroup analyses, because it did not qualify for the subgroups. The effects of COCs with a continuous regimen were also not evaluated in the current meta-analysis. However, studies comparing COCs using a continuous regimen with COCs using the 21:7 regimen report that T and SHBG levels are affected to the same extent (Legro et al., 2008; Sänger et al., 2008). This shows that the maximal effect of a COC on T and SHBG is already reached within 3 weeks of use.
Based on the outcome of this review, it seems reasonable to conclude that the suppression of T is caused by all three mechanisms of action mentioned in the introduction of this paper, i.e. suppression of both ovarian and adrenal androgen synthesis, along with increased SHBG levels. The average decrease of total T was 31% and for free T it was 61%, which cannot solely be explained by direct inhibition of ovarian androgen synthesis. Under normal conditions, ∼25% of T is produced by the ovaries, whereas the adrenals synthesize another 25% and the remaining 50% is derived from peripheral conversion (Longcope, 1986; Bachmann et al., 2002; Burger, 2002). Thus, the other two mechanisms of action, the increase in SHBG increase and the inhibition of adrenal androgen synthesis, seem to be relevant too. Possibly the peripheral conversion of T from (pre)androgens is diminished due to lower levels of (pre)androgens. This contention is supported by the fact that many studies have also found decreased levels of dihydrotestosterone, AD, DHEA, DHEA-S and androstanediol glucuronide after COC use (Gaspard et al., 1984; Wiegratz et al., 2003; Ågren et al., 2011a, b). AD is the major source of peripheral T production (Longcope, 1986; Burger, 2002). The lower levels of AD, DHEA and DHEA-S are possibly the result of suppression of the adrenal androgen synthesis (Carr et al., 1979; Klove et al., 1984; Coenen et al., 1996).
This review also confirms that SHBG significantly increases during COC use, an effect which varied depending on the dose of the EE component. COCs with the second generation progestin combined with the lowest EE dose (20–25 µg) had the least effect on SHBG. A separate analysis between the monophasic COCs with a second or third generation progestin, both containing 30–35 µg EE, revealed that the third generation progestin had a stronger effect on SHBG. All data confirm that COCs containing a second generation progestin increase SHBG considerably less compared with all other progestins (∼50% versus 150–250% elevation). Since SHBG is the main carrier protein of T one would expect more T to be bound when the SHBG increases are higher (Anderson, 1974; Van der Vange et al., 1990; Wiegratz et al., 2003; Ågren et al., 2011a). However, all progestin types reduce total and free T to the same extent and no significant difference in lowering T levels was found when comparing the 20–25 µg with the 30–35 µg EE dose except for a small but significant difference (P = 0.02) when the lower EE dose was combined with a first or second generation progestin. Similar results have been found in other studies (Jung-Hoffmann and Kuhl, 1987; Heuner et al., 1995; Boyd et al., 2001; Sänger et al., 2008). Apparently, the moderate SHBG increase caused by second generation progestins already exerts the maximal effect on additional binding of T and further lowering of free T levels.
Apart from estrogens and androgens, progestins can also bind to SHBG and albumin (Schindler et al., 2003). Competition with T or E2 for SHBG might decrease the circulating concentration of the respective progestin in the circulation and, concomitantly, increase the free T and E2 levels, thereby indirectly influencing the SHBG synthesis, be it to an as yet undetermined extent.
As mentioned above, T deficiency is thought to be associated with a broad range of undesired effects including diminished well-being and quality of life, mood changes (depression, irritation, moodiness), loss of energy, cognitive disturbances, interference with optimal sexual function, declining muscle mass and strength and lowering of bone mass and bone density (Bachmann et al., 2002; Traish et al., 2007). The clinical consequences of the suppressed T levels during COC use have so far gained little attention. Only a few studies have combined the investigation of the effects of COCs on androgens with the occurrence of side effects which could be attributed to suppressed androgen levels. These studies report conflicting results (Bancroft et al., 1991b; Schaffir, 2006; Graham et al., 2007; Greco et al., 2007; Strufaldi et al., 2010; Caruso et al., 2011; Battaglia et al., 2012; Elaut et al., 2012; Pastor et al., 2013). Although estrogens have been clearly identified as key hormones for maintaining bone mass (Ott et al., 2001; Riggs et al., 2002; Ott, 2008), androgens are also important regulators of skeletal growth and maturation (Martel et al., 1998; Riggs et al., 2002; Hartard et al., 2006). Data on the effect of androgens in women are scarce but T may exert an effect on protein synthesis and increase muscle mass (Gower and Nyman, 2000; Ružić et al., 2003). Therefore, the negative effect of COCs on T levels may have some impact on bone mass and also on muscle strength.
In conclusion, the current systematic literature review and meta-analysis demonstrates that COCs decrease circulating levels of total T and free T and increase SHBG concentrations. Due to the SHBG increase, free T levels decrease twice as much as total T. The estrogen dose and progestin type of the COC do not influence the decline of total or free T, but both affect the magnitude of the effect on SHBG. The clinical implications of suppressed androgen levels during COC use remain to be elucidated. Further research investigating the COC's effects on androgens combined with their effects on other clinical outcomes is warranted.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors' roles
Y.Z. made substantial contributions to the conception and design of the study, the acquisition of data, the analysis and interpretation of the data, the drafting of the article and the adjustments after the review by other authors and gave final approval of the submitted version of the manuscript. M.J.C.E. made substantial contributions to the conception and design of the study, the analysis and interpretation of the data, the critical review of the article and gave final approval of the submitted version of the manuscript. M.A.B. made substantial contributions to the interpretation of the data, the critical review of the article and gave final approval of the submitted version of the manuscript. H.J.T.C.B. and B.C.J.M.F. made substantial contributions to the conception and design of the study, the interpretation of the data, the critical review of the article and gave final approval of the submitted version of the manuscript.
Funding
This systematic review was funded by Pantarhei Bioscience (PRB).
Conflict of interest
Y.Z. is employed by PRB. H.J.T.C.B. is CEO of PRB and has equity interests in the company. M.J.C.E. and M.A.B. have nothing to declare. B.C.J.M.F has received fees and grant support from the following companies (in alphabetic order): Andromed, Ardana, Auxogyn, Ferring, Genovum, Merck (MSD), Merck Serono, Organon, Pantharei Bioscience, PregLem, Schering, Schering Plough, Serono, Uteron Pharma, Watson Pharmaceuticals and Wyeth.
Supplementary Material
Acknowledgements
We are very grateful to Prof. J.H.H. Thijssen, who provided us with valuable advice concerning the classification of progestins and assays. We would also like to thank Dr W. Wouters for kindly reviewing the abstracts during the selection process.
References
- Aden U, Jung-Hoffmann C, Kuhl H. A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception. 1998;58:75–81. doi: 10.1016/s0010-7824(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Sherwin BB, Bancroft J, Davidson DW. Testosterone and sexual behavior in oral contraceptive users and nonusers: a prospective study. Horm Behav. 1990;24:388–402. doi: 10.1016/0018-506x(90)90017-r. [DOI] [PubMed] [Google Scholar]
- Aliyeva NO. Endocrine status of women against a background of hormonal contraceptives. Azerbaijan Med J. 2002;1:77–80. [Google Scholar]
- Ågren UM, Anttila M, Mäenpää-Liukko K, Rantala M-L, Rautiainen H, Sommer WF, Mommers E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17ß-oestradiol in comparison to one containing levonorgestrel and ethinylestradiol on markers of endocrine function. Eur J Contracept Reprod Health Care. 2011a;16:458–467. doi: 10.3109/13625187.2011.614363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågren UM, Anttila M, Mäenpää-Liukko K, Rantala M-L, Rautiainen H, Sommer WF, Mommers E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17ß-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolism. Eur J Contracept Reprod Health Care. 2011b;16:444–457. doi: 10.3109/13625187.2011.604450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerlund M, Almström E, Högstedt S, Nabrink M. Oral contraceptive tablets containing 20 and 30 micrograms of ethinyl estradiol with 150 micrograms desogestrel: their influence on lipids, lipoproteins, sex hormone binding globulin and testosterone. Acta Obstet Gynecol Scand. 1994;73:136–143. doi: 10.3109/00016349409013416. [DOI] [PubMed] [Google Scholar]
- Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Bancroft J, Braunstein G, Burger H, Davis S, Dennerstein L, Goldstein I, Guay A, Leiblum S, Lobo R, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Davidson DW, Warner P, Tyrer G. Androgens and sexual behaviour in women using oral contraceptives. Clin Endocrinol (Oxf) 1980;12:327–340. doi: 10.1111/j.1365-2265.1980.tb02718.x. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: I. A comparison of sexual experience, sexual attitudes, and gender role in oral contraceptive users and nonusers. Arch Sex Behav. 1991a;20:105–120. doi: 10.1007/BF01541938. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Arch Sex Behav. 1991b;20:121–135. doi: 10.1007/BF01541939. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Battaglia B, Mancini F, Busacchi P, Paganotto MC, Morotti E, Venturoli S. Sexual behavior and oral contraception: a pilot study. J Sex Med. 2012;9:550–557. doi: 10.1111/j.1743-6109.2011.02597.x. [DOI] [PubMed] [Google Scholar]
- Benton SC, Nuttall M, Nardo L, Laing I. Measured dehydroepiandrosterone sulfate positively influences testosterone measurement in unextracted female serum: comparison of 2 immunoassays with testosterone measured by LC-MS. Clin Chem. 2011;57:1074–1075. doi: 10.1373/clinchem.2010.158600. [DOI] [PubMed] [Google Scholar]
- Bergink EW, Holma P, Pyörälä T. Effects of oral contraceptive combinations containing levonorgestrel or desogestrel on serum proteins and androgen binding. Scand J Clin Lab Invest. 1981;41:663–668. doi: 10.3109/00365518109090512. [DOI] [PubMed] [Google Scholar]
- Boyd RA, Zegarac EA, Posvar EL, Flack MR. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71–76. doi: 10.1016/s0010-7824(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl. 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Carlström K, Karlsson R, Von Schoultz B. Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scand J Clin Lab Invest. 2002;62:361–368. doi: 10.1080/00365510260296519. [DOI] [PubMed] [Google Scholar]
- Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Plasma levels of adrenocorticotropin and cortisol in women receiving oral contraceptive steroid treatment. J Clin Endocrinol Metab. 1979;49:346–349. doi: 10.1210/jcem-49-3-346. [DOI] [PubMed] [Google Scholar]
- Caruso S, Agnello C, Romano M, Cianci S, Lo Presti L, Malandrino C, Cianci A. Preliminary study on the effect of four-phasic estradiol valerate and dienogest (E2 V/DNG) oral contraceptive on the quality of sexual life. J Sex Med. 2011;8:2841–2850. doi: 10.1111/j.1743-6109.2011.02409.x. [DOI] [PubMed] [Google Scholar]
- Coenen CM, Thomas CM, Borm GF, Rolland R. Comparative evaluation of the androgenicity of four low-dose, fixed-combination oral contraceptives. Int J Fertil Menopausal Stud. 1995;40(Suppl. 2):92–97. [PubMed] [Google Scholar]
- Coenen CMH, Thomas CMG, Borm GF, Hollanders JMG, Rolland R. Changes in androgens during treatment with four low-dose contraceptives. Contraception. 1996;53:171–176. doi: 10.1016/0010-7824(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Cullberg G, Dovré P-A, Lindstedt G, Steffensen K. On the use of plasma proteins as indicators of the metabolic effects of combined oral contraceptives. Acta Obstet Gynecol Scand. 1982;111(Suppl.):47–54. [Google Scholar]
- Davison SL, Bell R. Androgen physiology. Semin Reprod Med. 2006;24:71–77. doi: 10.1055/s-2006-939565. [DOI] [PubMed] [Google Scholar]
- De Leo V, Lanzetta D, Vanni AL, D'Antona D, Severi FM. Low estrogen oral contraceptives and the hypothalamo-pituitary axis. Contraception. 1991;44:155–161. doi: 10.1016/0010-7824(91)90115-v. [DOI] [PubMed] [Google Scholar]
- Duijkers I, Klipping C, Grob P, Korver T. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17ß-oestradiol on ovarian function in comparison to a monophasic combined oral contraceptive containing drospirenone and ethinylestradiol. Eur J Contracept Reprod Health Care. 2010;15:314–325. doi: 10.3109/13625187.2010.504313. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- Elaut E, Buysse A, De Sutter P, De Cuypere G, Gerris J, Deschepper E, T'Sjoen G. Relation of androgen receptor sensitivity and mood to sexual desire in hormonal contraception users. Contraception. 2012;85:470–4792. doi: 10.1016/j.contraception.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Darensbourg C, Ogden B, Ogden LF, Hindelang P. Contraceptive vaginal ring use for women has less adverse metabolic effects than an oral contraceptive. Contraception. 2007;76:348–356. doi: 10.1016/j.contraception.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Endrikat J, Blode H, Gerlinger C, Rosenbaum P, Kuhnz W. A pharmacokinetic study with a low-dose oral contraceptive containing 20 µg ethinylestradiol plus 100 µg levonorgestrel. Eur J Contracept Reprod Health Care. 2002;7:79–90. [PubMed] [Google Scholar]
- Erdmann D, Schindler EM, Schindler AE. Ovarian suppression with Diane 35/50. Geburtshilfe Frauenheilkd. 1994;54:627–633. doi: 10.1055/s-2007-1022354. German. [DOI] [PubMed] [Google Scholar]
- Falsetti L, Schivardi MR, Prandini BD. A new low-dose estrogen oral contraceptive combination: effect on endocrine parameters and lipid status. Contraception. 1987;36:489–497. doi: 10.1016/0010-7824(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Fern M, Rose DP, Fern EB. Effect of oral contraceptives on plasma androgenic steroids and their precursors. Obstet Gynecol. 1978;51:541–544. doi: 10.1097/00006250-197805000-00005. [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–590. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Dubois M, Gillain D, Franchimont P, Duvivier J. Ovarian function is effectively inhibited by a low-dose triphasic oral contraceptive containing ethinylestradiol and levonorgestrel. Contraception. 1984;29:305–318. doi: 10.1016/0010-7824(84)90064-7. [DOI] [PubMed] [Google Scholar]
- Graham CA, Bancroft J, Doll HA, Greco T, Tanner A. Does oral contraceptive-induced reduction in free testosterone adversely affect the sexuality or mood of women? Psychoneuroendocrinology. 2007;32:246–255. doi: 10.1016/j.psyneuen.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Granger LR, Roy S, Mishell DR., Jr Changes in unbound sex steroids and sex hormone binding globulin–binding capacity during oral and vaginal progestogen administration. Am J Obstet Gynecol. 1982;144:578–584. doi: 10.1016/0002-9378(82)90231-9. [DOI] [PubMed] [Google Scholar]
- Greco T, Graham CA, Bancroft J, Tanner A, Doll HA. The effects of oral contraceptives on androgen levels and their relevance to premenstrual mood and sexual interest: a comparison of two triphasic formulations containing norgestimate and either 35 or 25 µg of ethinyl estradiol. Contraception. 2007;76:8–17. doi: 10.1016/j.contraception.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Groenestege WMT, Bui HN, Ten Kate J, Menheere PPCA, Oosterhuis WP, Vader HL, Heijboer AC, Janssen MJW. Accuracy of first and second generation testosterone assays and improvement through sample extraction. Clin Chem. 2012;58:1154–1156. doi: 10.1373/clinchem.2011.181735. [DOI] [PubMed] [Google Scholar]
- Gower BA, Nyman L. Associations among oral estrogen use, free testosterone concentration, and lean body mass among postmenopausal women. J Clin Endocrinol Metab. 2000;85:4476–4480. doi: 10.1210/jcem.85.12.7009. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Nisker JA, Jones LA, Siiteri PK. Estimation of the percentage of free steroid in undiluted serum by centrifugal ultrafiltration-dialysis. J Biol Chem. 1980;255:5023–5026. [PubMed] [Google Scholar]
- Hammond GL, Langley MS, Robinson PA, Nummi S, Lund L. Serum steroid binding protein concentrations, distributions of progestins, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil Steril. 1984;42:44–51. doi: 10.1016/s0015-0282(16)47956-2. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Abrams LS, Creasy GW, Natarajan J, Allen JG, Siiteri PK. Serum distribution of the major metabolites of norgestimate in relation to its pharmacological properties. Contraception. 2003;67:93–99. doi: 10.1016/s0010-7824(02)00473-0. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Hogeveen KN, Visser M, Coelingh Bennink HJ. Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells. Climacteric. 2008;11(Suppl. 1):41–46. doi: 10.1080/13697130701851814. [DOI] [PubMed] [Google Scholar]
- Haring R, Hannemann A, John U, Radke D, Nauck M, Wallaschofski H, Owen L, Adaway J, Keevil BG, Brabant G. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2012;97:408–415. doi: 10.1210/jc.2011-2134. [DOI] [PubMed] [Google Scholar]
- Hartard M, Kleinmond C, Luppa P, Zelger O, Egger K, Wiseman M, Weissenbacher ER, Felsenberg D, Erben RG. Comparison of the skeletal effects of the progestogens desogestrel and levonorgestrel in oral contraceptive preparations in young women: controlled, open, partly randomized investigation over 13 cycles. Contraception. 2006;74:367–375. doi: 10.1016/j.contraception.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Heiman JR, Rupp H, Janssen E, Newhouse SK, Brauer M, Laan E. Sexual desire, sexual arousal and hormonal differences in premenopausal US and Dutch women with and without low sexual desire. Horm Behav. 2011;59:772–779. doi: 10.1016/j.yhbeh.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Heuner A, Kuhnz W, Heger-Mahn D, Richert K, Hümpel M. A single-dose and 3-month clinical-pharmacokinetic study with a new combination oral contraceptive. Adv Contracept. 1995;11:207–225. doi: 10.1007/BF01978421. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. 2011 The Cochrane Collaboration Version 5.1.0 [updated March 2011] www.cochrane-handbook.org . [Google Scholar]
- Janaud A, Rouffy J, Upmalis D, Dain MP. A Comparison Study of Lipid and Androgen Metabolism with Triphasic Oral Contraceptive Formulations Containing Norgestimate or Levonorgestrel. Acta Obstet Gynecol Scand. 1992;71(Suppl. 156):33–38. doi: 10.3109/00016349209156513. [DOI] [PubMed] [Google Scholar]
- Janaud A, Rouffy J, Upmalis D, Dain MP. Effects on blood lipid and androgen levels of two triphasic oral contraceptives. Int J Fertil Menopausal Stud. 1993;38(Suppl. 3):114–116. [PubMed] [Google Scholar]
- Jung-Hoffmann C, Kuhl H. Divergent effects of two low-dose oral contraceptives on sex hormone-binding globulin and free testosterone. Divergent effects of two low-dose oral contraceptives on sex hormone-binding globulin and free testosterone. Am J Obstet Gynecol. 1987;156:199–203. doi: 10.1016/0002-9378(87)90238-9. [DOI] [PubMed] [Google Scholar]
- Jung-Hoffmann C, Heidt F, Kuhl H. Effect of two oral contraceptives containing 30 mcg ethinylestradiol and 75 mcg gestodene or 150 mcg desogestrel upon various hormonal parameters. Contraception. 1988a;38:593–603. doi: 10.1016/0010-7824(88)90044-3. [DOI] [PubMed] [Google Scholar]
- Jung-Hoffmann C, März W, Heidt F, Gross W, Kuhl H. Effect of 2 low-dosage gestodene or desogestrel containing ovulation inhibitors on sex hormones and lipid metabolism. Geburtshilfe Frauenheilkd. 1988b;48:215–219. German. [PubMed] [Google Scholar]
- Klove KL, Roy S, Lobo RA. The effect of different contraceptive treatments on the serum concentration of dehydroepiandrosterone sulfate. Contraception. 1984;29:319–324. doi: 10.1016/0010-7824(84)90065-9. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Gahn G, Romberg G, März W, Taubert HD A randomized cross-over comparison of two low-dose oral contraceptives upon hormonal and metabolic parameters: I. Effects upon sexual hormone levels. Contraception. 1985;31:583–593. doi: 10.1016/0010-7824(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, Weber J, Boehm BO. The effect of a biphasic desogestrel-containing oral contraceptives on carbohydrate metabolism and various hormonal parameters. Contraception. 1993;47:55–68. doi: 10.1016/0010-7824(93)90109-k. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Sostarek D, Gansau C, Louton T, Mahler M. Single and multiple administration of a new triphasic oral contraceptive to women: pharmacokinetics of ethinyl estradiol and free and total testosterone levels in serum. Am J Obstet Gynecol. 1991;165:596–602. doi: 10.1016/0002-9378(91)90292-y. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Baumann A, Staks T, Dibbelt L, Knuppen R, Jütting G. Pharmacokinetics of gestodene and ethinylestradiol in 14 women during three months of treatment with a new tri-step combination oral contraceptive: serum protein binding of gestodene and influence of treatment on free and total testosterone levels in the serum. Contraception. 1993a;48:303–322. doi: 10.1016/0010-7824(93)90077-k. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Staks T, Jütting G. Pharmacokinetics of cyproterone acetate and ethinylestradiol in 15 women who received a combination oral contraceptive during three treatment cycles. Contraception. 1993b;48:557–575. doi: 10.1016/0010-7824(93)90118-q. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Staks T, Jütting G. Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum. Contraception. 1994;50:563–579. doi: 10.1016/0010-7824(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Legro RW, Pauli JG, Kunselman AR, Meadows JW, Kesner JS, Zaino RJ, Demers LM, Gnatuk CL, Dodson WC. Effects of Continuous Versus Cyclical Oral Contraception: a Randomized Controlled Trial. J Clin Endocrinol Metab. 2008;93:420–429. doi: 10.1210/jc.2007-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ, et al. Reproductive Medicine Network. Total testosterone assays in women with polycycstic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levell MJ, Siddall JK, Rowe E, Glashan RW, Robinson MR, Pidcock NB. Relationship of testosterone, sex hormone binding globulin, and calculated free testosterone to subsequent clinical progress in patients with carcinoma of the prostate treated with bilateral orchidectomy or estrogens. Prostate. 1987;11:17–21. doi: 10.1002/pros.2990110103. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986;15:213–228. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- Madden JD, Milewich L, Parker CR, Carr BR, Boyar RM, MacDonald PC. The effect of oral contraceptive treatment on the serum concentration of dehydroisoandrosterone sulfate. Am J Obstet Gynecol. 1978;132:380–384. doi: 10.1016/0002-9378(78)90771-8. [DOI] [PubMed] [Google Scholar]
- Martel C, Sourla A, Pelletier F, Labrie C, Fournier M, Picard S, Li S, Stojanovic M, Labrie F. Predominant androgenic component in the stimulatory effect of dehydroepiandrosterone on bone mineral density in the rat. J Endocrinol. 1998;157:433–442. doi: 10.1677/joe.0.1570433. [DOI] [PubMed] [Google Scholar]
- Masters A, Hahnel R. Investigation of sex hormone-binding globulin interference in direct radioimmunoassays for testosterone and estradiol. Clin Chem. 1989;35:979–984. [PubMed] [Google Scholar]
- Middle JG. Dehydroepiandrostenedione sulphate interferes in many direct immunoassays for testosterone. Ann Clin Biochem. 2007;44:173–177. doi: 10.1258/000456307780118082. [DOI] [PubMed] [Google Scholar]
- Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endo Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutos D, Smith S, Zacur H. The effect of monophasic combinations of ethinyl estradiol and norethindrone on gonadotropins, androgens and sex hormone binding globulin: a randomized trial. Contraception. 1995;52:105–109. doi: 10.1016/s0010-7824(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Murphy AA, Cropp CS, Smith BS, Burkman RT, Zacur HA. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertil Steril. 1990;53:35–39. [PubMed] [Google Scholar]
- Odlind V, Milson I, Persson I, Victor A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81:482–490. [PubMed] [Google Scholar]
- Oettel M, Carol W, Gräser T, Klinger G, Mellinger U, Moore C, Schindler AE, Winkler UH. Effect of ethinyl estradiol-dienogest combination on serum androgen concentrations. Zentralbl Gynakol. 1997;119:597–606. German. [PubMed] [Google Scholar]
- Oranratanaphan S, Taneepanichskul S. A double blind randomized control trial, comparing effect of drospirenone and gestodene to sexual desire and libido. J Med Assoc Thailand. 2006;89(Suppl. 4):S17–S22. [PubMed] [Google Scholar]
- Ott SM. Reproductive hormones and skeletal health in young women. J Clin Endocrinol Metab. 2008;93:1175–1177. doi: 10.1210/jc.2008-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SM, Scholes D, Lacroix AZ, Ichikawa LE, Yoshida CK, Barlow WE. Effects of contraceptive use on bone biochemical markers in young women. J Clin Endocrinol Metab. 2001;86:179–185. doi: 10.1210/jcem.86.1.7118. [DOI] [PubMed] [Google Scholar]
- Pasinetti E, Falsetti L. Triphasic pills: variability of endocrine parameters and of sex steroid-binding globulins. Acta Eur Fertil. 1993;24:67–70. [PubMed] [Google Scholar]
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27–43. doi: 10.3109/13625187.2012.728643. [DOI] [PubMed] [Google Scholar]
- Pesant M-H, Desmarais G, Fink GD, Baillargeon J-P. 2012 Reference ranges for total and calculated free and bioavailable testosterone in a young healthy women population with normal menstrual cycles or using oral contraception. Clin Biochem. 2012;45:148–150. doi: 10.1016/j.clinbiochem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Refn H, Kjær A, Lebech A-M, Borggaard B, Schierup L. Clinical and hormonal effects of two contraceptives: correlation to serum concentrations of levonorgestrel and gestodene. Contraception. 1990;41:259–269. doi: 10.1016/0010-7824(90)90067-6. [DOI] [PubMed] [Google Scholar]
- Rickenlund A, Carlström K, Ekblom B, Brismar TB, Von Schoultz B, Hirschberg AL. Effects of oral contraceptives on body composition and physical performance in female athletes. J Clin Endocrinol Metab. 2004;89:4364–4370. doi: 10.1210/jc.2003-031334. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of Free Testosterone and Free Estradiol Determinations in Serum Samples from Postmenopausal Women by Theoretical Calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–1071. [PubMed] [Google Scholar]
- Rosenfield RL, Ehrlich EN, Cleary RE. Adrenal and ovarian contributions to the elevated free plasma androgen levels in hirsute women. J Clin Endocr. 1972;34:92–98. doi: 10.1210/jcem-34-1-92. [DOI] [PubMed] [Google Scholar]
- Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95:4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus JR, Azziz R, Sluss PM, Hershel R. Utility, limitations and pitfall in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Ružić L, Matković BR, Leko G. Antiandrogens in hormonal contraception limit muscle strength gain in strength training: comparison study. Croat Med J. 2003;44:65–68. [PubMed] [Google Scholar]
- Sanam M, Ziba O. Desogestrel+ethinylestradiol versus levonorgestrel +ethinylestradiol: Which one has better affect on acne, hirsutism, and weight change. Saudi Meds J. 2011;32:23–26. [PubMed] [Google Scholar]
- Sänger N, Stahlberg S, Manthey T, Mittmann K, Mellinger K, Mellinger U, Lange E, Kuhl H, Wiegratz I. Effects of an oral contraceptiva containing 30 mcg ethinyl estradiol and 2 mg dienogest on thyroid hormones and androgen parameters: conventional vs. extended-cycle use. Contraception. 2008;77:420–425. doi: 10.1016/j.contraception.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Schaffir J. Hormonal contraception and sexual desire: a critical review. J Sex Marital Ther. 2006;32:305–314. doi: 10.1080/00926230600666311. [DOI] [PubMed] [Google Scholar]
- Schaffir JA, Isley MM, Woodward M. Oral contraceptives vs injectable progestin in their effect on sexual behavior. Am J Obstet Gynecol. 2010;203:545.e1–e5. doi: 10.1016/j.ajog.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JHH. Classification and pharmacology of progestins. Maturitas. 2003;46S1:S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Siegberg R, Nilsson CG, Stenman UH, Widholm O. Sex hormone profiles in oligomenorrheic adolescent girls and the effect of oral contraceptives. Fertil Steril. 1984;41:888–893. doi: 10.1016/s0015-0282(16)47903-3. [DOI] [PubMed] [Google Scholar]
- Sobbrio GA, Granata A, Granese D, D'Arrigo F, Panacea A, Nicita R, Pullè C, Trimarchi F. Sex hormone binding globulin, cortisol binding globulin, thyroxine binding globulin, ceruloplasmin: changes in treatment with two oral contraceptives low in oestrogen. Clin Exp Obstet Gynecol. 1991;18:43–45. [PubMed] [Google Scholar]
- Song S, Chen JK, Yang PJ, He ML, Li LM, Fan BC, Rekers H, Fotherby K. A cross-over study of three oral contraceptives containing ethinyloestradiol and either desogestrel or levonorgestrel. Contraception. 1992;45:523–532. doi: 10.1016/0010-7824(92)90103-z. [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17ß to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Speroff L, Fritz M. Clinical gynecologic endocrinology and infertility. 7th edn. Philadelphia, USA: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- Spona J, Huber J, Schmidt JB. Inhibition of ovulation with 35 micrograms of ethinyl estradiol and 2 mg of cyproterone acetate (Diane 35) Geburtshilfe Frauenheilkd. 1986;46:435–438. doi: 10.1055/s-2008-1026659. German. [DOI] [PubMed] [Google Scholar]
- Spona J, Feichtinger W, Kindermann C, Wünsch C, Brill K. Inhibition of ovulation by an oral contraceptive containing 100 micrograms levonorgestrel in combination with 20 micrograms ethinylestradiol. Contraception. 1996;54:299–304. doi: 10.1016/s0010-7824(96)00183-7. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Leyden J, Kempers S, Shalita A, Harrison DD. The effects of an oral contraceptive containing 20 µg ethinyl estradiol and 100 µg levonorgestrel (EE 20/LNG 100) on ovarian, adrenal and peripheral androgens. Gynecol Endocrinol. 2000;14(Suppl. 2):137. [Google Scholar]
- Strufaldi R, Pompei LM, Steiner ML, Cunha EP, Ferreira JAS, Peixoto S, Fernandes CE. Effects of two combined hormonal contraceptives with the same composition and different doses on female sexual function and plasma androgen levels. Contraception. 2010;82:147–154. doi: 10.1016/j.contraception.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Sulak P, Lippman J, Siu C, Massaro J, Godwin A. Clinical comparison of triphasic norgestimate/35 micrograms ethinyl estradiol and monophasic norethindrone acetate/20 micrograms ethinyl estradiol: cycle control, lipid effects, and user satisfaction. Contraception. 1999;59:161–166. doi: 10.1016/s0010-7824(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Taieb J, Mathian B, Millot F, Patricot M-C, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, Ballagh SA, Nichols M, Weber ME. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255–262. doi: 10.1016/s0010-7824(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Traish A, Guay AT, Spark RF the Testosterone Therapy in Women Study Group. Are the Endocrine Society's clinical practice guidelines on androgen therapy in women misguided? A commentary. J Sex Med. 2007;4:1223–1235. doi: 10.1111/j.1743-6109.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JHH. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–352. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- Van Kammen E, Thijssen JHH, Rademaker B, Schwarz F. The influence of hormonal contraceptives on sex hormone binding globulin (SHBG) capacity. Contraception. 1975;11:53–59. doi: 10.1016/0010-7824(75)90050-5. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Thiery M. Metabolic effects of the triphasic oral contraceptive Trigynon®. Contraception. 1982;26:505–513. doi: 10.1016/0010-7824(82)90149-4. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Stoïca T, Verdonck L. The apparent free testosterone concentration, an index of androgenicity. J Clin Endocrinol Metab. 1971;33:759–767. doi: 10.1210/jcem-33-5-759. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Vesper HW, Botelho JC, Shacklady C, Smith A, Myers GL. CDC project on standardizing steroid hormone measurements. Steroids. 2008;73:1286–1292. doi: 10.1016/j.steroids.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Vesper HW, Bhasin S, Wang C, Tai SS, Dodge LA, Singh RJ, Nelson J, Ohorodnik S, Clarke NJ, Salameh WA, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids. 2009;74:498–503. doi: 10.1016/j.steroids.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol. 2010;121:513–519. doi: 10.1016/j.jsbmb.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Volpe A, Silferi M, Mauri A, Deiana P, Angioni S, Grasso A, Genazzani AR. Efficacy on hyperandrogenism and safety of a new oral contraceptive biphasic formulation containing desogestrel. Eur J Obstet Gynecol Reprod Biol. 1994;53:205–209. doi: 10.1016/0028-2243(94)90120-1. [DOI] [PubMed] [Google Scholar]
- White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. Am J Obstet Gynecol. 2005;192:2055–2059. doi: 10.1016/j.ajog.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Jung-Hoffmann C, Kuhl H. Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception. 1995;51:341–346. doi: 10.1016/0010-7824(95)00098-u. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, Kuhl H. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. doi: 10.1016/s0010-7824(02)00436-5. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N. Androgen therapy in women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- Winkler UH, Sudik R. The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. 2009;79:15–23. doi: 10.1016/j.contraception.2008.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.