Abstract
200 years of research with carbon-rich molecules have shaped the development of modern chemistry. Research pertaining to the chemistry of boron-rich species has historically trailed behind its more distinguished neighbor (carbon) in the periodic table. Notably, a potentially rich and, in many cases, unmatched field of coordination chemistry using boronrich clusters remains fundamentally underdeveloped. Our work has been devoted to examining several basic concepts related to the functionalization of icosahedral boron-rich clusters and their use as ligands, aimed at designing fundamentally new hybrid molecular motifs and materials. Particularly interesting are icosahedral carboranes, which can be regarded as 3D analogs of benzene. These species comprise a class of boron-rich clusters that were discovered in the 1950s during the “space race” while researchers were developing energetic materials for rocket fuels. Ultimately, the unique chemical and physical properties of carborane species, such as rigidity, indefinite stability to air and moisture, and 3D aromaticity, may allow one to access a set of properties not normally available in carbon-based chemistry. While technically these species are considered as inorganic clusters, the chemical properties they possess make these boron-rich species suitable for replacing and/or altering structural and functional features of the organic and organometallic molecules—a phenomenon best described as “organomimetic”. Aside from purely fundamental features associated with the organomimetic chemistry of icosahedral carboranes, their use can also provide new avenues in the development of systems relevant to solving current problems associated with energy production, storage, and conversion.
Keywords: boron clusters, dicarbollides, dye-sensitized solar cells, icosahedral carboranes, ligand design, metal–organic frameworks, organomimetic chemistry
HISTORICAL BACKGROUND
Around 1910, Alfred Stock began his pioneering work on boranes (boron hydrides), which, at that time, were considered to be extremely exotic and elusive species [1]. The pyrophoric nature of these materials required air-free conditions, making their syntheses and the elucidation of these borane structures particularly challenging. Consequently, this led to the invention of the vacuum line by Stock and co-workers, thus allowing for the separation and characterization of elementary boranes via vacuum distillation (Fig. 1).
Fig. 1.
Alfred Stock circa 1932 (left) and Stock’s original vacuum line apparatus featuring U-traps (right). Notably, the invention of such vacuum lines served as a prototype for the conventionally used Schlenk line for handling air- and moisture-sensitive compounds today. Images were taken from ref. [1].
Another challenge Stock and his co-workers faced was the lack of a pure source of elemental boron. Therefore, magnesium boride (Mg3B2) was treated with strong inorganic acids to obtain boron hydrides. However, Mg3B2 still contained a large amount of magnesium silicide impurity, leading to the concomitant formation of silane by-products. This prompted Stock to examine the properties of many silane species as well, thus pioneering the field of organosilicon chemistry.
Writing to the British Chemical Society in 1917, Stock was comparing existing carbon-based chemistry with recently explored silicon, noting [2]:
“To prevent misunderstandings the meaning of several words used here must be explained. Affinity is the expression for the firmness with which one element binds other elements or radicals (generally: “Ligands” (ligare[Latin], to bind); the introduction of a word hitherto lacking simplifies the manner of expression for this immediately clear concept). Valence (Valenz) means the unit of force which can bind a univalent ligand; positive valencies bind negative ligands, negative valencies bind positive ligands. Atomicity (Wertigkeit) is the number of valencies which an atom manifests; the highest atomicity is the highest number of valencies observed for an element.”
Therefore, Stock can be also credited in coining the word “ligand” roughly 30 years prior to the widespread use of such terminology within the chemical community. While Stock and co-workers are credited for the isolation and characterization of the “simple” boron hydride species (e.g., B2H6, B4H10, B5H9, B5H11, B6H10, and B10H14), structural studies revealing their unique bonding characteristics were only accomplished several decades later by Lipscomb and co-workers [3]. The fundamental work conducted by Lipscomb preceded a so-called renaissance era for boron chemistry, which originated as a part of the Manhattan project aimed at producing U(IV) borohyride [U(BH4)4]—the most volatile uranium-based material known at that time [4]. Though the use of U(BH4)4 for nuclear fuel enrichment was not implemented, several immensely important discoveries emerged from its research effort, including the synthesis of sodium borohydride (NaBH4) [5], hydroboration chemistry developed later by Brown [6], and even the serendipitous discovery of polyfluorinated polymers [7].
The military-funded boron saga continued after WWII, where the need for high-energy fuels led researchers to revisit Stock’s chemistry. Given the fact that the B–H bond contains more energy than the carbon analog, it was thought that fuels based on boranes could be used for long-range bomber planes (such as the XB-70 Valkyrie) [8]. To continue the trend of military-funded failures regarding boron chemistry, the effort was abandoned after engineers came to the realization that borates formed as a result of borane combustion clogged the jet engines, thus preventing boranes in becoming efficient rocket fuels [8]. Yet, as a consequence of substantial funding directed towards exploratory chemistry during this time, a fundamental paradigm shift in the field of boron clusters occurred. This was sparked with a seminal discovery in 1960 led by Hawthorne and co-workers, who reported the synthesis of the dodecaborate (B12H122−) anion (Fig. 2) [9]. Strikingly, at the time of the discovery made by Hawthorne and co-workers, carbon-based chemistry featured more than a million known molecules, whereas boron-rich cluster chemistry at that point of time only explored a handful of structurally exotic species (at maximum 50) [3].
Fig. 2.
Original syntheses of the dodecaborate B12H122− anion: (i) vacuum pyrolysis, 120 °C; (ii) I2/AlCl3, CH2Cl2 (iii) Et3N, benzene, reflux; and ortho-carborane species.
Hawthorne’s discovery demonstrated that robust boron clusters could be synthesized. In fact, the cesium salt of B12H122− could be heated up to 600 °C in air without decomposition! Perhaps even more important and striking were the developments demonstrating the unique derivatization reactivity of B12H122− and several similar boron clusters. In many instances, the development of B12H122− chemistry resembled the early days of organic chemistry, based on its reactivity similarities with aromatic hydrocarbons [3].
B12H12-based polyhedral structures with different heteroatoms were subsequently developed with resembling chemistries of the parent icosahedral parent platform [10]. In these species, one or more boron vertices are commonly replaced by various atoms such as N, S, Al, P, or C. Of particular interest is the C2B10H12 carborane structural platform (Fig. 2), which is represented by three constitutional isomers (carbon atoms either in the ortho, meta, or para position and further abbreviated in the text as o-, m-, and p-carborane, respectively) [11]. Carboranes do not exclusively consist of two carbon atoms in its skeletal framework, but can have between one to five carbon atoms. C2B10H12 clusters will be discussed further in this manuscript, as they represent a molecular motif that will be a focal point in this review (vide infra).
Over the years, fundamentally new theories describing the nature of chemical bonding had to be developed as a result of the discovery of stable neutral boranes by Stock, and subsequently the synthesis of polyhedral boron-rich anions and their various derivatives containing heteroatoms (such as carborane, metallaboranes, and main-group heteroboranes) all have profoundly influenced many areas of inorganic and organic chemistry. However, given the vast structural and functional diversity presented by these structures, their application has remained sparse in most branches of chemistry. Several recent review articles highlight the potential usefulness of boron-rich clusters in varying fields of chemistry, ranging from medicine to nanoscience [12–14]. It is my hope that the work highlighted in this review will convey to the organometallic and coordination chemistry communities that utilizing carboranes as building blocks for ligands may lead to molecules and materials that do not have structural and functional analogs to carbon-rich (organic) building blocks.
BORON-BASED LIGANDS
From the early discoveries of Prussian blue [15], to the seminal work by Frankland on alkyl-zinc reagents [16], coordination and organometallic chemistry historically have been dominated by carbon-based chemistry. The aforementioned examples are considered to be the earliest known synthetic examples of complexes containing transition-metal–carbon bonds. In stark comparison, transition-metal boron-based complexes were only first reported in the early 1960s by Shriver and Parshall [17,18]. Independently, they reported molecules containing boron moieties coordinated to metal centers in two distinct ligand systems (BF3 and BH3), pioneering boron–metallic chemistry (Fig. 3). Despite this progress, species containing the nucleophilic boron moiety (boryl) were structurally unknown until 1990 [19], in contrast to the thousands of analogous complexes with nucleophilic carbon-, nitrogen-, and oxygen-based ligands [20].
Fig. 3.
Representative structures of several important boron-based ligands.
Recent efforts in designing metal complexes where electron-precise transition-metal–boron bonds are featured suggest that B–M interactions give rise to many unique properties [21]. A particularly interesting discovery made by Braunschweig and co-workers in 2009 pertaining to the oxoboryl ligand (BO−), in fact, suggests a drastic difference between this species and its carbon analog—carbon monoxide (Fig. 3) [22].
One can also argue that progress in boron-based coordination chemistry may have been impeded largely as a consequence of its lack of direct biological relevance. Metal–carbon bonds are prominent in biological systems as a result of the abundance of CN−, CO, and other carbon-based ligands in metallo enzymes [23]. On the other hand, boron species, such as oxoboryl, are purely abiotic and no biologically-inspired predictive forces could be used to justify its potential existence. While such elements of uncertainty in boron chemistry certainly diminish our capacity in target-based research, discoveries like the case of oxoboryl are extremely exciting, since boron research can redefine our fundamental knowledge and perceptions of chemical bonding and reactivity.
LIGAND PLATFORMS FEATURING ICOSAHEDRAL CARBORANES
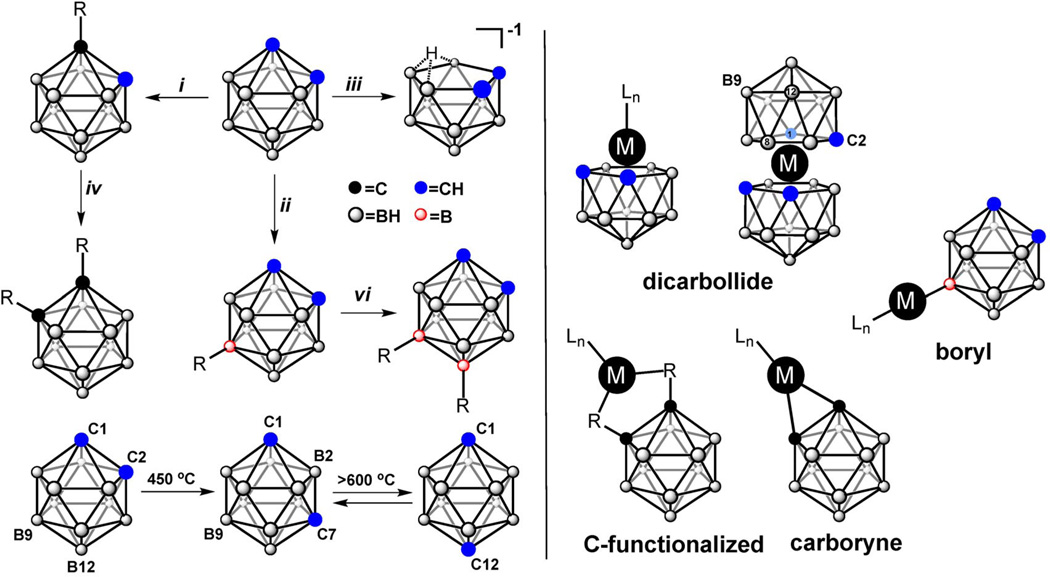
The development of carborane-based ligands has spanned a period of nearly 50 years, and advances in functionalization chemistry pertaining to these species have subsequently followed. o-Carborane and its derivatives can be conveniently synthesized from decaborane and alkyne-based precursors in the presence of a Lewis base; a reaction amendable to both industrial and laboratory bench settings (Fig. 2) [24]. An important feature of o-carborane chemistry is in its ability to undergo isomerization upon heating (Fig. 4). Cage isomerization involves the thermally induced migration of skeletal carbon atoms away from each other, as exemplified in the quantitative conversion of the 1,2-isomer to the 1,7-isomer at 450 °C, and subsequently, the partial rearrangement of the 1,7 species to the 1,12-isomer at higher temperatures (T > 600 °C) [25].
Fig. 4.
Outline of three major reaction types involving icosahedral carboranes based on o-carborane (left): (i), (iv) base-promoted functionalization of the CH vertices; (ii), (vi) electrophilic functionalization of the BH vertices; (iii) nucleophile-promoted deboronation leading to the nido species. Existing coordination complexes and corresponding binding modes involving carborane-based species as ligands (right).
An important structural aspect of carboranes is the presence of one or more carbon atoms in an electron-delocalized cluster framework. As the skeletal carbon and boron atoms are involved in delocalized binding, each atom has five or six neighbors, including hydrogens or other attached functional groups. These molecules create a new class of nonclassical bonding motifs and, therefore, their structures cannot be described through the classical organic bond diagrams, where a connecting line between two atoms would indicate a single two-electron bonding interaction. Instead, proper description of the bonding scenarios exhibited by these species are best described by applying the Wade–Mingos rules (derived from the polyhedral skeletal electron pair theory (PSEPT)) [8,26].
All three C2B10H12 isomers share a common reactivity pattern centered around three major types of reactions. First, cage degradation can occur through the removal of the skeletal boron atoms, where upon reaction with a strong and nonbulky nucleophile, 1,2-C2B10H12 can be converted almost quantitatively to nido anions (C2B9H12−1) (Fig. 4) [27]. The second reaction, which was established in the initial development of carborane research, relies on the weakly acidic character of the C–H hydrogens in the parent clusters [8]. This reactivity property is a consequence of the cluster polarity induced by the electronegative carbon atoms, allowing the use of reagents such as n-butyllithium to generate C–Li species, which can then react with a wide variety of reagents to give mono- and di-substituted derivatives (Fig. 4). The third reaction type arises from the low electronegativity of boron vertices (especially those located farthest away from carbon vertices), making the B–H moieties susceptible to attack by electrophiles (Fig. 4) [8]. Furthermore, B–I carborane-based species, which can be efficiently produced from carboranes via electrophilic iodination, can be coupled with aryl- and some alkyl-based reagents via conventional Pd-catalyzed catalytic methods, making this overall process an attractive strategy for introducing a wide range of substituents onto the boron vertices (vide infra) [28]. Based on their unique properties, carborane species are best described as “organomimetic”, which implies that while many aspects of the carborane chemistry bear similarities to organic and organometallic chemistry, utilization of these cluster molecules may give rise to some fundamentally new or enhanced properties in the system of particular interest. Herein, we describe several recent advances featuring icosahedral carborane species as organomimetic components of ligands utilized in coordination, supramolecular, and materials chemistry.
Ni(III/IV) BIS(DICARBOLLIDE) REDOX SHUTTLES
The ability of placing functional groups at specific carbon and boron vertices of the carborane clusters creates several important organometallic and coordination motifs. In 1965, Hawthorne and co-workers opened a new chapter in boron cluster chemistry by utilizing carboranes as ligands in metal-sandwich complexes [29]. These species incorporate C2B9H112− ligands (referred to as dicarbollide, Fig. 4) and are electronically related to metallocene complexes, though they feature very unique distinctions from their carbon-based surrogates partially due to the (–2) charge formally carried by the dicarbollide species. Several hundreds of metallacarborane complexes have been prepared to date, holding promise in areas ranging from catalysis to nuclear waste remediation [8].
Interestingly, given the plethora of studies in metallocene chemistry demonstrating electronic tenability of these ligands via functionalization, significantly less work has been done with dicarbollide species. Several studies previously showed that substitution can affect properties of the dicarbollide ligands. For example, Hawthorne and co-workers have developed several derivatization routes via CH and B(8) vertex functionalization [30]. The influence on electrochemical properties of dicarbollide complexes by substituents was also studied for Co(II)/Co(III)-based bis(dicarbollide) species functionalized at B(8) positions by Teixidor and co-workers [31]. The same group also reported a series of electrochemically tunable Co-based clusters synthesized via sequential halogenation at multiple boron vertices [32]. Yet, a majority of these strategies only lead to functionalized bis(dicarbollide) species featuring sterically encumbered arrangement of the functional groups respectively to each other [33].
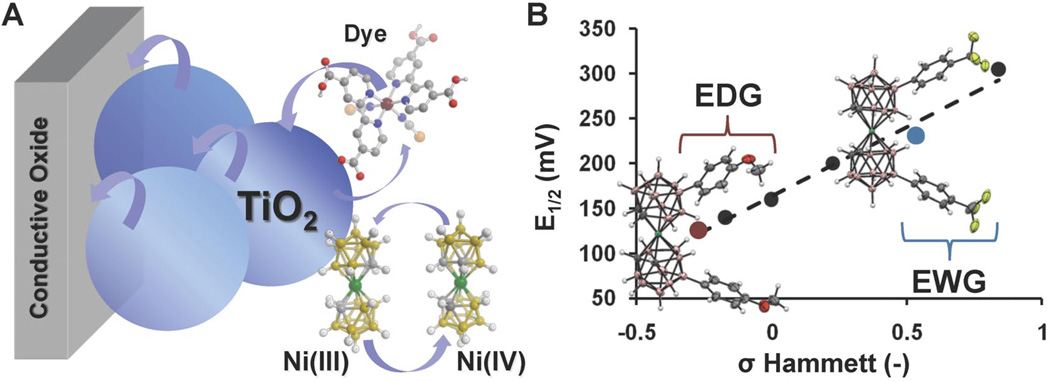
Recently, we reported a discovery pertaining to the molecular design of third-generation solar cell technology [34]. This particular photovoltaic system (referred to as dye-sensitized solar cells, or DSCs), consists of a porous, dye-coated titanium dioxide photoelectrode and a counter electrode (Fig. 5A) [35]. These electrodes typically encapsulate an electrolyte solution containing an iodide/triiodide (I−/I3−) redox shuttle, where I− mediates the regeneration of the dye molecule [36]. However, the iodine-based system is corrosive to many materials, including silver, which is commonly used as a metal inter connect material in commercial modules. We have discovered that unfunctionalized Ni(III/IV) bis(dicarbollide) complex represents a promising new class of DSC redox shuttles, since these compounds can undergo reversible redox processes, are thermally stable, and are noncorrosive [37]. Given the success of incorporating these materials as active components in DSCs, next generation of Ni bis(dicarbollides) was synthesized in order to rationally tune and access a broader range of electrochemical properties to improve the kinetics in functional DSC devices.
Fig. 5.
(A) Schematic representation of a DSC architecture featuring an unfunctionalized Ni(III/IV) bis(dicarbollide) redox shuttle. (B) Hammett plot for Ni(III/IV) redox potentials in B(9/10) arylated bis(dicarbollide) species and two representative molecular structures of Ph-4-OMe and Ph-4-CF3 metallacarborane derivatives.
Aryl functionalization of the Ni-based bis(dicarbollide) species through the B(9/12) vertices of the clusters was chosen in order to minimize repulsive interaction in the resulting carborane ligands (Fig. 5B) [38]. Syntheses of these Ni bis(dicarbollide) derivatives were accomplished in five high-yielding steps, starting from commercially available o-carborane. Through this synthetic route, B(9/12)-arylated bis(dicarbollide) species featuring six different substituents [Ph-4-OMe, Ph-4-Me, Ph, Ph-4-Cl, Ph-4-CF3, and Ph-3,5-(CF3)2] were prepared as stable solids in both their reduced and oxidized forms [Ni(III) and Ni(IV), respectively]. Electrochemical transformations of a series of Ni(III)/Ni(IV) species were studied via cyclic voltammetry. In all cases, the functionalized complexes demonstrated quasireversible, one-electron processes, similar to the parent Ni bis(dicarbollide). Distinct potentials were observed for each metallacarborane derivative, prompting us to establish a correlation of redox potentials with respect to the chemical substituents (Fig. 5B). Plotting these potentials against their Hammett constants yielded a linear relationship, supporting the direct impact of varying the degree of electron donation to or from the dicarbollide results with the molecule’s electrochemical potential (Fig. 5), in contrast to steric effects that may arise with bulky substituents. With this new system, not only we were able to study how systematically varying redox shuttle potentials affects the competing kinetic processes in working devices, but also probe the effects of the different chemical interactions by modifying the periphery of the shuttle with electron-withdrawing or -donating functional groups. As a result of such modifications, higher voltages in these cells were obtained as compared to that of traditional liquid I−/I3− DSCs. As one of the first examples of such a comprehensive study, the importance of redox shuttle chemical compatibility to other internal components in the device and the consequences of a rational molecular design were realized. These studies have provided a deeper understanding of DSCs, helping to pave the way for highly efficient, next-generation solar cells.
LIGAND PLATFORMS BASED ON C-FUNCTIONALIZED ICOSAHEDRAL CARBORANES
Another important class of carborane-based ligands are mono- or bis-C-functionalized o-carboranes, which can be used for a wide variety of tailored metal chelate complexes (Fig. 4). These species are generally kinetically stabilized by the steric bulkiness of the parent cage located in proximity to the metal center. Among widely explored motifs are species featuring phosphine- [39], chalcogenate- [40], nitrogen-based donors [41], and cyclopentadienyl moieties [42]. The latter have been employed effectively as ligands in stable, isolable organolanthanide complexes [43]. Similar complexes having dimethylsilylindenyl units connecting the carborane and metal center have been prepared as well. Among these, several constrained-geometry compounds of this type (analogous to metallocene surrogates) demonstrated promising catalytic activities in olefin polymerization reactions [44,45]. Other interesting coordination motifs utilizing carboranes have been recently explored. Among these is a benzyne analog of o-carborane–carboryne, which can serve as an interesting ligand platform, as well as a useful synthon for metal-catalyzed cycloaddition derivatization. Since its discovery as an active intermediate in the 1950s, benzynes have found many applications in organic synthesis [46], mechanistic studies [47], and the synthesis of functional materials. Even so, it was not until 1990 that the carboryne species were first reported by Jones and co-workers [48]. Similar to benzyne, the in situ generated carboryne reacts with alkenes, dienes, and alkynes in [2 + 2] cycloaddition and ene reaction fashions [49]. However, the chemistry of metal-carboryne complexes has been largely unexplored until recent contributions by Xie and co-workers, who isolated and studied a significant number of these species (Fig. 4) [50].
CARBORANE-BASED METAL–ORGANIC FRAMEWORKS (MOFs)
The examples in the previous section highlight an important stability feature the carborane-based metal–organic hybrid molecules generally possess. While icosahedral carboranes have been extensively utilized previously as geometrically precise building blocks in polymer and oligomer construction, only a few reports have disclosed merging metal-directed supramolecular assembly using carborane-based species. One particular example includes the work of Hawthorne and Stang, who utilized linear pyridine-functionalized p-carborane clusters for the self-assembly of Pt(II) macrocycles [51]. In the past several years, we have developed and explored the possibility of gas storage and separation with carborane-based MOFs (also known as coordination polymers). In general, MOFs represent an infinite array of organic-based ligands, interconnected with metal nodes, collectively forming a 3D framework of channels and nanometer-sized pores, which can serve as molecular wells to store and separate gases and small molecules [52,53]. Thermal instability, however, is a significant drawback of MOFs, which precludes efficient host–guest exchange at elevated temperatures. We envisioned that utilizing p-carborane as a robust building block for these materials would significantly improve the overall thermal stability of these materials (Fig. 6).
Fig. 6.
(A) Explored p-carborane-based ligands for MOF synthesis. (B) Unactivated and (C) activated Zn(II)-based MOF featuring ligand I as a building block.
As such, we discovered that the synthesized carborane coordination polymer based on Zn(II) structures exhibit unprecedented stability with respect to thermal degradation, inherited from the carborane ligand. This phenomenon qualitatively arises due to increased thermal stability of the carborane linker itself, as well as its sterically encumbering nature capable of protecting metal-based secondary building unit (SBU) [54]. As a result, these new MOFs show no collapse of the framework even at elevated temperatures (500 °C), which was unprecedented, and ultimately allows the activation of these materials by heating under vacuum. Such activation also allows generating uncoordinated metal sites within the pores, and significantly enhancing hydrogen uptake. Additionally, the presence of such sites influenced selectivity in gas mixture separations [55]. In particular, this process works well for mixtures of methane and carbon dioxide, potentially making these materials promising in gas purifications and carbon dioxide sequestration at a significantly lower energy cost. A similar MOF composed of Co-based metal SBU exhibited interesting gas sorption selectivities between O2 and N2 gases [56,57].
Notably, utilization of carborane-based building blocks for the construction of porous coordination polymers has been recently extended to lanthanide-based SBUs [58] and diacid-functionalized meta-carborane [59] ligand by Jin and co-workers. Similar to MOFs featuring carbon-rich linkers, one can utilize available functionalization chemistry of carboranes to extend the available ligand pool to generate a diverse array of porous materials (Fig. 6, ligands II and III) [60,61]. While technically carborane-based MOFs featuring ligand I can be classified as purely “inorganic” materials, their structural resemblance to the carbon-rich MOFs further highlights how the concept of organomimetic chemistry is pertinent to these boron-rich clusters.
CARBORANE-BASED PINCER LIGANDS FEATURING METAL–BORYL INTERACTION
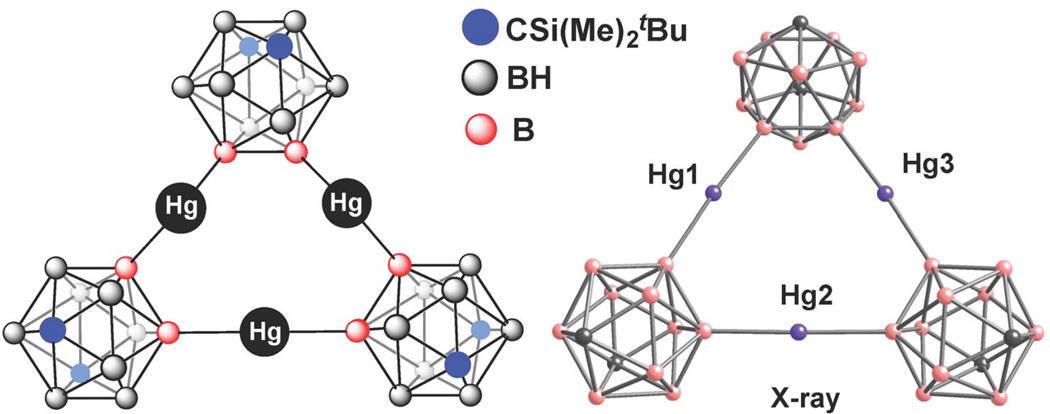
In contrast to the C-functionalized derivatives, only a few well-characterized derivatives of carboranes having exo-polyhedral transition-metal atoms directly bonded to boron are known. These boron-metallated species are important intermediates in the transition-metal-catalyzed coupling reactions. Several notable B-functionalized systems were reported originally by Hawthorne and co-workers including a metal-based complex featuring an Ir–B interaction characterized in solution [62a] and a macrocyclic species with three Lewis acidic Hg(II) centers [62b] (Fig. 7). In light of the overall scarcity of B-based carborane ligand platforms [62c], we embarked on a quest to develop new ligand platforms featuring these clusters, possessing unique structural and/or functional properties.
Fig. 7.
Mercurocarborand complex featuring a unique B-Hg-B coordination motif. The crystal structure is adapted from ref. [62b] (hydrogen atoms, solvents, and solubilizing groups [Si(Me)2tBu] functionalized on C atoms of the carboranyl cages are omitted for clarity).
Pincer complexes represent an exciting platform for such development due to its generally high rigidity and stability. Tunability of a pincer ligand arises from its molecular design, hierarchically starting from base ligand, to donor arms and to anchoring and auxiliary sites [63]. Until recently, the base has been made primarily from hydrocarbons, including aromatic groups such as benzene and the corresponding heteroatom variants [64], aliphatic moieties [65], and more recently developed carbene-type species (Fig. 8A) [66]. Surprisingly, it was not until our report in 2009 where icosahedral carboranes were first explored as base units in pincer complexes, where we presented the carborane-based pincer ligand motif and its respective Pd(II) complexes [67,68]. The structures obtained represented a new class of compounds with a previously unobserved Pd–B boryl σ-coordination within a pincer ligand framework.
Fig. 8.
(A) Representative examples and general anatomy of “pincer” complexes. (B) Crystallographically derived X-ray structure of the SeBSe pincer Pd(II) complex and (C) 11B NMR spectral changes associated with the palladation of the SeBSe ligand.
The carborane-based SeBSe pincer ligand can be synthesized in four steps starting with commercially available m-carborane and further metallated with Pd(II) precursor under nonforcing conditions. The molecular structure of the resulting SeBSe–Pd(II) complex in the solid state (Fig. 8B) was confirmed by single-crystal X-ray diffraction analysis. Compared to similar motifs featuring aryl-based pincer ligands, the Pd–Cl bond length in this carborane-based complex is somewhat longer, indicating a stronger trans influence of the carborane boryl moiety on the m-carborane cage than the phenyl-based analog. This structure represents the first crystallographically characterized Pd–B interaction for m-carborane species and metal–B(2) bond in general. As a proof of generality we also were able to synthesize and characterize a similar SBS pincer analog ligand and its corresponding Pd(II) complex. In both cases, we demonstrated that the electronic structure of the Pd–B interaction is substantially different from the previously studied hydrocarbon-based pincer analogs through density functional theory (DFT) calculations. Calculated Mulliken charge densities suggest that there is an overall negative charge localized on the Pd atom, and a positive one concentrated on B(2). The opposite trend is observed for the conventional aryl C–Pd motif charge distribution, wherein the net negative charge is localized on the carbon and the positive charge on the metal. This phenomenon can be explained by a combination of electron-donating property of the carboranyl cage functionalized through the B(2) moiety as well as the shift in the electronegativity of a Pd–B interaction vs. Pd–C congener.
Owing to a straightforward synthesis of these pincer ligands, we anticipate the possibility of creating a wide-range library of carborane-based pincer complexes using other metals and heteroatoms. Furthermore, diverse methods of introducing an anchoring modification through the B–H vertices of the carborane can be envisioned. Such structures, owing to the high stability, rigidity, and unique electronic properties imposed by the cage, should serve as interesting models for a variety of complexes featuring boryl–metal interactions. Importantly, recent work by Nakamura and co-workers demonstrated a possibility of utilizing related carborane-based NBN pincer complexes for catalytic applications [69].
Simultaneously with our work, Yamashita, Nozaki, and co-workers extended the chemistry on boryllithium and related isolable boryl precursors [70], and reported an elegant synthesis of a bis(phosphine)-functionalized hydro(diamino)borane pincer ligand (PBP) [71]. Oxidative addition of the B–H bond to an Ir-based precursor generated the first boryl-based pincer scaffold, coordinated to an Ir(III) metal center (Fig. 9). Yamashita and co-workers further explored this concept for the development of a Pt-based PBP pincer hydrosilylation catalyst [72].
Fig. 9.
Tridentate PBP pincer complex featuring boryl moiety prepared by Yamashita, Nozaki, and co-workers (H atoms are omitted for clarity in the crystallographically derived structure of PBP Ir(III) complex).
It remains to be seen whether the described boryl-based pincer species possess any advantages over existing ligand platforms for transition-metal complexes relevant to catalysis. Nevertheless, the introduction of boron as a new element in the family of hetero-donor-functionalized tridentate pincer ligands is a fundamentally new concept and deserves further attention from the chemistry community. Given that boron–metal interactions derived from the use of carboranes are fundamentally different from the carbon–metal analogs, boryl-based species described in this section can ultimately lead to isolation of metal-based species with unique reactivity.
CARBORANES AS DICHOTOMOUS LIGAND SUBSTITUENTS
The properties of most ligands currently employed by chemists are modulated by the attachment of electron-donating or -withdrawing groups to the heteroatom moiety involved in metal binding. A similar strategy is utilized to influence steric features of these ligands. The majority of functional groups capable of performing these modulations have historically been carbon-based [73]. Normally, alkyl-based moieties possess inductive electron-donating properties, whereas aryl-based species are generally more electron-withdrawing. Furthermore, additional substituents attached to aryl and alkyl moieties are used to alter and fine-tune the steric constraints or electronic parameters of a desired ligand. Yet, it is very challenging to keep the steric features of a specific ligand constant while affecting the electronic properties, and it is nearly impossible to do so when significant changes in electronics are necessary (e.g., changing a ligand from being very electron-rich to very electron-poor). Such modulation, for example, can be potentially important for tuning the catalytic activity of metal-based species. It is thus desirable to develop a fundamentally new chemical platform, which would possess properties allowing the adjustment of electron-donating or -withdrawing properties of ligands without drastically altering their steric parameters. Previous studies by Hawthorne and co-workers on mercurocarborand complexes (Fig. 7) suggested that this modulation in principle could be accomplished by using the electronic differences associated with inductive electronic effects exerted by B- and C-bound carboranyl species, respectively [62b].
In our recent work [74], we developed a new class of ligands that demonstrated a proof-of-concept examples, where we challenged a fundamental paradigm of ligand design via tuning steric and electronic properties of ligands with carbon-based substituents. With a series of phosphine-thioether chelating hemilabile ligands featuring m- or o-carborane substituents on the sulfur atom, we showed how the platinum–sulfur interaction of Pt(II)-thioether complexes can be tuned depending on the positional attachment of the sulfur at the specific vertex of the boron-rich cluster. Experimental results comparing a series of carbon-based and boron-cluster-based complexes featuring these hemilabile ligands showed that icosahedral carboranes can act either as strong electron-withdrawing ligands or bulky electron-donating moieties (similar to aryl- or alkyl-based groups, respectively), depending on the specific atom of the carborane cage that is attached to the thioether moiety. This work was preceded by the observation of Plešek et al. who discovered that o- and m-carborane thiol species functionalized on different vertices of these clusters exhibit drastic pKa differences in these analogs, where B(9)-based thiols have an acidity ~ 105–106 times lower than the corresponding carboranes functionalized on the C(1) atom (note that this range in pKa values is similar to the case of alkyl- and aryl-based thiols) [75]. This work is also consistent with the previous studies by Teixodor and Viňas and co-workers, who showed that a methyl substituent acts as an inductively electron-withdrawing group when attached to several vertices in carborane clusters [76]
Following our initial effort related to the thio- and selenoether-based ligands, we have expanded the generality of this concept to phosphine species by developing a new class of stable and extremely electron-rich carborane phosphines featuring the B9-functionalized m-carboranyl substituent [77]. These species were prepared via Pd-catalyzed phosphination of 9-iodo-m-carborane and were found to be air-stable in their pure form. While the syntheses of pentavalent phosphorous compounds containing carboranyl moieties connected through boron atom were previously pioneered by the work of Bregadze and co-workers [77b–d], these new phosphinoboranes represent the first examples of trivalent alkyl and/or aryl phosphines featuring B-connected carborane-based substituent. Importantly, we observed that the B9-based m-carboranyl moiety exhibited more electron-releasing character than any carbon-based substituent. Crystallographic and IR spectroscopy studies conducted with Rh(I) complexes featuring these ligands further suggested that several of the synthesized B(9)-based phosphinoborane species are more electron-rich than any made to date trivalent phosphines containing only carbon-rich substituents (Fig. 10). There results are also in agreement with the trends observed by Bregadze et al. for related phosphonate species [77c,d]. In combination with the previously developed carbon-based carborane phosphines [8], it should be now possible to envision a library of phosphine ligands, where electronic properties can be adjusted without affecting their steric profiles.
Fig. 10.
(A) Sterically invariant, electronically tunable carborane-based hemilabile ligands and their relations to ligands containing carbon-rich substituents. (B) Synthetic route towards B-connected m-carboranyl phosphines. Corresponding Rh(I) complexes featuring these phosphinoborane ligands were used to probe their extremely electron-rich character via IR spectroscopy.
Overall, our work suggests that a heteroatom attached to different vertices of icosahedral carboranes can experience a drastically different electronic influence. The magnitude of this effect can be really substantial, where both strongly electron-withdrawing (similar to perfluorinated aryl moieties) and strongly electron-donating (similar to electron-donating bulky alkyl moieties) influences could be observed. Furthermore, given several recent developments enabling synthetic routes towards precise functionalization of the vertices in these clusters previously considered difficult or impossible to attain, one can anticipate a much broader scope of ligand tuning [78–82]. Therefore, this unique property of icosahedral carboranes may potentially provide a way for chemists to overcome a fundamental limitation associated with the electronic tunability window and inability to orthogonalize steric and electronic properties of ligands when using classical carbon-based substituents. Furthermore, as was recently shown by Lavallo and co-workers [83], utilization of perhalogenated carborane substituted ligands can drastically improve stability of the corresponding metal-based complexes leading to species with unprecedentedly high catalytic reactivity.
CONCLUSIONS
The examples described in this manuscript highlight some recent developments demonstrating how carboranes and metallacarboranes have been successfully employed as organomimetic substituents within several ligand platforms (Fig. 11). These examples demonstrate how properly designed carborane derivatives, by virtue of their enhanced stability, tailorability, electronic properties, and other important properties, can provide performance superior to that of conventional organic or organo - metallic systems. However, the potential for application of carborane chemistry may ultimately extend well beyond the bonding motifs and applications presented. The most intriguing developments that can be achieved with boron clusters are likely going to be the ones involving molecular architectures or properties that are not encountered in the absence of boron. Unique properties of carborane species, such as rigidity, stability towards air and moisture, and 3D aromaticity, generated ligands not normally available through classical carbon-based chemistry. The chemistry presented herein has also highlighted how these clusters can be utilized in the development of porous materials for gas storage and separations, and as active molecular components in photovoltaic devices.
Fig. 11.
Summary of organomimetic ligand platforms featuring carboranyl clusters (M = metal, X/R = heteroatom-based substituents).
ACKNOWLEDGMENTS
I would like to thank Prof. Chad A. Mirkin for providing me with an intellectually stimulating and supportive environment in his research group during the course of my graduate studies at Northwestern University and Profs. Stephen L. Buchwald and Bradley L. Pentelute for their help and support during my postdoctoral stint in their laboratories at MIT. I am indebted to all my colleagues and collaborators whose names are mentioned in the references. I furthermore thank Dr. Tina C. Li, Ms. Ekaterina V. Vinogradova, and anonymous referees of this manuscript for their critical input during the preparation of this work. A. M. S. is currently a recipient of the National Institutes of Health post-doctoral fellowship (1F32GM101762). The content of this manuscript is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Pure Appl. Chem. 85, 883–956 (2013). A collection of invited, peer-reviewed articles by the winners of the 2012 IUPAC Prize for Young Chemists.
This article is dedicated to Prof. M. Frederick Hawthorne for his pioneering studies in the field of boron-cluster chemistry, and for sparking my interest in this field during my undergraduate studies at UCLA.
REFERENCES
- 1.Stock A. Hydrides of Boron and Silicon (The George Fisher Baker Non-Resident Lectureship in Chemistry at Cornell University) Ithaca, NY: Cornell University Press; 1933. [Google Scholar]
- 2.Brock WH, Jensen KH, Jørgensen CK. Polyhedron. 1983;2:1. [Google Scholar]
- 3.a Lipscomb WN. The Boranes and Their Relatives, Nobel Lecture, December. 1976;11 [Google Scholar]; b Muetterties E. Boron Hydride Chemistry. New York: Academic Press; 1975. [Google Scholar]
- 4.Ephritikhine M. Chem. Rev. 1997;97:2193. doi: 10.1021/cr960366n. [DOI] [PubMed] [Google Scholar]
- 5.Schlesinger HI, Brown HC, Abraham B, Bond AC, Davidson N, Finholt AE, Gilbreath JR, Hoekstra H, Horvitz L, Hyde EK, Katz JJ, Knight J, Lad RA, Mayfield DL, Rapp L, Ritter DM, Schwartz AM, Sheft I, Tuck LD, Walker AO. J. Am. Chem. Soc. 1953;75:186. [Google Scholar]
- 6.Brown HC, Rao BC. J. Org. Chem. 1957;22:1137. [Google Scholar]
- 7.Biographical Memoirs of the National Academy of Sciences. Vol. 64. Washington, DC: The National Academies Press; 1994. pp. 368–396. [Google Scholar]
- 8.Grimes R. Carboranes. 2nd ed. Elsevier; 2011. [Google Scholar]
- 9.Original synthesis: Pitochelli AR, Hawthorne MF. J. Am. Chem. Soc. 1960;82:3228. recently optimized process amenable to laboratory synthesis: Geis V, Guttsche K, Scherer H, Uzun R. Dalton Trans. 2009:2687. doi: 10.1039/b821030f.
- 10.Grimes RN. Metal Interactions with Boron Clusters. New York: Plenum Press; 1982. [Google Scholar]
- 11.Original synthesis: Heying TL, Ager JW, Jr, Clark SL, Mangold DJ, Goldstein HL, Hillman M, Polak RJ, Szymanski JW. Inorg. Chem. 1963;2:1089.
- 12.Scholz M, Hey-Hawkins E. Chem. Rev. 2011;111:7035. doi: 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- 13.Dash BP, Satapathy R, Maguire JA, Hosmane NS. New J. Chem. 2011;35:1955. [Google Scholar]
- 14.Issa F, Kassiou M, Rendina LM. Chem. Rev. 2011;111:5701. doi: 10.1021/cr2000866. [DOI] [PubMed] [Google Scholar]
- 15.Review article (see references within): Kraft A. Bull. Hist. Chem. 2008;33:61. structural study (see references within): Buser HJ, Schwarzenbach D, Petter W, Ludi A. Inorg. Chem. 1977;16:2704.
- 16.a Frankland E. Experimental Researches in Pure, Applied and Physical Chemistry. London: John Van Voorst; 1877. [Google Scholar]; b Seyferth D. Organometallics. 2001;20:2940. [Google Scholar]
- 17.Shriver DF. J. Am. Chem. Soc. 1963;85:3509. [Google Scholar]
- 18.Parshall GW. J. Am. Chem. Soc. 1964;86:361. [Google Scholar]
- 19.a Merola JS, Knorr JR. Abstracts of Papers; 199th National Meeting of the American Chemical Society; Boston, MA. Washington, DC: American Chemical Society; 1990. p. 392. INOR. [Google Scholar]; b Knorr JR, Merola JS. Organometallics. 1990;9:3008. [Google Scholar]; c Baker RT, Ovenall DW, Calabrese JC, Westcott SA, Taylor NJ, Williams ID, Marder TB. J. Am. Chem. Soc. 1990;112:9399. [Google Scholar]
- 20.Crabtree RH. The Organometallic Chemistry of the Transition Metals. 3rd ed. John Wiley; 2001. [Google Scholar]
- 21.Braunschweig H, Dewhurst RD, Schneider A. Chem. Rev. 2010;110:3924. doi: 10.1021/cr900333n. [DOI] [PubMed] [Google Scholar]
- 22.Braunschweig H, Radacki K, Schneider A. Science. 2010;328:345. doi: 10.1126/science.1186028. [DOI] [PubMed] [Google Scholar]
- 23.Ibers JA, Holm RH. Science. 1980;209:223. doi: 10.1126/science.7384796. [DOI] [PubMed] [Google Scholar]
- 24.a Fein MM, Paustian JE, Sarakwash M. Ind. Eng. Chem. Proc. Des. Dev. 1966;5:380. [Google Scholar]; b Fein MM, Paustian JE. Ind. Eng. Chem. Proc. Des. Dev. 1965;4:129. [Google Scholar]; c Ditter JF, Klusmann EB, Oakes JD, Williams RD. Inorg. Chem. 1970;9:889. [Google Scholar]
- 25.Papetti S, Obenland C, Heying TL. Ind. Eng. Chem. Prod. Res. Dev. 1966;5:334. [Google Scholar]
- 26.a Wade K. J. Chem. Soc. D. 1971:792. [Google Scholar]; b Mingos DMP. Nature. 1972;236:99. [Google Scholar]; c Welch AJ. Chem. Commun. 2013;49:3615. doi: 10.1039/c3cc00069a. [DOI] [PubMed] [Google Scholar]
- 27.Hawthorne MF, Young DC, Garrett PM, Owen DA, Schwerin S, Tebbe FN, Wegner PA. J. Am. Chem. Soc. 1968;90:862. [Google Scholar]
- 28.B-C bond formation: Zakharkin LI, Kovredov AI, Olshevskaya VA, Shaungumbekova ZS. J. Organomet. Chem. 1982;226:217. B-O bond formation: Kabytaev KZ, Mukhin SN, Glukhov IV, Starikova ZA, Bregadze VI, Beletskaya IP. Organometallics. 2009;28:4758. B-N bond formation: Mukhin SN, Kabytaev KZ, Zhigareva GG, Glukhov IV, Starikova ZA, Bregadze VI, Beletskaya IP. Organometallics. 2008;27:5937.
- 29.Hawthorne MF, Young DC, Wegner PA. J. Am. Chem. Soc. 1965;87:1818. doi: 10.1021/ja00949a014. [DOI] [PubMed] [Google Scholar]
- 30.Hawthorne MF, Dunks GB. Science. 1972;178:462. doi: 10.1126/science.178.4060.462. [DOI] [PubMed] [Google Scholar]
- 31.Rojo I, Teixidor F, Viňas C, Kivekäs R, Sillanpää R. Chem.—Eur. J. 2003;9:4311. doi: 10.1002/chem.200304970. [DOI] [PubMed] [Google Scholar]
- 32.a González-Cardoso P, Stoica A, Farràs P, Pepiol A, Viñas C, Teixidor F. Chem.—Eur. J. 2010;16:6660. doi: 10.1002/chem.200902558. [DOI] [PubMed] [Google Scholar]; b Pepiol A, Teixidor F, Sillanpää R, Lupu M, Viñas C. Angew. Chem., Int. Ed. 2011;50:12491. doi: 10.1002/anie.201105668. [DOI] [PubMed] [Google Scholar]
- 33.Sivaev IB, Bregadze VI. J. Organomet. Chem. 2000;614-615:27. [Google Scholar]
- 34.Li TC, Spokoyny AM, She C, Farha OK, Mirkin CA, Marks TJ, Hupp JT. J. Am. Chem. Soc. 2010;132:4580. doi: 10.1021/ja100396n. [DOI] [PubMed] [Google Scholar]
- 35.O’Regan B, Grätzel M. Nature. 1991;353:737. [Google Scholar]
- 36.Hamann TW, Jensen RA, Martinson ABF, Van Ryswyk H, Hupp JT. Energy Environ. Sci. 2008;1:66. [Google Scholar]
- 37.Li TC, Fabregat-Santiago F, Farha OK, Spokoyny AM, Raga SR, Bisquert J, Mirkin CA, Marks TJ, Hupp JT. J. Phys. Chem. C. 2011;115:11257. [Google Scholar]
- 38.Spokoyny AM, Li TC, Farha OK, Machan CW, She C, Marks TJ, Hupp JT, Mirkin CA. Angew. Chem., Int. Ed. 2010;49:5339. doi: 10.1002/anie.201002181. [DOI] [PubMed] [Google Scholar]
- 39.Tamao K, Kiso Y, Sumitani K, Kumada M. J. Am. Chem. Soc. 1972;94:9268. [Google Scholar]
- 40.Jin G-X. Coord. Chem. Rev. 2004;248:587. [Google Scholar]
- 41.Dröse P, Hrib CG, Edelmann FT. J. Am. Chem. Soc. 2010;132:15540. doi: 10.1021/ja108051u. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Dang L, Sun Y, Chan H-S, Lin Z, Xie Z. J. Am. Chem. Soc. 2008;130:16103. doi: 10.1021/ja8067098. [DOI] [PubMed] [Google Scholar]
- 43.Shen H, Chan H-S, Xie Z. Organometallics. 2008;27:5309. [Google Scholar]
- 44.Wang H, Wang Y, Li H-W, Xie Z. Organometallics. 2001;20:5110. [Google Scholar]
- 45.Wang X, Jin G-X. Organometallics. 2004;23:6319. [Google Scholar]
- 46.Heaney H. Chem. Rev. 1962;62:81. [Google Scholar]
- 47.Roberts JD, Semenow DA, Simmons HE, Jr, Carlsmith LA. J. Am. Chem. Soc. 1956;78:601. [Google Scholar]
- 48.Gingrich HL, Ghosh T, Huang Q, Jones M., Jr J. Am. Chem. Soc. 1990;112:4082. [Google Scholar]
- 49.Deng L, Chan H-S, Xie Z. J. Am. Chem. Soc. 2006;128:7728. doi: 10.1021/ja061605j. [DOI] [PubMed] [Google Scholar]
- 50.Qiu Z, Ren S, Xie Z. Acc. Chem. Res. 2011;44:299. doi: 10.1021/ar100156f. [DOI] [PubMed] [Google Scholar]
- 51.Jude H, Disteldorf H, Fischer S, Wedge T, Hawkridge AM, Arif AM, Hawthorne MF, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2005;127:12131. doi: 10.1021/ja053050i. [DOI] [PubMed] [Google Scholar]
- 52.Long JR, Yaghi OM. Chem. Soc. Rev. 2009;38:1213. doi: 10.1039/b903811f. and refs. Cited within. [DOI] [PubMed] [Google Scholar]
- 53.Farha OK, Hupp JT. Acc. Chem. Res. 2010;43:1166. doi: 10.1021/ar1000617. [DOI] [PubMed] [Google Scholar]
- 54.Farha OK, Spokoyny AM, Mulfort KL, Hawthorne MF, Mirkin CA, Hupp JT. J. Am. Chem. Soc. 2007;129:12680. doi: 10.1021/ja076167a. [DOI] [PubMed] [Google Scholar]
- 55.Bae Y-S, Farha OK, Spokoyny AM, Mirkin CA, Hupp JT, Snurr RQ. Chem. Commun. 2008:4135. doi: 10.1039/b805785k. [DOI] [PubMed] [Google Scholar]
- 56.Farha OK, Spokoyny AM, Mulfort KL, Galli S, Hupp JT, Mirkin CA. Small. 2009;5:1727. doi: 10.1002/smll.200900085. [DOI] [PubMed] [Google Scholar]
- 57.Bae Y-S, Spokoyny AM, Farha OK, Snurr RQ, Hupp JT, Mirkin CA. Chem. Commun. 2010;46:3478. doi: 10.1039/b927499e. [DOI] [PubMed] [Google Scholar]
- 58.Huang S-L, Lin Y-J, Yu W-B, Jin G-X. ChemPlusChem. 2012;77:141. [Google Scholar]
- 59.Huang S-L, Weng L-H, Jin G-X. Dalton Trans. 2012;41:11657. doi: 10.1039/c2dt30708a. [DOI] [PubMed] [Google Scholar]
- 60.Müeller J, Baše K, Magnera TF, Michl J. J. Am. Chem. Soc. 1992;114:9721. [Google Scholar]
- 61.Spokoyny AM, Farha OK, Mulfort KL, Hupp JT, Mirkin CA. Inorg. Chim. Acta. 2010;364:266. [Google Scholar]
- 62.a Hoel EL, Hawthorne MF. J. Am. Chem. Soc. 1975;97:6388. [Google Scholar]; b Zheng Z, Diaz M, Knobler CB, Hawthorne MF. J. Am. Chem. Soc. 1995;117:12338. [Google Scholar]; c Kalinin VN, Usatov AV, Zakharkin LI. Proc. Indian Natl. Sci. Acad. 1989;55:293. [Google Scholar]
- 63.Morales-Morales D, Jensen CM. The Chemistry of Pincer Compounds. Elsevier; 2007. [Google Scholar]
- 64.Gunanathan C, Shimon LJW, Milstein D. J. Am. Chem. Soc. 2009;131:3146. doi: 10.1021/ja808893g. [DOI] [PubMed] [Google Scholar]
- 65.Zhao J, Goldman AS, Hartwig JF. Science. 2005;307:1080. doi: 10.1126/science.1109389. [DOI] [PubMed] [Google Scholar]
- 66.Lin G, Jones ND, Gossage RA, McDonald R, Cavell RG. Angew. Chem., Int. Ed. 2003;42:4054. doi: 10.1002/anie.200351320. [DOI] [PubMed] [Google Scholar]
- 67.Spokoyny AM, Reuter MG, Stern CL, Ratner MA, Seidman T, Mirkin CA. J. Am. Chem. Soc. 2009;131:9482. doi: 10.1021/ja902526k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Vlugt JI. Angew Chem., Int. Ed. 2010;49:252. doi: 10.1002/anie.200904795. [DOI] [PubMed] [Google Scholar]
- 69.El-Zaria MH, Arii H, Nakamura H. Inorg. Chem. 2011;50:4149. doi: 10.1021/ic2002095. [DOI] [PubMed] [Google Scholar]
- 70.Segawa Y, Yamashita M, Nozaki K. Science. 2006;314:113. doi: 10.1126/science.1131914. [DOI] [PubMed] [Google Scholar]
- 71.Segawa Y, Yamashita M, Nozaki K. J. Am. Chem. Soc. 2009;131:9201. doi: 10.1021/ja9037092. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa H, Yamashita M. Dalton Trans. 2013;45:625. doi: 10.1039/c2dt31892j. [DOI] [PubMed] [Google Scholar]
- 73.Tolman CA. Chem. Rev. 1977;77:313. [Google Scholar]
- 74.Spokoyny AM, Machan CW, Clingerman DC, Rosen MS, Wiester MJ, Kennedy RD, Sarjeant AA, Stern CL, Mirkin CA. Nat. Chem. 2011;3:590. doi: 10.1038/nchem.1088. [DOI] [PubMed] [Google Scholar]
- 75.Plešek J, Heřmánek S, Štíbr B. J. Less Common Met. 1979;67:225. [Google Scholar]
- 76.Teixidor F, Barberà G, Vaca A, Kivekäs R, Sillanpää R, Oliva J, Viñas C. J. Am. Chem. Soc. 2005;127:10158. doi: 10.1021/ja052981r. [DOI] [PubMed] [Google Scholar]
- 77.a Spokoyny AM, Lewis CD, Teverovskiy G, Buchwald SL. Organometallics. 2012;31:8478. doi: 10.1021/om301116x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kampel VTs, Bregadze VI, Ermanson LV, Antonovich VA, Matrosov EI, Godovikov NN, Kabachnik MI. Metalloorg. Khim. 1992;5:1024. [Google Scholar]; c Yanovskii AI, Struchkov Yu. T, Ts. Kampel V, Bregadze VI, Godovikov NN, Kabachnik MI. Izv. Akad. Nauk SSSR, Ser. Khim. 1986;10:2299. [Google Scholar]; d Bregadze VI, Ts. Kampel V, Matrosov EI, Antonovich VA, Yanovskii AI, Struchkov YuT, Godovikov NN, Kabachnik MI. Dokl. Akad. Nauk SSSR. 1985;285:1127. [Google Scholar]
- 78.Safronov AV, Sevryugina YV, Jalisatgi SS, Kennedy RD, Barnes CL, Hawthorne MF. Inorg. Chem. 2012;51:2629. doi: 10.1021/ic2025846. [DOI] [PubMed] [Google Scholar]
- 79.Bondarev O, Sevryugina YV, Jalisatgi SS, Hawthorne MF. Inorg. Chem. 2012;51:9935. doi: 10.1021/ic3014267. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Tang X, Yang J, Li Y, Yan H, Bregadze VI. Organometallics. 2013;32:2014. [Google Scholar]
- 81.Kabytaev KZ, Everett TA, Safronov AV, Sevryugina YV, Jalisatgi SS, Hawthorne MF. Eur. J. Inorg. Chem. 2013 doi: 10.1021/acs.inorgchem.5b00414. Online: http://dx.doi.org/10.1002/ejic.201201518. [DOI] [PubMed] [Google Scholar]
- 82.Olid D, Núñez R, Viñas C, Teixidor F. Chem. Soc. Rev. 2013;42:3318. doi: 10.1039/c2cs35441a. [DOI] [PubMed] [Google Scholar]
- 83.Lavallo V, Wright JH, Tham FS, Quinlivan S. Angew. Chem., Int. Ed. 2013;52:3172. doi: 10.1002/anie.201209107. [DOI] [PubMed] [Google Scholar]