Abstract
The field of visual analytics continues to expand into the realm of electronic health record (EHR) analysis. At the National Institutes of Health (NIH), Lifelines2, a visualization program from the University of Maryland’s Human-Computer Interaction Laboratory, was recently integrated into the NIH’s clinical repository, the Biomedical Translational Research Information System (BTRIS). To explore the functionality of Lifelines2, two markers of inflammation – erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) – were compared on patients both with and without the rare immune disorder chronic granulomatous disease (CGD) using ten years of de-identified patient data. We used Lifelines2 to visualize correlations among 12,266 laboratory events on 622 patients. The findings from our unrestricted sample identify areas warranting further studies on population subsets in which clinical features (such as disease activity) are known. Overall, visual aids such as Lifelines2 allowed for both rapid and powerful interpretation of EHR data.
Introduction
As electronic health record (EHR) use continues to rise across the nation, de–identified patient data are becoming increasingly available for use in research 1 , 2 . Researchers are now able to quickly generate retrospective studies based on professional interest, limited only by the quality of EHR data and the amount of professional workload.
To help digest such large data sets, the emerging field of visual analytics employs the human eye as a statistical tool for quickly recognizing patterns and dissimilarities 3 . Typically used as a means of overview, researchers can now use tools to “see” large data sets and rapidly make statistical inferences. They may then perform further analyses to answer more focused questions. While doing so requires additional training and a slightly modified approach, the potential speed and depth of each analysis is substantial.
At the National Institutes of Health (NIH), the visual analytics program Lifelines2 4 , 5 has recently been integrated with the NIH’s research data repository, the Biomedical Translational Research Information System (BTRIS) 6 . As Lifelines2 is still a relatively new tool to the NIH community, the purpose of this paper is to directly explore the program’s power and functionality by applying it to a clinical context. Through example, the overall goal is to help ease the learning curve of using abstract data visualization approaches.
Background
BTRIS includes a repository of clinical research data collected from many different source systems at the NIH’s Bethesda, Maryland campus 6 , including EHR systems used continuously from 1976 to present. BTRIS contains many of the typical types of data found in EHRs, such as laboratory test results and patient diagnoses.
Lifelines2 is a platform–independent visual analytics program developed at the University of Maryland’s Human–Computer Interaction Laboratory (HCIL) for means of rapid, simultaneous comparisons of sets of timestamped events 5 . It has been used in both medical and non–medical realms for administrative and quality assurance analyses 4 , but to date no studies have been published using this program in a translational research context.
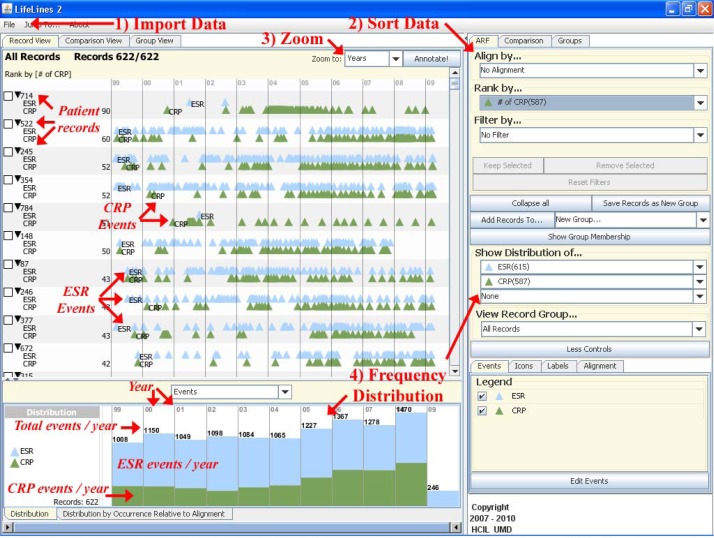
The basic premise behind Lifelines2 is to Import time–stamped event data from multiple EHRs, to Sort based on an event of interest, to Zoom to a desired time scale, and to check the Frequency Distribution of similar events across EHRs ( Figure 1 ). Numerous tandem analyses can be performed by manipulating any of the above features with a few clicks of the mouse. Figure 2 illustrates the basic features of Lifelines2 using the dataset employed in this study.
Figure 1 .
Outline of the basic functions found in Lifelines2.
Figure 2 .
Visual overview of Lifelines2 using the Test Only dataset. (ESR = erythrocyte sedimentation rate, CRP = C-reactive protein)
One powerful sorting feature in Lifelines2 is the ability to Align the time axis (x–axis) by a single event of interest. As a basic clinical example, the date and time of a hospital admission is unique to each patient’s EHR, and thus comparisons of hospital events among patients are limited when performed on a standard timeline. A better approach would be to Align each patient’s record by the date and time of admission, thus setting this event as a zero point for each patient. Any further analyses on hospital course or discharge can be made relative to each patient’s zero point, though the actual timestamp for these admissions may vary by months or even years. Further example scenarios and training videos are posted on HCIL’s website 5 .
In this study, we used Lifelines2 to aid a single researcher with her work on patients with chronic granulomatous disease (CGD) – a rare, inherited disorder of the immune system. Without regular prophylaxis and aggressive antimicrobial treatment during suspected infection, the disease can quickly become fatal 7 . The researcher’s current work within this population is in evaluating two non–specific markers of immune system activation – erythrocyte sedimentation rate (ESR) and C–reactive protein (CRP) – with an overall goal to search for correlations that may assist with medical decision making. Focusing on the first arm of the study, we directly compared ESR and CRP values that were measured within the same 24–hour period.
Materials and Methods
Using BTRIS, de–identified patient data were acquired for all patients enrolled in CGD protocols as well as non–CGD patients enrolled in two overlapping protocols. The study range was from March 7, 1999 to March 30, 2009, chosen to ensure consistency amongst ESR and CRP values. This was determined by two respective factors: 1) since ESR normal ranges were consistently reported only after the adoption of the NIH’s current laboratory system in 1999; and 2) since the units and normal ranges for reporting CRP results changed in 2009. This study period allowed for an optimal evaluation of both ESR and CRP results without the potential error induced by variations in reference ranges.
Data acquired from BTRIS included ESR and CRP values, date and time of each laboratory study, whether patients were diagnosed with CGD, year of birth, and gender. Age during the time of each laboratory event was calculated by subtracting the year the study was performed by the patient’s birth year, giving a resolution of ± 0.5 years. These data were acquired and used in compliance with the IRB–approved protocols for each of the studies.
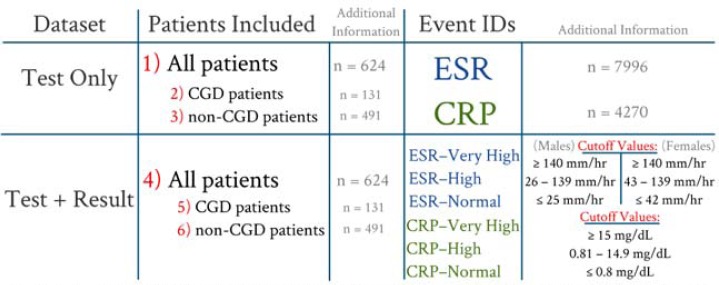
This study was conducted using datasets derived from six tab– delineated files (numbered in Figure 3 ). The Test Only datasets reported the date and time each test was performed, whereas the Test + Result datasets included test result information. For import into Lifelines2, numerical laboratory values were converted into categorical events (or “bins”) based on BTRIS observation comments and historical reference ranges 8 . “Very High” results and “High” results were determined by the point where laboratory observation comments were altered (e.g. “HH” instead of “H”). Figure 3 shows a summary of the cutoff values used. Each dataset was then viewed using Lifelines2.
Figure 3 .
Derivation of datasets ( Test Only , Test + Result ) for use in Lifelines2. Event IDs for the Test + Result dataset was determined by the values listed above.
Results
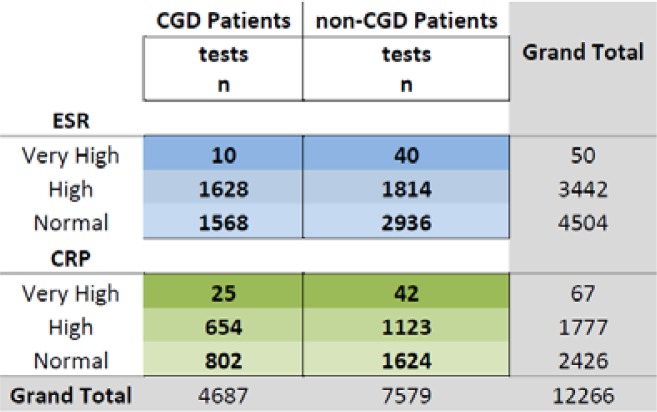
Over the ten–year study period, a total of 12,266 ESR and CRP tests were performed on 622 patients (131 CGD patients, 491 non-CGD patients). As a whole, ESR was performed approximately twice as often as CRP (7,996 versus 4,270, respectively). This remained true for both CGD and non–CGD patients. Looking at the Lab + Result dataset ( Figure 4 ), we noted that very few ESR and CRP results were “Very High” (50 and 67, respectively). As mentioned above, the results placed in the “Very High” category were determined solely by the laboratory’s observation comments and do not necessarily reflect a true, validated cutoff for critically–elevated values.
Figure 4 .
ESR and CRP Lab + Result event occurrences.
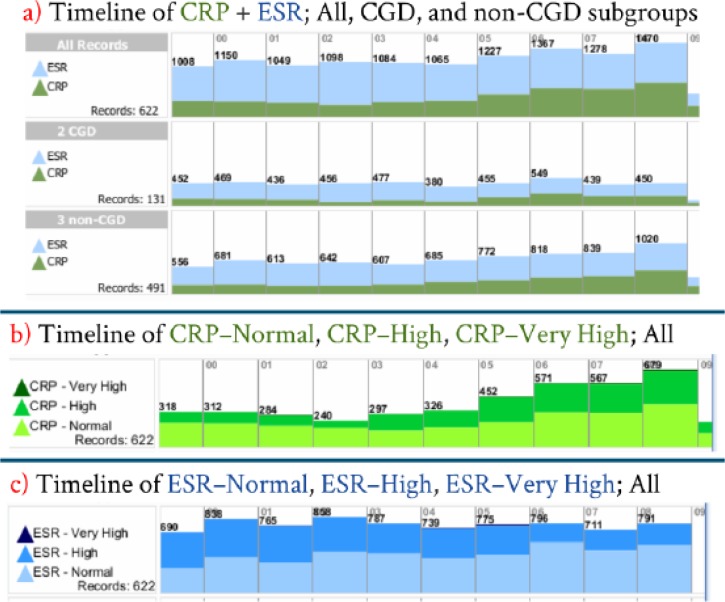
To gain familiarity with the frequency distribution feature of Lifelines2, it is perhaps easiest to begin with a ten–year timeline (no alignment). This timeline can be separated into CGD and non–CGD subgroups ( Figure 5a ) or by laboratory events ( Figures 5b and 5c ). On this figure, each bar on the x–axis represents one year in this ten–year study period, while the y–axis shows the relative frequency of tracked events. The total number of tracked events is listed per year.
Figure 5 .
Timeline frequency distribution of all datasets (no alignment). a) Test Only timeline split into CGD and non-CGD subgroups. Test + Result timeline showing into b) ESR and c) CRP events.
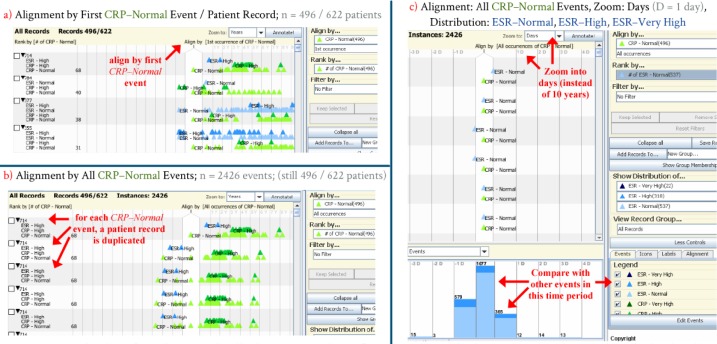
Figure 6 illustrates the process of alignment and comparison from the perspective of all CRP – Normal events. Since each patient record is unique, it is highly unlikely that two patients will have every lab draw and result occur at the exactly the same times. Rather, the focus of this study was to look at all labs drawn within a 24–hour period of another lab. Patient records were initially aligned by the first occurrence of CRP–Normal events ( Figure 6a ). Since each patient had a variable number of lab draws, the alignment was changed to all instances of CRP–Normal events ( Figure 6b ), which then duplicated 496 records into 2,426 “instances”. Finally, after zooming into a time scale of days (rather than the default of ten years), the relative frequency and occurrence of ESR–Normal , ESR–High , and ESR–Very High events was compared to the baseline CRP–Normal alignment. From this perspective, of the 2,426 times a CRP test was drawn and resulted “Normal”, by far the majority of the 1,077 ESR tests drawn at the same time were also resulted “Normal”. In a similar fashion, the majority of the ESR tests drawn up to 24 hours before (n = 579) and up to 24 hours after (n = 365) this time period were also “Normal”.
Figure 6 .
Derivation of an alignment distribution. a) Records are first aligned by a single event (CRP–Normal). b) Aligning by all CRP–Normal events creates duplicate “instances”, c) which may then be zoomed and compared to other events. (y = years, d = days)
Discussion
The primary endpoint of this analysis was to gauge the utility of Lifelines2 in a translational research setting. As such, the current study makes no claim about increasing understanding of CGD or the use of laboratory tests in its diagnosis or management. Rather, we used these data as a convenience sample for illustrating how Lifelines2 could be applied to data sets that are typically obtained from clinical data repositories. CGD is a complex disease with at least four genetic variants and a variety of clinical presentations. Our findings serve as the beginning point for asking deeper questions of data from populations in which much more detail of a patient’s clinical status is known. One such scenario would be to compare events where infection was suspected (either directly by infectious disease diagnosis codes or inferred by administration of antibiotics) within 24 hours of documented ESR and CRP values.
Perhaps most significant about this study was the relative speed at which each analysis was performed. Though abstract features such as Align and Frequency Distribution initially seemed foreign, this was mitigated through online videos and example scenarios outlined in the Background section 5 . This initial learning curve was overcome after several hours of practice, and with time it became evident as to just how quickly a query could be conducted. The comparison outlined in Figure 6c shows three separate pairwise analyses ( CRP–Normal versus ESR–Normal , ESR–High , and ESR–Very High ), but Lifelines2 is not limited to three simultaneous analyses. By utilizing the Comparison View tab (such as in Figure 5a ), frequency distributions may be performed on any number of events simultaneously on different population subsets. During the initial comparison of all ESR and CRP events in a 24– hour period (not shown), over 90 separate analyses were performed with only a few clicks of a mouse.
Once the eye has been trained, Lifelines2 holds incredible potential as an analytic aid. Some limitations of this program, however, include its learning curve and its data input requirements. While the sorting features such as Align , Rank , and Filter are extremely robust and show a high potential for isolating and analyzing population subgroups, knowing exactly how and when to use these tools poses a bit of a challenge. Unless the logical approach to a specific scenario or question has been previously outlined, determining exactly how to apply a scenario to Lifelines2 requires a much deeper understanding of the program. Additionally, data input is limited to “temporal categorical events” – meaning no duration qualifiers or numerical data may be imported 3 , 4 . For use in this study, ESR and CRP lab values were first converted into categories (such as CRP–Normal ) by necessity.
While previous analytic tools in this category have been limited in scope to a single medical scenario per program 4 , Lifelines2 has had no such restrictions. To date, it has been applied in twelve separate scenarios (nine of which are medical 4 ), and the aforementioned CGD study represents a unique approach that has not yet been described in prior studies. Finally, thanks to a simplified input format, further analyses were performed using Lifelines2’s direct successor, Lifeflow 11 , and additional visual tools called Treemaps and Temporal Event Tracker 12 . These analyses are outside the scope of this paper.
Conclusions
Our study outlines some of the potential applications of Lifelines2 on de-identified patient data. This by no means describes the program’s full utility, as a number of features were not employed in this analysis. Additionally, while Lifelines2 is limited to temporal categorical events, a number of visual analytic tools have no such restrictions. Due to its robust usage and its native integration in BTRIS, however, Lifelines2 appears an effective and accessible tool for NIH researchers. Additionally, the program can be easily attained for academic use by directly contacting the HCIL.
The data obtained from BTRIS in this study are typical of the kinds of data available from an emerging number of similar repositories. Regional consortia funded by Clinical and Translational Science Awards (CTSA) now exist as a compendium of pooled patient data made available to interested researchers in a de–identified form 1 . Currently represented at 60 research institutions in 30 states, the CTSA has gained steady interest among multidisciplinary research venues 2 . As these databases provide more sophisticated tools for researchers, so too should the means by which their studies are conducted. Visual analytic programs such as Lifelines2 have great potential to help satisfy this new role.
Acknowledgments
This work was performed in part with intramural research support from the NIH Clinical Center and the National Library of Medicine. The authors thank Dr. Harry Malech and Dr. Steven Holland for sharing their data for this analysis and Dr. John Gallin for advice on presentation of the findings. The authors also thank Dr. Ben Shneiderman, Dr. Catherine Plaisant, and PhD students Krist Wongsuphasawat, Megan Monroe, and Hsueh-Chien Cheng from the University of Maryland’s Human–Computer Interaction Laboratory for providing the programs Lifelines2, Lifeflow, Treemaps, and Temporal Event Tracker software as well as general guidance on the programs’ use.
References
- 1. Clinical and Translational Research Awards (CTSA) Homepage. Available at: http://www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards/ . Accessed Mar 02, 2012 .
- 2. Hollander JE , Gaulton GN , Courtney DM , et al. Facilitating emergency care research networks: Integration into the Clinical Translational and Science Award (CTSA) infrastructure . Acad Emerg Med . 2009 Oct ; 16 ( 10 ): 1005 – 9 . doi: 10.1111/j.1553-2712.2009.00524.x. [DOI] [PubMed] [Google Scholar]
- 3. Shneiderman B . The eyes have it: A task by data type taxonomy for information visualizations . VL ‘96 Proceedings of the 1996 IEEE Symposium on Visual Languages . 1996 Sep ; 3 : 336 – 43 . [Google Scholar]
- 4. Wang TD , Wongsuphasawat K , Plaisant C , Shneiderman B . Extracting insights from electronic health records: case studies, a visual analytics process model, and design recommendations . J Med Syst . 2011 Oct ; 35 ( 5 ): 1135 – 52 . doi: 10.1007/s10916-011-9718-x. [DOI] [PubMed] [Google Scholar]
- 5. Lifelines2: Discovering temporal categorical patterns across multiple records. Homepage. Available at: http://www.cs.umd.edu/hcil/lifelines2/ . Accessed Mar 10, 2012
- 6. Cimino JJ , Ayres EA . The clinical research data repository of the US National Institutes of Health . Stud Health Technol Inform . 2010 ; 160 ( Pt 2 ): 1299 – 303 . [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffman . Hematology: Basic Principles and Practice . 5th ed . Churchill Livingstone ; 2008 . pp. 687 – 720 . “Chapter 50: Disorders of phagocyte function and number” . [Google Scholar]
- 8. Test Guide – NIH Laboratory Medicine / Transfusion Medicine Homepage. Available at: http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index?OpenForm . Accessed Mar 05, 2012 .
- 9. Frost F , Roach MJ , Kushner I , Schreiber P . Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury . Arch Phys Med Rehabil . 2005 Feb ; 86 : 312 – 7 . doi: 10.1016/j.apmr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 10. Batlivala SP . Focus on Diagnosis: The erythrocyte sedimentation rate and the C-reactive protein test . Pediatrics in Review . 2009 ; 30 : 72 – 4 . doi: 10.1542/pir.30-2-72. [DOI] [PubMed] [Google Scholar]
- 11. Wongsuphasawat K , G’omez JA , Plaisant C , Wang TD , et al. LifeFlow: Visualizing an overview of event sequences . ACM Conference on Human Factors in Computing (CHI) ; Vancouver, BC, Canada . 2011 . May . [Google Scholar]
- 12. Shneiderman B , Dunne C , Sharma P , Wang P . Information Visualization . Innovation trajectories for information visualizations: Comparing treemaps, cone trees, and hyperbolic trees . Published online before print November 16, 2011 . [DOI] [Google Scholar]