Abstract
Informed consents are a critical and essential component of the clinical research process. Currently, most consents and research privacy authorizations are being captured on paper. In this paper we describe a novel method of capturing this information electronically. The objective is to allow easier tracking of research participants’ intent for current and future research involvement, enhance consent comprehension and facilitate the research workflow. After multidisciplinary analysis in key hospital registration areas and research participant enrollment, an open source software product was designed to capture this data through a user-friendly touch screen interface. The data may then be fed into a clinical data warehouse for use in cohort discovery or consent tracking. Despite ethical, legal and informatics challenges in clinical and research environments, we propose that this technology opens new avenues for significantly enhancing the consent process and positively impacting recruitment.
Introduction and Background
Informed consents are ethical and legal obligations that need to be executed prior to enrollment in clinical studies. Closely related are the authorizations required by the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule for using an individual’s protected healthcare information for research. Most health care institutions collect consents and privacy authorizations using paper-based methods. However, informed consents are fraught with readability and comprehension issues ( 1 – 3 ) and recruitment of adequate numbers of research participants is often problematic. Failure to recruit an adequate number in a reasonable time frame increases the cost of studies ( 4 ) and reduces statistical power due to smaller sample size. One mechanism to increase participation in research is to solicit patients’ general willingness to participate in clinical research. Most medical care facilities inform patients about institutional privacy practices upon registration, and in some clinic areas ask for their permission for future contact should new clinical studies become available that they may be eligible for ( 5 ). The challenge is in tracking those permissions currently collected on paper and scanned into medical records in a non-computable format. Here we describe the design and testing of the Research Permissions Management System (RPMS), a novel and comprehensive mechanism for electronically capturing and managing informed consents and privacy authorizations (“research permissions”). In recent years, mobile tablet computing devices have become readily available and familiar to the general population. Such devices have been examined for use by patients in primary care setting for self-administered questionnaires ( 6 ) and computerized interfaces have been used by physicians to assist in the delivery of screening and preventative health care ( 7 , 8 ). In RPMS we examine the use of touch screen interface on a tablet form factor in the consenting process. Information technology has transformed health care in recent years. Electronic medical records are widely being adopted across the nation and are being leveraged for translational research ( 9 ). The collection of research permissions electronically adds a new dimension of data that can be linked to clinical data to enhance the recruitment of willing research participants. Moreover, the use of electronic media creates an opportunity for enhancing the informed consent process with audio, video or other rich media.
System Design and Implementation
The project was undertaken in South Carolina with a Grand Opportunity grant funding from the National Library of Medicine to Health Sciences South Carolina (HSSC), a statewide biomedical research collaborative of three principal research universities and four major health systems; Clemson University, Greenville Hospital System University Medical Center, the Medical University of South Carolina (MUSC), Palmetto Health, Spartanburg Regional Healthcare System, and the University of South Carolina (USC). The collaboration included an Ethical, Legal, and Social Issues (ELSI) committee lead by Duke University. Project execution followed a multidisciplinary approach and included team members with Informatics, systems engineering, software engineering, project management, ethics and regulatory expertise. Following the specific aims of the grant and instituted software development practices, the team employed the following iterative software development lifecycle throughout the project: analysis, requirements gathering, design, development, testing, implementation, and training. The initial phase involved substantial analysis focused on two key areas: 1) systematic analysis of existing business practices, registration processes, and consent collection workflows at each of the HSSC member health care facilities, and 2) investigative analysis of best practices for presenting information to users via tablet technology and capturing permissions and consent data electronically. The exact details of the methods and procedures the team used are reported elsewhere ( 5 , 10 ).
Workflow Analysis:
The analysis performed on the existing processes at each location informed the team of specific areas in research participant enrollment and patient registration process where the electronic forms would be best interjected to replace paper-based forms. This analysis influenced the design for the detailed workflow embedded into the RPMS application. The general workflow included presentation of the information to a patient or participant, collection of information from checkbox options and signatures, registrant review of the information, and witness signatures. Institutional business requirements included the ability to select appropriate forms applicable for each patient or patient visit, and the need to accommodate multiple languages.
User Interface Analysis:
Best practices for presenting and collecting information electronically were investigated. For example the use of long forms with scrolling pages vs. the presentation of the information one page at a time with the use of navigation buttons, and the use of a tablet-based device (Apple iPad®) vs. a fixed touch screen. Results showed a preference by both registration clerks and patients for a portable device (the iPad in this case). In addition, the paginated interface was preferred over the scrolling interface with less handling errors ( 10 ). These results influenced the design of RPMS user interface. The design was also informed by the results of a Video Assisted Consent (VAC) study conducted at MUSC in collaboration with regulatory knowledge experts using mockup RPMS code ( 11 , 12 ).
Architecture:
RPMS utilizes a core ontology that describes the relationships between consenters and the policies they have consented to, which includes: 1) Policy - a collection of policy rules and descriptions that can be consented to or not; 2) Policy Rule - the smallest action that can be consented to, e.g. contact for research; 3) Consenter - the patient or participant who is being asked to consent to a policy; 4) Encounter - the event that triggered the consent process, e.g. hospital visit; and 5) Consent - relates whether a consenter has granted consent or not to a policy. The RPMS architecture allows multi-tenancy, authentication and authorization, a library of reusable policy elements, text internationalization, and workflow. RPMS has been partitioned into 3 deployable modules to allow for flexibility, security, and integration capabilities: 1) The Consent Services Application provides RESTful service endpoints required to perform all data and business processing actions; 2) The Consent Administration Application provides a web-based rich user interface (UI) for managing multi-tenancy and security, defining consent policies, as well as designing and managing the lifecycle of consent forms used to collect consents; 3) The Consent Collector Application is used to present digital forms to collect consents using a web-based native UI experience specifically stylus/touch screen on a tablet form factors. A pluggable and extensible framework is at the core implementation of the RPMS modules. Every process, service, workflow, UI component, and form widget is capable of being extended or overridden in order to customize RPMS for the organization using it, whether it is in a patient care or research setting. Customization may be applied to extensions globally, for specific organizations or for specific users.
System Piloting and Release
The Environment:
HSSC has been working with its member institutions to establish a state wide Clinical Data Warehouse (CDW) system as part of its mission to improve the health of all South Carolinians by collaborating across the state with the goal of enabling evidence-based research. The legal and regulatory framework including business associate agreements and Institutional Review Board (IRB) approvals have already been established for this data sharing project and the restricted use of the CDW for research. The legal agreements were extended to encompass work on RPMS including the analysis described in the above section. The CDW and Enterprise Master Patient Index (EMPI) employed in this project were designed to accept data from multiple HSSC member institutions across the state of South Carolina including MUSC.
Implementation in Patient Registration Areas:
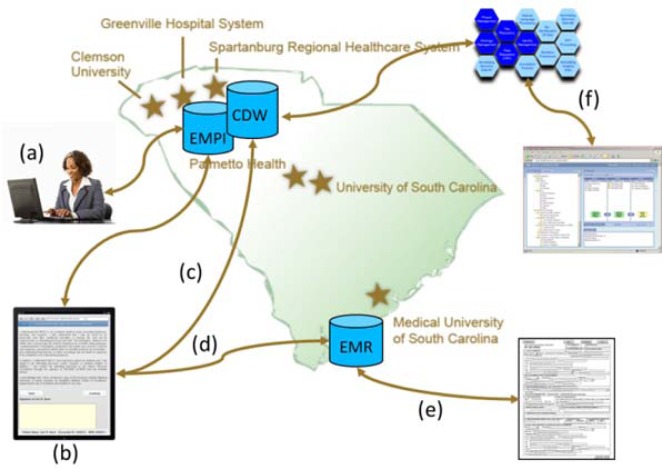
Prior to the release of the open source version of RPMS, a customized implementation of the software was piloted in select patient registration clinics at MUSC starting in November of 2011. The implementation was designed specifically for the state-wide infrastructure that is being established by HSSC. The stand-alone RPMS software was extended to communicate with the EMPI to optimize patient lookups and to store collected data in the CDW. As a result the final extended RPMS system was distributed in nature with data transmitted from source registration areas at the member institution to the centralized HSSC CDW ( Figure 1 ). Upon patient registration at MUSC clinics, HL7 messages with registration information are instantaneously transmitted to a central HSSC data store. The information is matched against the EMPI. After registration, the clerk moves to the RPMS interface on the tablet device and enters the visit number. The system retrieves the patient information and displays it on the device. The clerk verifies the retrieved patient record and hands the device to the patient for review and consent. The patient signs the electronic consent form using a stylus. After completion of the form the system is automatically locked requiring the patient to return the device to the clerk who is required to enter the unlock code as a security measure. The clerk reviews the entered information with the patient and signs as a witness. An electronic version of the completed and signed form identical to the paper version is dynamically generated in PostScript (PS) document format and pushed into the Electronic Medical Record (EMR) patient folder. This bypasses the necessity of scanning and storing the paper forms, thus simplifying the workflow for registration clerks. The signed PS formatted consent form may be printed and given as a copy to the patient. After completing the forms, research permission data is loaded into the CDW along with the patient’s clinical data. By supporting the persistence of discrete data elements for captured permissions in the CDW, the information can be leveraged for research purposes based on clinical criteria combined with patient permission status (for example the permission to be contacted for future research). This is accomplished by expressing the data using Informatics for Integrating Biology and the Bedside (i2b2) an open source software platform which provides a researcher-friendly clinical data exploratory and cohort discovery tools ( 13 ). Consent data terms are added to the i2b2 ontology.
Figure 1:
The RPMS workflow. a) The patient is registered. Patient demographics are pushed to a central data store and matched against an EMPI. b) Patient information is pulled up on an iPad and the patient is consented. c) The consent data is transmitted to the CDW. d) A PostScript consent form is generated and stored in the EMR and may be e) printed and given to the patient. f) The consent data is expressed in i2b2 along with clinical data.
For this pilot implementation, information from several paper-based forms typically used for consenting during patient registration were converted into electronic format and displayed on an iPad via the RPMS UI. These forms include: the Consent for Medical Treatment, Lewis Blackman Hospital Patient Safety Act Acknowledgement, Medicare, and Tricare. One example of a general permission that was collected in the Consent for Medical Treatment form is the permission to be contacted for future research studies. The layout of the forms was optimized for a touchscreen tablet device. For example: boxes used for patient initials for each section on paper were converted to an “accept” button, and prolonged text sections which require scrolling across multiple pages were divided into discrete single-screen sections with “Back” and “Continue” navigation buttons eliminating the need for scrolling. RPMS also offers the option to display forms in other languages when translated versions are available. As of August 2012, over 2300 patients have been registered using the RPMS pilot at MUSC.
Informed Consent Implementation:
The form designer module in RPMS allows organizations to design their own consent forms to capture consent information from patients and/or study participants. Using the web-based Administration Application, users may design form layout and select language and interactive controls that are displayed on the iPad. Form design may also include rich media components to enhance patient or participant comprehension. We have successfully created mock consent forms using the form designer module and have used these forms for system demonstration to researchers at MUSC. More work is needed to incorporate this process into the workflow of informed consent form submission to and approval by the IRB along with the research protocol.
Release as Open Source:
The final version of RPMS, which offers new features such as the support for the workflow of the informed consent to participate in research and consent form design tools, has been completed and is being released under an open source license at www.healthsciencessc.org/rpms . The platform is extensible and configurable with the ability to be used in multiple consenting environments such as patient registration for routine care or informed consenting for clinical trials.
Discussion
There were a number of challenges in deploying the RPMS pilot in busy registration areas. The deployment process involved replacing the entire paper process with the electronic system since it was impossible to separate out research permissions from the entire set of clinical permissions collected during that encounter while preserving an efficient clinic workflow. Since this was a gradual deployment in a pilot setting, in order to preserve consistency between clinics using paper form and those using RPMS, the existing processes and exact wording had to be preserved. Rendered RPMS paper-equivalent PS documents are pushed into the EMR in compliance with the current practices. Other challenges that need to be addressed in the deployment of such an electronic system are the ethical, legal and social implications, for example, the question of opt-in vs. opt-out presentation of research related questions to patients. Since the RPMS authoring tool allows institutions to design electronic forms in compliance with their own policies and regulations, such a discussion on ethical issues, although important, is outside the scope of this manuscript. Despite these challenges, reaction to the new technology has been overwhelmingly positive. Patients, registration clerks, researchers and the hospital legal department have eagerly participated in piloting, testing and feedback of the new system. Moreover, the fact that a broader set of permissions (in addition to research permissions) are now being captured and stored electronically will likely be of major value in coordinating, tracking and archiving clinical permissions as well.
A variety of new technologies and circumstances are increasingly available to consumers that allow them to indicate their desire to participate in diverse aspects of research ( 14 , 15 ). However, in most hospitals, collecting research consents is not standardized in process or content. Paper-based mechanisms of managing research consents will eventually limit the ability of a geographically dispersed set of practices/hospitals to perform efficiently as a clinical trials network. Efforts in standardizing research permission data and privacy directives have already been reported ( 16 – 18 ) and will help in contextualizing patients’ intent during the consumption of data in various research use cases. For example linking the permission to be contacted for future research with clinical information in the clinical data warehouse may allow researchers to identify cohorts of patients who are more likely to participate in research.
In addition to the clinical patient registration use cases, RPMS software was designed for the presentation of informed consents and collection of consent data in the research setting. This data includes information about the research protocol, demographic information, consent date, consent version, expiration date, and elements of the consent, components of the research that a participant may agrees or disagree to. For example if a protocol includes a clinical trial component and a registry component a participant may consent to one but not the other. This data can then be fed to a clinical trials management system or CDW or both. The data can be used for linking certain components of a protocol with clinical data in a CDW. This could be particularly advantageous in cases when a research protocol contributes biospecimens to a biorepository for research. Capturing consent data electronically can also facilitate tracking of consent form versions. This is particularly problematic when a research protocol undergoes an amendment which requires a newer version of the consent form often requiring re-consenting. Version tracking can help researchers identify participants consented using older versions.
Our intention is to replace the current paper-based informed consent process used in clinical studies with an electronic system with minimal disruption to the research workflow. The introduction of this new medium to the consenting process has several potential benefits. Patients are often presented with increasingly complicated informed consent forms during enrollment in clinical studies. The forms are often verbose and difficult to comprehend ( 19 ). The electronic format opens new avenues for richer content in consent forms, such as audio or video that can enhance the information presented and improve comprehension of the complex concepts and/or procedures being researched. RPMS enables study coordinators to embed rich media into consent forms. The results of the VAC study suggest that using videos to convey details about study procedures may increase participant understanding ( 12 ).
Finally consents collected electronically can be presented back for patient or research participant review via a patient research portal allowing them to review the list of research protocols and research authorizations they have consented to. The information presented in this manner empowers patients by allowing them to be better informed and make informed decisions about research, and facilitates access to the research team.
Conclusion
In conclusion, we have developed an electronic system designed to simplify the collection and management of research authorizations and informed consents. The captured data is fed to the CDW with associated clinical data. The result is a system that can facilitate the versioning and tracking of informed consents, enhance the consent process and facilitate research participant recruitment as well as the coordination and management of a state-wide clinical trials network. Future plans include the development of best practices for the presentation of multimedia in consents; the incorporation of IRB workflow; linking of policies and rules to other consent ontologies ( 17 , 18 ); a detailed analysis of the impact of RPMS on the consent process including retained comprehension, recruitment, enrollment and user satisfaction; and the addition of other components to RPMS such as a patient portal to empower patients and research participants to be more informed about their decisions on consents and authorizations that accrue during their interactions with the research enterprise.
Acknowledgments
This work was supported by Health Sciences South Carolina and funded by Grant number RC2 LM010796 from the National Library of Medicine, and the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, National Institute of Health Grant numbers UL1 RR029882 and UL1 TR000062. We would like to acknowledge the work of the Duke University ELSI team: Laura Beskow, PhD, Lawrence (Doc) Muhlbaier, PhD and Kevin Weinfurt, PhD.
References
- 1. Tarnowski KJ , Allen DM , Mayhall C , Kelly PA . Readability of pediatric biomedical research informed consent forms . Pediatrics . 1990 ; 85 ( 1 ): 58 – 62 . Epub 1990/01/01 . [PubMed] [Google Scholar]
- 2. Philipson SJ , Doyle MA , Gabram SG , Nightingale C , Philipson EH . Informed consent for research: a study to evaluate readability and processability to effect change . J Investig Med . 1995 ; 43 ( 5 ): 459 – 67 . Epub 1995/10/01 . [PubMed] [Google Scholar]
- 3. Reidenberg MM . Informed consent or acknowledgment of disclosure . Clinical pharmacology and therapeutics . 2005 ; 78 ( 4 ): 439 – 40 . doi: 10.1016/j.clpt.2005.07.008. Epub 2005/10/04 . [DOI] [PubMed] [Google Scholar]
- 4. Nasser N , Grady D , Balke CW . Commentary: Improving participant recruitment in clinical and translational research . Academic medicine : journal of the Association of American Medical Colleges . 2011 ; 86 ( 11 ): 1334 – 5 . doi: 10.1097/ACM.0b013e3182302831. Epub 2011/10/28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalil Madathil K , Koikkara R , Gramopadhye AK , Fryar K . An Analysis of the General Consenting Process in an Emergency Department at a Major Hospital: Challenges for Migrating to an Electronic Health Record . Proceedings of the 2011 Industrial Engineering Research Conference ; Reno, NV . 2011 . [Google Scholar]
- 6. Hess R , Santucci A , McTigue K , Fischer G , Kapoor W . Patient difficulty using tablet computers to screen in primary care . Journal of general internal medicine . 2008 ; 23 ( 4 ): 476 – 80 . doi: 10.1007/s11606-007-0500-1. Epub 2008/04/01 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliven BD , Kaufman SE , Spertus JA . Electronic collection of health-related quality of life data: validity, time benefits, and patient preference . Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation . 2001 ; 10 ( 1 ): 15 – 22 . doi: 10.1023/a:1016740312904. Epub 2001/08/18 . [DOI] [PubMed] [Google Scholar]
- 8. Bachman JW . The patient-computer interview: a neglected tool that can aid the clinician . Mayo Clinic proceedings Mayo Clinic . 2003 ; 78 ( 1 ): 67 – 78 . doi: 10.4065/78.1.67. Epub 2003/01/17 . [DOI] [PubMed] [Google Scholar]
- 9. McCarty CA , Chisholm RL , Chute CG , Kullo IJ , Jarvik GP , Larson EB , et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies . BMC medical genomics . 2011 ; 4 : 13 . doi: 10.1186/1755-8794-4-13. Epub 2011/01/29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koikkara R , Chalil Madathil K , Fryar K , Moskowitz J , Sanderson I , Greenstein JS , et al., editors. Human Factors Applied to the Development of a State-wide Electronic Consenting System: A Healthcare Information Technology Case Study . The 4th International Conference on Applied Human Factors and Ergonomics ; San Francisco, CA . 2012 . [Google Scholar]
- 11. Obeid JS , Magruder K , Sonne SC , Gentilin S , Andrews JO , Sanderson IC , editors. Enhancing the informed consent process using video and new electronic media . 2012 AMIA Summit on Clinical Research Informatics ; 2012 March 21, 2012 ; San Francisco, CA . [Google Scholar]
- 12. Sonne S , Andrews JO , SG , Oppenheimer S , Obeid J , Brady K , et al., editors. Development and Pilot Testing of a Video Assisted Informed Consent Process . 5th Annual CRM Workshop ; New Haven, CT . 2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy SN , Weber G , Mendis M , Gainer V , Chueh HC , Churchill S , et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) . J Am Med Inform Assoc . 2010 ; 17 ( 2 ): 124 – 30 . doi: 10.1136/jamia.2009.000893. Epub 2010/03/02 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA , Scott KW , Lebo L , Hassan N , Lightner C , Pulley J . ResearchMatch: a national registry to recruit volunteers for clinical research . Academic medicine : journal of the Association of American Medical Colleges . 2012 ; 87 ( 1 ): 66 – 73 . doi: 10.1097/ACM.0b013e31823ab7d2. Epub 2011/11/23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zarin DA , Tse T , Ide NC . Trial Registration at ClinicalTrials.gov between May and October 2005 . The New England journal of medicine . 2005 ; 353 ( 26 ): 2779 – 87 . doi: 10.1056/NEJMsa053234. Epub 2005/12/31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Obeid J , Gabriel D , Sanderson I . A biomedical research permissions ontology: cognitive and knowledge representation considerations . 26th Annual Computer Security Applications Conference, Governance of Technology, Information and Policies ; 2010 Dec 2010 ; Austin, TX : ACM New York, NY, USA ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weida T , Blobel B , Davis M , Moehrke J , Gonzales-Webb S , Thoreson R , et al. HL7 Security and Privacy Ontology . 2011 [cited 2012]; Available from: http://wiki.hl7.org/index.php?title=Security_and_Privacy_Ontology .
- 18. Grando MA , Schwab R , Boxwala A , Alipanah N . Ontological approach for the management of informed consent permissions . 2012 IEEE Second International Conference on Healthcare Informatics, Imaging and Systems Biology ; 2012 . [Google Scholar]
- 19. Fortun P , West J , Chalkley L , Shonde A , Hawkey C . Recall of informed consent information by healthy volunteers in clinical trials . QJM : monthly journal of the Association of Physicians . 2008 ; 101 ( 8 ): 625 – 9 . doi: 10.1093/qjmed/hcn067. Epub 2008/05/20 . [DOI] [PubMed] [Google Scholar]