Abstract
Prior adverse experience alters behavioral responses to subsequent stressors. For example, exposure to a brief swim increases immobility in a subsequent swim test 24 h later. In order to determine if qualitative differences (e.g. 19 °C versus 25 °C) in an initial stressor (15 min swim) impact behavioral, physiological, and associated neural responses in a 5 min, 25 °C swim test 24 h later, rats were surgically implanted with biotelemetry devices one week prior to experimentation then randomly assigned to one of 6 conditions (Day 1 (15 min)/Day 2 (5 min)): 1) home cage (HC)/HC, 2) HC/25 °C swim, 3) 19 °C swim/HC, 4) 19 °C swim/25 °C swim, 5) 25 °C swim/HC, 6) 25 °C swim/25 °C swim. Core body temperature (Tb) was measured on Days 1 and 2 using biotelemetry; behavior was measured on Day 2. Rats were transcardially perfused with fixative 2 h following the onset of the swim on Day 2 for analysis of c-Fos expression in midbrain serotonergic neurons. Cold water (19 °C) swim on Day 1 reduced Tb, compared to both 25 °C swim and HC groups on Day 1, and, relative to rats exposed to HC conditions on Day 1, reduced the hypothermic response to the 25 °C swim on Day 2. The 19 °C swim on Day 1, relative to HC exposure on Day 1, increased immobility during the 5 min swim on Day 2. Also, 19 °C swim, relative to HC conditions, on Day 1 reduced swim (25 °C)-induced increases in c-Fos expression in serotonergic neurons within the dorsal (DRD) and interfascicular (DRI) parts of the dorsal raphe nucleus (DR). These results suggest that exposure to a 5 min 19 °C cold water swim, but not exposure to a 5 min 25 °C swim alters physiological, behavioral and serotonergic responses to a subsequent stressor.
Keywords: swim stress, forced swim test, core body temperature, depression, serotonin, raphe
The stress-diathesis model of depression proposes that vulnerability to depression is a combination of genetic vulnerability and an additional trigger by an environmental event, such as stress, to unveil depressive symptomology (Caspi et al., 2003; Munafo et al., 2009). Several animal models of depression involve stress exposure that results in observable behavioral signs, including shifts from proactive to more reactive emotional coping styles, which have been proposed to represent behavioral depression. These models include inescapable shock/learned helplessness (Henn et al., 1993; Maier and Watkins, 2005; Vollmayr and Henn, 2001; Weiss et al., 1981), forced swimming (Christianson and Drugan, 2005; Detke et al., 1995; Porsolt et al., 1977; Weiss et al., 1998) chronic social stress (Rygula et al., 2006), and resident/intruder defeat (Krishnan et al., 2007; Paul et al., 2011).
The serotonergic system has been shown to be critically involved in the majority of the stress-induced behavioral responses in the models mentioned above (Detke et al., 1995; Maier and Watkins, 2005; Rygula et al., 2006) although certain models indicate significant noradrenergic involvement as well (Christianson et al., 2008; Drugan et al., 2010; Weiss et al., 1981). The full spectrum of stress reactivity is important to consider, such as proactive versus reactive emotional coping behavior, which is thought to be controlled in part by serotonergic systems (Chung et al., 1999; Chung et al., 2000; Koolhaas et al., 2007). For example, male mice that respond with high aggression in response to an intruder (proactive coping) respond with active swimming and climbing in the forced swim test, whereas non-aggressive males with low aggression in response to an intruder (reactive coping) respond with predominantly immobility (Veenema et al., 2005). Mice with a proactive emotional coping style have higher 5-HT1A receptor expression and binding capacity in forebrain limbic structures (Korte et al., 1996), and a higher sensitivity of postsynaptic 5-HT1A receptors (van der Vegt et al., 2001). Activation of 5-HT1A receptors in these mice with a proactive emotional coping style induces a shift toward a more reactive emotional coping style (Veenema et al., 2005). Although the anatomical origin of protective responses to aversive stimuli (e.g. active behavioral coping, behavioral immunization), may originate in areas of the brain such as the prefrontal cortex (Amat et al., 2006), their ultimate protection against subsequent stress effects has to do with their modulatory effect on midbrain serotonergic systems (Amat et al., 2006; Amat et al., 2010; Greenwood et al., 2003). Therefore, evaluation of midbrain serotonergic systems is important because they are important in all aspects of these models including stress, coping, and behavioral depression.
Continuous swim stress has been shown to recruit serotonergic activity in brainstem and hippocampal regions during an initial 15 min continuous swim in a water temperature and time-dependent manner (Kelly et al., 2011; Linthorst et al., 2008). For example, in the hippocampus, 25 °C or 35 °C swim results in an immediate elevation of extracellular serotonin (5-hydroxytryptamine; 5-HT), while a 19 °C swim results in a delayed increase of hippocampal 5-HT one hour later (Linthorst et al., 2008). Prior research has shown that serotonergic neurons in the dorsal raphe nucleus (DR) are thermosensitive (Hale et al., 2011a). Indeed, Kelly et al., (2011) have reported that cold swim, relative to swimming at a warmer temperature, increases numbers of double immunostained c-Fos/tryptophan hydroxylase (Tph) neurons in subregions of the DR. Acute cold swim, relative to swim at higher temperatures, increased c-Fos expression in serotonergic neurons within the ventrolateral part of the dorsal raphe nucleus/ventrolateral periaqueductal gray (DRVL/VLPAG) as well as the interfascicular part of the dorsal raphe nucleus (DRI), subregions of the DR that are thought to be involved in thermoregulatory processes and stress-related behavioral coping mechanisms (Hale et al., 2011a; Lowry et al., 2007). Serotonergic neurons within the DRI project to the hippocampus (Köhler and Steinbusch, 1982; McKenna and Vertes, 2001; Pierce et al., 1976), and therefore this subset of serotonergic neurons may account for the effects of temperature on hippocampal serotonin release observed by Linthorst and colleagues (Linthorst et al., 2007). The midbrain 5-HT system appears to be implicated in the psychological (exteroceptive) stress- as well as the physiological (interoceptive) stress-induced alterations associated with forced swim in cold water. However, as noted by Kelly et al., (2011), since widespread activation in the DR was observed independent of water temperature, these DR serotonergic neurons are likely to be responding to both the psychological stress of forced swimming as well as the physiological stress of hypothermia.
The above findings provide direct support for the role of 5-HT in several brain regions that mediate the responses to either hyperthermia or hypothermia. However, no work to date has evaluated the impact of prior exposure to differing water temperatures on the subsequent reactivity of midbrain serotonergic systems to a subsequent forced swim exposure. This reactivity may be very different from the studies reported above, because now all groups of subjects are experiencing the same water temperature in the subsequent acute and brief (5 min) forced swim. The test situation is therefore identical for all subjects and would allow determination of the impact of prior swim exposure at cold or warm temperatures on this subsequent identical swim stress challenge for all groups.
Few studies have examined the impact of different temperatures in the swim stress-induced behavioral depression and serotonergic correlates in the forced swim test (for exceptions see Kelly et al., 2011; Linthorst et al., 2008). Since forced swimming involves both psychological (anxiety, panic) and physiological (hypothermia) stress (Abel, 1993; Stone, 1970a; Stone, 1970b), we sought to examine the behavioral, physiological, and cellular effects of different water temperatures on Day 1 swim stress by measuring behavior, Tb, and c-Fos/Tph double immunostaining in response to a second swim exposure (25 °C water, 5 minutes) 24 hours later.
2. Experimental Procedures
2.1 Animals
Adult male Wistar rats (HSD-WI, Harlan Labs, Indianapolis, IN, USA; 250-275 g) were used throughout the course of the experiment. Eighty experimental rats were pair housed in transparent polycarbonate cages (26 cm W × 47.6 cm L × 20.3 cm H; Cat. No., RC88D-PC, Alternative Designs, Siloam Springs, AR, USA) using standard cage bedding (Teklad Laboratory Grade Aspen Bedding, Harlan, Madison, WI, USA). Both food (Cat No. 8640, Teklad22/5 Rodent Diet, Harlan, Madison, WI, USA) and tap water were provided ad libitum for the duration of the experiment. Rats were kept on a standard 12 h: 12 h light/dark cycle, with lights on at 0700 h. Rats were allowed to acclimate to housing conditions for 5 days prior to any experimental procedures. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, D.C., 2011) and were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee. All possible efforts were made to minimize the number of animals used and their suffering.
2.2 Surgical procedures
Telemetric transmitters were implanted into the peritoneal cavity for measurement of Tb in freely moving animals. Surgery was performed under inhaled isoflurane anesthesia (5% initial and 2% maintenance). Briefly, following anesthesia the rat was placed onto its back, the rat’s abdominal hair was shaved, and its skin was disinfected with a 10% povidone-iodine solution (Qualitest Pharmaceuticals, Huntsville, AL, USA), and with 70% ethanol. A 1 cm midline incision was made through both the dermis and abdominal muscle wall, starting just caudal to the xiphoid process of the sternum. A telemetric probe (Model No. TA-F40; Data Sciences International (DSI), St. Paul, MN, USA) was sterilized with Actril according to the manufacturer’s recommendations (Cat. No. 176-02-046, Minnetech, Minneapolis, MN, USA) and implanted into the peritoneal cavity. Following probe implantation the abdominal wall was sutured using 3-0 non-absorbable silk surgical suture (Cat. No.MV-684, Med-Vet International, Mettawa, IL, USA) in a simple interrupted pattern (3 stitches). The dermal tissue was rejoined using two sterile stainless steel wound clips (Cat. No. 12032-09, Fine Science Tools Inc., Foster City, CA, USA). Antibacterial wound wash (Nolvasan, Fort Dodge Animal Health, Fort Dodge, IA, USA) was applied following suturing, and the rat was placed on a 37 °C heating pad until full recovery. Upon recovery a 5 mg/kg dose of the post-surgical analgesic metacam (meloxicam, Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO, USA) was administered subcutaneously. After surgery, all rats were single-housed in clean cages with fresh bedding. Rats were allowed to recover from surgery and acclimate for 10 days post-surgery before behavioral testing.
2.3 Experimental design
Six treatment groups were used in the experiment: 1) a home cage Day 1/home cage Day 2 group (HC/HC; n = 7) that was left undisturbed during the forced swim sessions, 2) a HC Day 1/25 °C 5 min swim on Day 2(HC/25; n = 9), 3) a 19 °C 15 min swim on Day 1/HC on Day 2 (19/HC; n = 8), 4) a 19 °C 15 min swim on Day 1/25 °C 5 min swim on Day 2 (19/25; n = 8), 5) a 25 °C 15 min swim on Day 1/HC on Day 2 (25/HC; n = 9), and 6) a 25 °C 15 min swim on Day 1/25 °C 5 min swim on Day 2 (25/25; n = 7). All rats in this study were implanted with biotelemetry probes to measure core body temperature (Tb; °C) and motor activity (arbitrary units). Forced swim consisted of a 15 minute swim period on Day 1 and a 5 minute swim period on Day 2. Two hours following the onset of the swim session on Day 2 rats were deeply anesthetized with sodium pentobarbital (90 mg/kg; Fatal-Plus, Vortech Pharmaceutical, Dearborn, MI, USA) prior to perfusion and collection of brain tissue for immunohistochemical procedures.
2.4 Forced swim test
All forced swim tests (FST) were performed using a Pyrex® glass cylinder (45.7 cm H × 30.5 cm diameter; Cat. No. 36360-201, VWR, West Chester, PA, USA), and a water depth of 30 cm. FSTs consisted of a 15 minute “pretest” swim session on Day 1, followed 24 hours later by a 5 minute “test” swim session on Day 2. Digital video cameras (Sony Handycam Model No. DCR-SR68, Sony Corporation of America, New York, NY, USA) were used to record behavior from a horizontal viewing plane during the 5 min swim session, and the rats’ behaviors were scored later using a behavioral computer software (The Observer® XT, Noldus Information Technology, Wageningen, The Netherlands). Briefly, the rat’s behavior during forced swim was categorized into ‘swimming’, ‘climbing’, and ‘immobility’. Behavioral analysis was analyzed using continuous sampling. Swimming was defined as any movement across the water surface that was more than necessary for the rat to keep its head above water. Climbing was defined as upward movement directed to the sides of the forced swim chamber, with the front paws breaking the water surface. Immobility was defined as lack of activity other than that required to maintain the rat’s head above water (Detke et al., 1995). The scorer was blind to assignment of treatment groups.
2.4.1 Validation of forced swim test methods
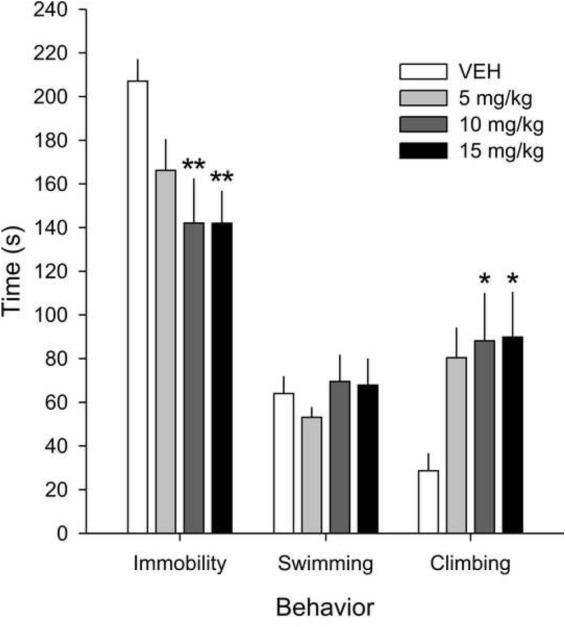
To validate our methods of using a 30.5 cm swim tank (compared to 20 cm diameter, as used by Detke and Lucki, 1996) we performed a separate-dose response study to show that the larger swim tank is sensitive to the effects of an antidepressant. We performed the dose response study using three doses of the antidepressant drug, desipramine, a tricyclic antidepressant that inhibits the reuptake of norepinephrine and serotonin (Bolden-Watson and Richelson, 1993; Nelson, 1999). Following the protocol outlined by Detke and Lucki (1996), rats received 3 subcutaneous injections in the interscapular region with desipramine hydrochloride (Cat. No. D3900, Sigma-Aldrich, St. Louis, MO, USA) at a dose of 5, 10, or 15 mg/kg or sterile 0.9% saline vehicle (VEH) injection (2 ml/kg injection volume, n = 8 for all groups) 1 hour following onset of the 15 min swim session, and then again 5 hours and 1 hour before the 5 min swim session (water temp = 25 °C ± 1 °C for both swim sessions). Drug doses were based on those used in previous studies (Molina-Hernandez et al., 2008; Molina-Hernandez et al., 2012) that have shown that 10 and 15 mg/kg doses of desipramine, but not a 5 mg/kg dose, have antidepressant-like effects in the FST. Detke and Lucki (1996) also showed that 10 mg/kg of desipramine specifically increased climbing behavior, without affecting swimming, and decreased time spent immobile.
2.4.2 Experimental forced swim test
Briefly, rats were placed into a glass cylinder filled with water at the appropriate temperature (19 °C ± 1 °C or 25 °C ± 1 °C) for 15 minutes and allowed to explore the environment. Following the 15 minute swim session, rats were dried with a towel and placed back into their home cages. On Day 2 rats were placed into the same glass cylinder filled with 25 °C ± 1 °C water for 5 minutes and allowed to explore the environment. Following the 5 minute swim session, rats were dried with a towel and placed back into their home cages. Rat’s behavior during the 5 min swim session was analyzed as described above.
2.5 Biotelemetry
Tb (°C) and motor activity (recorded as arbitrary units) were monitored on Days 1and 2 continuously at a frequency of 64 Hz during ten-second samples at one-minute intervals. Recording began 55 min prior to the onset of the forced swim on Days 1 and 2, and continued for 110 minutes after termination of forced swim. Telemetric receivers (Physiotel® Receiver, Model No. RPC-1, DSI) placed below the rats’ home cages transmitted signals to a computer before and after the swim session. During the forced swim sessions, Tb was recorded with the receivers placed below the forced swim cylinder. Telemetric signals were processed and recorded using the software ‘Dataquest ART’ (version 3.0, DSI).
2.6 Tissue fixation and sectioning
Following the induction of anesthesia, rats were transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by an ice-cold 4% paraformaldehyde solution (prepared using 80 g paraformaldehyde, 4.8 mL sodium hydroxide, 808 ml 0.2 M Na2HPO4·7H2O, 192 ml NaH2PO4·H2O, 1 L distilled H2O (dH2O)). Following fixation, brains were removed and post-fixed in 4% paraformaldehyde solution for 2 h at 4 °C. Brains were then placed in 25% sucrose in 0.1 M PBS until they had sunk. Brains were then dissected into forebrain and hindbrain sections with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately −5.80 mm bregma) using a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA) to ensure a consistent coronal plane of sectioning, then rapidly frozen in isopentane, chilled on dry ice, and stored at −80 °C. The hindbrains were sectioned (30 μm) using a cryostat (Leica CM1900, North Central Instruments, Denver, CO, USA) and stored in cryoprotectant (prepared using 270 ml ethylene glycol, 160 ml glycerol, 202 ml 0.2 M Na2HPO4·7H2O, 48 ml 0.2 M NaH2PO4·H2O, and 320 ml dH2O) at −20 °C as 6 alternate sets of sections until immunohistochemical procedures were conducted.
2.7 Antibodies
For immunodetection of tryptophan hydroxylase (Tph), an affinity-isolated antibody (sheep anti-Tph antiserum, Cat. No. T8575, Lot No, 047K1223, Sigma-Aldrich, St. Louis, MO, USA), raised against recombinant rabbit Tph, was used. Briefly, the antibody was isolated as inclusions bodies from Escherichia coli and purified by preparative sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as immunogen. This antibody has been characterized previously, and has been shown to bind both Tph1 and Tph2 isoforms (Hale et al., 2011a). To determine if the sheep anti-Tph antibody is selective to the Tph1 isoform or the Tph2 isoform of the Tph protein, we conducted dual label immunofluorescence using the sheep anti-Tph antibody from Sigma-Aldrich (Cat. No. T8575, used in this study) and monospecific polyclonal antibodies raised against a conserved peptide sequence in rat Tph1 (rat, L435ARVSRWPSV444) or Tph2 (mouse, R15RGLSLDSAVPEDHQL30) peptide antigens from non-overlapping sequences in their respective proteins (for review, see Sakowski et al., 2006), kindly supplied by Professor Donald M. Kuhn. From the immunofluorescence experiments with the monospecific polyclonal antibodies in the DR, it appeared that Tph1 was not expressed in cell soma in the DR, whereas Tph2 was highly expressed in the DR and was co-localized with Tph immunostaining using the sheep anti-Tph antibody from Sigma-Aldrich used in this study. Meanwhile, for immunofluorescence experiments with the monospecific polyclonal antibodies in the pineal gland of the same rat, it appeared that Tph1 was highly expressed in presumed pinealocytes and was co-labeled with the sheep anti-Tph antibody from Sigma-Aldrich used in this study, whereas Tph2 was not found to be expressed in the pineal gland. The finding that the sheep anti-Tph antibody from Sigma-Aldrich binds with Tph in the DR and the pineal gland suggests that this antibody binds to both Tph1 and Tph2 isoforms of the Tph protein. These experiments are detailed in a previous manuscript (Hale et al., 2011b) and are further discussed in an additional review paper (Hale and Lowry, 2011).
For immunodetection of the protein product of the immediate-early gene c-fos, an affinity-isolated antiserum was used (rabbit anti-c-Fos polyclonal antibody, Cat. No. PC38 (Ab-5), Lot No. 88552, 1:3000; EMD Biosciences, San Diego, CA, USA). This antibody, formerly sold by Oncogene Science (Cambridge, MA) as anti-c-Fos [Ab-5] [4 –17] rabbit pAb, has previously been characterized and has been shown to bind specifically to amino acids 4-17 of the human c-Fos protein (Rinaman et al., 1997). Briefly, the immunogen was a synthetic peptide (S4GFNADYEASSSRC17) corresponding to amino acids 4-17 of human c-Fos (manufacturer’s information), which is identical to the sequence in rat and mouse. The antiserum recognizes the ~55 kDa c-Fos protein and 62-kDa v-Fos protein, a viral homologue of c-Fos, but does not cross-react with the ~39 kDa c-Jun protein (manufacturer’s information). In western blot analysis using fibroblast-like BHK 21 C13 cells, the antiserum stained a single band at ~45-50 kDa in stimulated and unstimulated cells, a band that was absent following incubation of the antiserum with the synthetic immunogen (Archer et al., 1999). Likewise, preadsorption of the antiserum with the synthetic peptide eliminates immunoreactivity in neural tissue (Serrats and Sawchenko, 2006; Wahlin et al., 2000). Additionally, in previous studies, a commercially available goat anti-c-Fos antiserum raised against amino acids 3–16 of the human c-Fos protein (Cat. No. SC-52; Santa Cruz Biotechnology, Santa Cruz, CA, USA) results in a staining pattern identical to that produced by the rabbit anti-c-Fos antibody used in this study (Reznikov et al., 2008; Stratford and Finger, 2011). Finally, specificity of this same antiserum has been determined by others using co-localization of immunostaining with c-fos mRNA expression (Serrats and Sawchenko, 2006).
2.8 Immunohistochemistry
Immunohistochemistry for c-Fos and Tph was conducted on free-floating tissue in 12-well tissue culture plates in 1.5 mL of solution and gently shaken on an orbital shaker throughout the staining process. Unless stated otherwise, tissue was washed for 15 min. Tissue was rinsed twice in 0.05 M PBS followed by washing in 0.05 M PBS containing 1% hydrogen peroxide (H2O2). Tissue was then rinsed twice in 0.05 M PBS followed by one wash in 0.05 M PBS containing 0.1% Triton X-100 and 0.01% sodium azide (NaN3) (0.1% PBST + 0.01% NaN3). Sections were then incubated overnight at room temperature with 1:3000 rabbit anti-c-Fos polyclonal antibody in 0.1% PBST + 0.01% NaN3. After 16 hours, tissue was washed twice in 0.05 M PBS followed by incubation with 1:200 biotinylated donkey anti-rabbit IgG (Cat. No. 711-065-152, Lot No. 89828, Jackson Immunolabs, West Grove, PA, USA) in 0.05 M PBS for 90 min. Tissue was then washed twice in 0.05 M PBS followed by incubation with an avidin-biotin peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:200; Vector Laboratories, Burlingame, CA, USA) in 0.05 M PBS for 90 min. Tissue was washed twice in 0.05 M PBS then placed in a peroxidase substrate solution (SG substrate, Cat. No. SK4700, Vector Laboratories, diluted as recommended by the vendor) in 0.05 M PBS for 28 min. Immediately after the chromogen reaction, sections were washed twice in 0.05 M PBS followed by one wash in 1% H2O2 in 0.05 M PBS. Tissue was then washed twice in 0.05 M PBS followed by an overnight incubation in sheep anti-Tph in 0.1% PBST+ 0.01% NaN3. After 18 hours, tissue was washed twice in 0.05 M PBS followed by a 90-min incubation in biotinylated rabbit anti-sheep IgG (Vectastain Elite, Cat. No. PK-6016, 1:200; Vector Laboratories) in 0.05 M PBS. After incubation, tissue was washed twice in 0.05 M PBS followed by incubation with an avidin-biotin peroxidase complex (Elite ABC reagent; Cat. No. 6100, 1:200; Vector Laboratories) in 0.05 M PBS for 90 min. Tissue was washed twice in 0.05 M PBS followed by a 27-minute incubation in 0.01% 3-3’-diaminobenzidine tetrahydrochloride (DAB, Cat. No. D9015, Sigma-Aldrich) in 0.05 M PBS with 0.0066% H2O2. Immediately following the chromogen reaction tissue was rinsed twice in 0.05 M PBS to stop the reaction. Brain sections were stored in 0.1 M PB with 0.01% NaN3 at 4 °C until tissue mounting. Lastly, brain sections were rinsed in 0.1 M PB and then in 0.15% gelatin in dH2O, before being mounted on glass microscope slides (VistaVision UniMark microscope slides, Cat. No. 16005-106; VWR Scientific, West Chester, PA, USA), dehydrated through an ascending alcohol series and cleared with xylene. Sections were preserved with cover slips and mounting medium (Entellan; Cat. No. RT14082, EM Sciences, Hatfield, PA, USA).
2.9 Cell counting
2.9.1 Dorsal raphe nucleus (DR)
Four rostrocaudal levels of the DR were chosen for analysis (−8.00, −8.18, −8.36, and −8.54 mm bregma; Figure 1). The subdivisions of the DR studied included the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG) at − 8.00 mm and −8.18 mm bregma, and the dorsal raphe nucleus, interfascicular part (DRI) at −8.36 mm and −8.54 mm bregma. We chose to study these subregions of the DR because previous studies have found that serotonergic neurons in the DRD subregion are associated with emotional behavior (Commons et al., 2003; Lowry et al., 2008) while the DRVL and DRI subregions are activated following exposure to cold water swim, relative to swim stress at 25 °C (Kelly et al., 2011). Rostrocaudal levels and anatomical divisions of the midbrain raphe complex were based on comparison of immunostained sections to a stereotaxic rat brain atlas (Paxinos and Watson, 1998), and an atlas of Tph immunostaining in the rat DR (Abrams et al., 2004). Cell counting of c-Fos-immunoreactive (ir)/Tph-ir, c-Fos-ir/Tph-immunonegative (Tph−), and total Tph-ir neurons was performed. Cells were counted from both left and right sides of the DRVL/VLPAG and summed to give a total number of cells. All remaining cell counts were from midline subdivisions. In a limited number of cases (N = 2 out of 82 sections) from all rats at all rostrocaudal levels sampled where one side of the DRVL/VLPAG was damaged, unilateral counts were doubled for use in statistical analysis and graphical representations of the data. Cell counts were performed under brightfield microscopy at a total magnification of 100x. If necessary, a total magnification of 400x was used to verify double immunostaining. The experimenter was blind to the treatment groups throughout cell counting.
Figure 1.
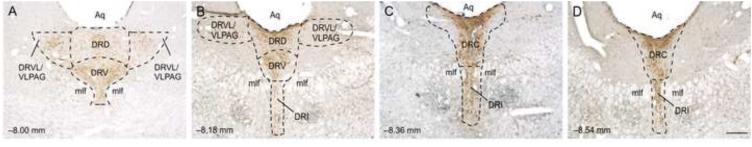
Photomicrographs of c-Fos/Tph-ir-immunostained sections illustrating the four rostrocaudal levels chosen for analysis. All rostrocaudal measurements are in reference to bregma. Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus. Scale bar = 250 μm.
2.10 Image capture
Representative photomicrographs were taken using a Nikon 90i microscope and a Nikon DS-Fi1 digital camera linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA).
2.11 Statistical analysis
All statistical analyses were performed using PASW statistics (Version 20 for Windows, SPSS Inc., Chicago, IL, USA). Data in figures are presented as group means + SEMs. Statistical significance is denoted in the graphs.
2.11.1 Telemetric data
A multifactor ANOVA with repeated measures was used to analyze telemetric data for Tb expressed as the average temperature in 5 minute time bins. Tb was analyzed using treatment (Day 1 three levels: HC, 19 °C, and 25 °C swim stress) as a between-subjects factor and time (52 levels) as a within-subjects factor for the repeated measures analysis. A Greenhouse-Geisser epsilon correction (ε) was used for repeated measures to correct for violation of the sphericity assumption. Grubbs’ test was used to identify and remove any outliers (Day 1 temperature, N = 4, 0.26%; Day 2 temperature, N = 3, 0.19%) (Grubbs, 1969). Replacement values for missing data (Day 1 temperature, N = 4, 0.26%; Day 2 temperature, N = 3, 0.19%) for the multifactor ANOVA with repeated measures were calculated using the Petersen method (Petersen, 1985). Replacement data were not used in graphical representations of the data or in planned pairwise comparisons. Significance was accepted at the level of p < 0.05. If significance was reached in the multifactor ANOVA with repeated measures, Bonferroni tests were used for planned pairwise comparisons.
2.11.2 FST data
2.11.2.1 Validation of FST methods
A one-way analysis of variance (ANOVA) with drug dose as a between-subjects factor was used to determine the effect of drug dose on swimming, climbing, and immobility behaviors during the 5 minute swim session. Grubbs’ test was used to identify any outliers (swimming, n = 2, 6.25%; climbing, n = 1, 3.13%; immobility, n = 1, 3.13%) and outliers were removed. When appropriate, Fisher’s protected least significant difference (LSD) tests were used for post hoc comparisons. Statistical significance was accepted at the level of p < 0.05 for both the ANOVA and post hoc comparisons.
2.11.2.2 Experimental FST
A one-way analysis of variance (ANOVA) with water temperature as a between-subjects factor was used to determine the effect of Day 1 water temperature on swimming, immobility, and climbing behaviors during swim stress on Day 2. Grubbs’ test was used to identify any outliers; no outliers were identified. When appropriate, Fisher’s protected least significant difference (LSD) tests were used for post hoc comparisons. Statistical significance was accepted at the level of p < 0.05 for both the ANOVA and post hoc comparisons.
2.11.3 Cell count data
Cell counts within the DR for the numbers of c-Fos-ir/Tph-ir (serotonergic) neurons, the numbers of c-Fos-ir/Tph− (non-serotonergic) cells and the total numbers of Tph-ir neurons sampled were analyzed separately using a three-way design using a linear mixed model analysis (Day 1, three levels: Home Cage (HC), 19 °C and 25 °C swim stress; Day 2, two levels: HC and 25 °C swim stress) as between-subjects factors and brain region (3 levels) as a within-subjects factor for the repeated measures analysis. Linear mixed model analysis is a statistical approach ideal for repeated measures data that efficiently accounts for multiple measures derived from the same unit of observation (i.e. rat). Furthermore, linear mixed model analysis asses both random effects and fixed effects while also accommodating missing data and complex covariance structures. In the analysis, we separately modeled standardized c-Fos- ir/Tph-ir, c-Fos-ir/Tph−, and total Tph-ir counts measured in different subdivisions of the midbrain raphe complex, subject to different treatment conditions described above, assuming an unstructured covariance structure. We followed the primary analysis with post hoc analysis using Fisher’s protected LSD tests when appropriate. Grubbs’ test was used to identify outliers (c-Fos-ir/Tph-ir neurons, N = 5, 3.47%; c-Fos- ir/Tph&− cells, N = 3, 2.08%; total Tph-ir neurons, N = 2, 1.39%) and outliers were removed; total missing data (c-Fos-ir/Tph-ir neurons, N = 21, 14.58%; c-Fos-ir/Tph− cells, N = 19, 13.19%; total Tph-ir neurons, N = 18, 12.5%).
3. Results
3.1 Core body temperature (Tb)
3.1.1 Effects of Day 1 HC control conditions or swim at 19 °C or 25 °C on Tb
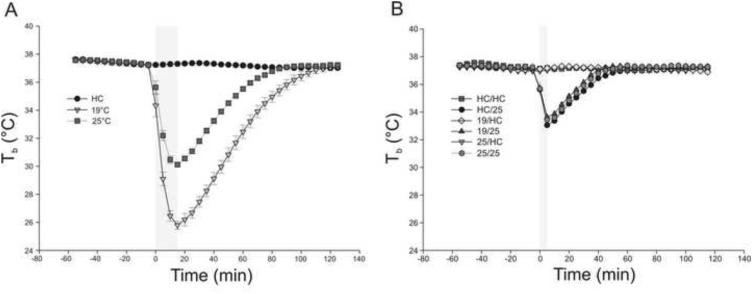
Swim stress at 19 °C and 25 °C on Day 1 decreased Tb (measured via biotelemetry) throughout the test (treatment × time point interaction: F(72,1404) = 287.68, p < 0.001, ε = 0.069; Figure 2). Swim stress at different water temperatures differentially affected Tb relative to the HC control condition (Figure 2). Swim stress at 19 °C, relative to HC controls, decreased Tb from 0 to 100 minutes after the onset of swim stress (p < 0.001 from 0-90 minutes; p < 0.01 at 95 minutes; p < 0.05 at 100 minutes). Swim stress at 25 °C, relative to HC controls, resulted in decreased Tb from 0 to 70 minutes after the onset of swim stress (p < 0.001 from 0-60 minutes; p < 0.01 at 65 minutes; p < 0.05 at 70 minutes). In addition, swim stress at 19 °C resulted in greater decreases in Tb relative to 25 °C. Swim stress exposure at 19 °C, relative to 25 °C, decreased Tb from 0 to 100 minutes after the onset of swim stress (p < 0.001 from 0-90 minutes, p < 0.01 at 95 minutes, and p < 0.05 at 100 minutes). Tb of HC control rats was not altered throughout the experiment (Figure 2).
Figure 2.
Graphs illustrating the effects of Days 1 and 2 swim stress on core body temperature (Tb; °C). A) Graph illustrating effect of home cage control (HC) conditions or exposure to 19 °C or 25 °C swim stress (15 min exposure) on Tb on Day 1 (HC, n = 14, 19 °C, n = 15; 25 °C, n = 13). B) Graph illustrating the effects of HC control conditions or exposure to 25 °C swim stress (5 min exposure) on Day 2 following exposure to HC control conditions, or exposure to 19 °C or 25 °C swim stress (15 min exposure) on Day 1 (HC/HC, n = 6; HC/25, n = 8; 19/HC, n = 7; 25/HC, n = 8; 19/25, n = 8; 25/25, n = 7). C) magnified view of the area highlighted in B. Gray bars indicate timing and duration of forced swim exposure. Symbols indicate significance for individual time points, or for a span of time points (where specified). * p < 0.05 vs HC at same time point, ** p < 0.01 vs HC at same time point, *** p < 0.001 vs HC at same time point. † p < 0.05 vs 25 °C at same time point, †† p < 0.01 vs 25 °C at same time point, ††† p < 0.001 vs 25 °C at same time point. # p < 0.05 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, at same time point, ## p < 0.01 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, at same time point, ### p < 0.001 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, at same time point. ‡ p < 0.05 vs Day 1 home cage (HC) control conditions under the same Day 2 condition, at same time point, ‡‡ p < 0.01 vs Day 1 home cage (HC) control conditions under the same Day 2 condition, at same time point.
3.1.2 Effects of Day 2, HC control conditions or 25 °C swim stress on Tb
Swim stress at 25 °C, relative to HC control conditions, on Day 2 decreased Tb (measured via biotelemetry) throughout the test (treatment × time point interaction: F(170,1292) = 38.72, p < 0.001, ε = 0.148) (Figure 2B).
3.1.3 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to HC control conditions on Day 2
Differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 did not affect Tb among rats exposed to HC treatment on Day 2.
3.1.4 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to 25 °C swim stress on Day 2
Differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 affected Tbamong rats exposed to 25 °C swim stress on Day 2. Among rats exposed to 25 °C swim stress on Day 2, prior exposure to 19 °C swim stress on Day 1, relative to prior exposure to HC control conditions on Day 1 resulted in higher Tb on Day 2 at 5 minutes (p < 0.05), 35 minutes (p < 0.01), and 40-45 minutes (p < 0.05) following the onset of swim stress. Exposure to 25 °C swim stress on Day 1, relative to exposure to HC control conditions on Day 1, did not affect Tb responses to 25 °C swim stress on Day 2.
3.1.5 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to HC control conditions on Day 1
Among rats exposed to HC control conditions on Day 1, exposure of rats to 25 °C swim stress on Day 2 resulted in lower Tb than rats exposed to HC control conditions on Day 2 following the onset of swim stress (0-35 minutes, p < 0.001; 40 minutes p < 0.05).
3.1.6 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 19 °C swim stress on Day 1
Among rats exposed to 19 °C swim stress on Day 1, exposure of rats to 25 °C swim stress on Day 2 resulted in lower Tb relative to rats exposed to HC control conditions on Day 2 following the onset of swim stress (0-30 minutes, p < 0.001).
3.1.7 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 25 °C swim stress on Day 1
Among rats exposed to 25 °C swim stress on Day 1, exposure of rats to 25 °C swim stress on Day 2 resulted in lower Tb relative to rats exposed to HC control conditions on Day 2, 0-30 minutes (p < 0.001) and 35 minutes (p < 0.01) following the onset of swim stress.
3.2 Swim stress behavior
3.2.1 Validation of FST methods
There was an effect of drug on climbing (F(3,27) = 3.14, p < 0.05) and immobility (F(3,27) = 4.84, p < 0.01) behavior during the 5 min swim session. Rats treated with 10 or 15 mg/kg doses of desipramine spent more time climbing (Fisher’s protected LSD test, p < 0.05; Figure 3) and spent less time immobile (Fisher’s protected LSD test, p < 0.01; Figure 3) compared to rats treated with vehicle. In contrast, desipramine had no effect on swimming behavior, at any dose tested.
Figure 3.
Graph illustrating the effects of different doses of desipramine (5, 10 or 15 mg/kg) or vehicle (VEH; sterile saline) on behavior in the forced swim test (FST). The 10 and 15 mg/kg doses of desipramine, but not the 5 mg/kg dose, increased climbing behavior and decreased time spent immobile, without affecting swimming behavior, compared to VEH-treated controls. Behavior was measured continuously and is expressed as mean total time +SEM. Data were analyzed using a one-way analysis of variance (ANOVA) with drug dose as a between-subjects factor. * p < 0.05 vs VEH, ** p < 0.01 vs VEH; Fisher’s protected least significant difference (LSD) test. n = 8 for all groups.
3.2.2 Experimental FST behavior
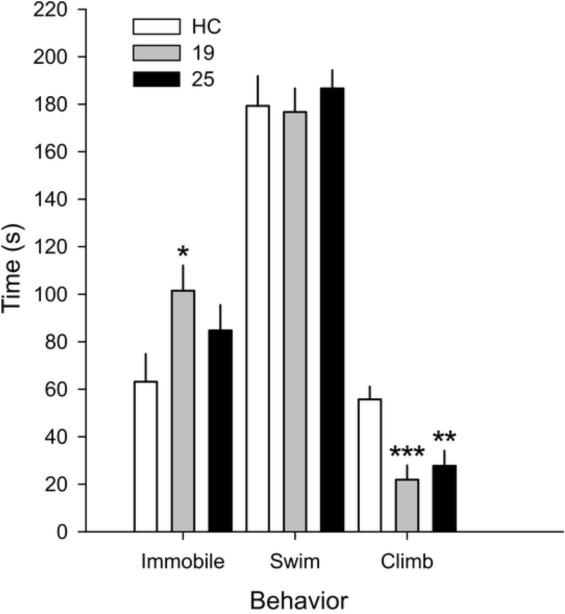
Among rats exposed to swim stress on Day 2, previous swim on Day 1 altered climbing behavior (F(2,21) = 9.94, p < 0.01). Rats exposed to 19 °C or 25 °C swim stress on Day 1 spent less time climbing when compared to rats exposed to HC control conditions on Day 1 (Figure 4). In addition, rats exposed to 19 °C swim stress on Day 1 (but not rats exposed to 25 °C on Day 1) spent more time immobile during the 5 minute swim on Day 2 compared to rats exposed to HC control conditions on Day 1 (Fisher’s protected LSD test, p < 0.05; Figure 4).
Figure 4.
Graph illustrating the effects of Days 1 and 2 swim stress on behavior. Graph displaying the effects of home cage (HC) control conditions or 15 minute swim stress at 19 °C or 25 °C on Day 1 on subsequent behavior following exposure to swim stress at 25 °C on Day 2. Behavior was measured continuously and is expressed as mean total time +SEM. * p < 0.05 vs HC, ** p < 0.01 vs HC, *** p < 0.001 vs HC; Fisher’s protected least significant difference (LSD) test. HC, n = 9; 19 °C, n = 8; 25 °C, n = 7.
3.3 Immunohistochemistry
3.3.1 c-Fos-ir/Tph-ir neurons in the DR
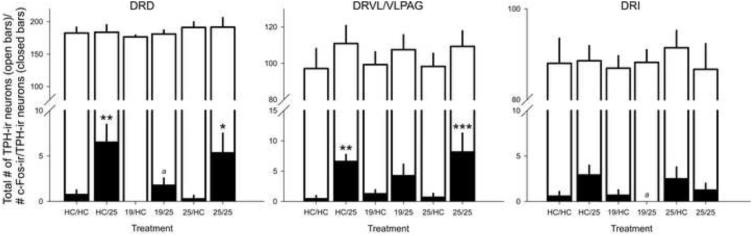
Exposure to swim stress on Day 2 increased c-Fos-ir/Tph-ir neurons in subdivisions of the DR (treatment × brain region interaction: F(10,32.51) = 2.433, p < 0.05; Figure 5 and Figure 6).
Figure 5.
Graphs illustrating the effects of Days 1 and 2 swim stress on numbers of c-Fos-immunoreactive (ir) serotonergic neurons in subregions of the dorsal raphe nucleus (DR). Graphs display the effects of either home cage control (HC) conditions or a 15 minute swim stress exposure at 19 °C or 25 °C on Day 1 on cellular responses to subsequent exposure to either HC conditions or a 5 min swim stress exposure at 25 °C on Day 2. Graphs display the effects of treatment on the numbers of c-Fos-immunoreactive (ir)/tryptophan hydroxylase (Tph)-ir neurons following swim stress on Day 2 (HC or 25 °C 5 minute swim) within different subdivisions of the dorsal raphe nucleus (DR). Open bars represent mean values for numbers of Tph-ir neurons, closed bars represent mean values for numbers of c-Fos-ir/Tph-ir neurons. * p < 0.05 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, ** p < 0.01 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, *** p < 0.001 vs Day 2 home cage (HC) control conditions under the same Day 1 condition, a p < 0.05 vs HC/25; Fisher’s protected least significant difference (LSD) test. Abbreviations: DRD, dorsal raphe nucleus, dorsal part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; DRI, dorsal raphe nucleus, interfascicular part.
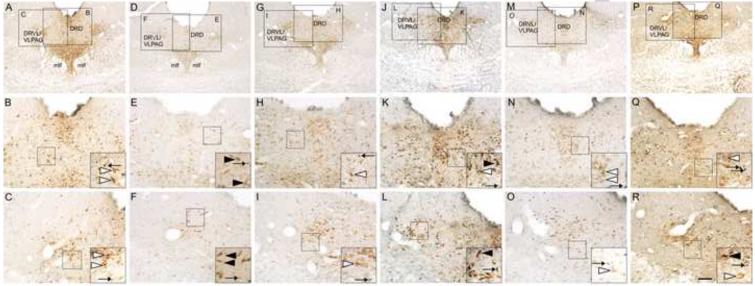
Figure 6.
Photomicrographs illustrating c-Fos/tryptophan hydroxylase (Tph) immunostaining in the mid-rostrocaudal dorsal raphe nucleus (DR; −8.00 mm bregma) in representative rats from each treatment group. Photomicrographs illustrate immunostaining in rats exposed to (A-C) HC/HC, (D-F) HC/25 °C, (G-I) 19 °C/HC, (J-L) 19 °C/25 °C, (M-O) 25 °C/HC, (P-R) 25 °C/25 °C. Black boxes in A, D, G, J, M and P represent regions shown at higher magnification in B, C, E, F, H, I, K, L, N, O, Q and R. Black boxes in B, C, E, F, H, I, K, L, N, O, Q and R indicate regions shown at higher magnification as insets located in the lower right-hand corner of these respective panels. Black arrows indicate representative examples of c-Fos-immunoreactive (ir) non-serotonergic cells (blue/black nuclear staining); white arrowheads indicate representative examples of Tph-ir/c-Fos-immunonegative neurons (red/brown cytoplasmic staining); black arrowheads indicate representative examples of c-Fos-ir/Tph-ir neurons (red/brown cytoplasmic staining with blue/black nuclear staining). Scale bar = 250 μm (A, D, G, J, M, and P); 100 μm (B, C, E, F, H, I, K, L, N, O, Q, and R); 50 μm (insets).
3.3.1.1 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to HC control conditions on Day 1
Among rats exposed to HC control conditions on Day 1, rats exposed to 25 °C swim stress on Day 2 (HC/25) had increased numbers of c-Fos-ir/Tph-ir neurons in the DRD (p < 0.01) and the DRVL/VLPAG (p < 0.01) when compared to rats exposed to HC control conditions (HC/HC) on Day 2 (Figure 5). An effect of differential treatment on Day 2 was not found to affect the number of c-Fos-ir/Tph-ir neurons in the DRI.
3.3.1.2 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 19 °C swim stress on Day 1
Among rats exposed to 19 °C swim stress on Day 1, exposure of rats to 25 °C swim stress on Day 2 (19/25) did not have an effect on the number of c-Fos-ir/Tph-ir neurons in the DRD, DRVL/VLPAG, or DRI when compared to rats exposed to HC control conditions (19/HC) on Day 2.
3.3.1.3 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 25 °C swim stress on Day 1
Among rats exposed to 25 °C swim stress on Day 1, rats exposed to 25 °C swim stress on Day 2 had increased numbers of c-Fos-ir/Tph-ir neurons in the DRD (p < 0.05) and the DRVL/VLPAG (p < 0.001) when compared to rats exposed to HC control conditions on Day 2 (Figure 5). An effect of differential treatment on Day 2 was not found to affect the number of c-Fos-ir/Tph-ir neurons in the DRI.
3.3.1.4 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to HC control conditions on Day 2
Among rats exposed to HC control conditions on Day 2, differential treatment on Day 1 did not affect the number of c-Fos-ir/Tph-ir neurons in the DRD, DRVL/VLPAG, or DRI (Figure 5).
3.3.1.5 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to 25 °C swim stress on Day 2
Among rats exposed to 25 °C swim stress on Day 2, rats exposed to 19 °C swim stress on Day 1 (19/25), relative to rats exposed to HC control conditions on Day 1 (HC/25) had decreased numbers of c-Fos-ir/Tph-ir neurons in the DRD (p < 0.05) and the DRI (p < 0.05) (Figure 5). Differential treatment on Day 1 was not found to affect the number of c-Fos-ir/Tph-ir neurons in the DRVL/VLPAG (Figure 5).
3.3.2 c-Fos-ir non-serotonergic cells in subdivisions of the DR
Exposure to swim stress increased c-Fos immunostaining in non-serotonergic cells in subdivisions of the DR (treatment × brain region interaction: F(10,41.53) = 3.61, p = 0.002) (Table 1).
Table 1.
Effects of differential treatment on Day 1 and Day 2 on the number of c-Fos-ir non-serotonergic cells in subregions of the DR
| c-Fos-ir/Tph-immunonegative cells |
||||||
|---|---|---|---|---|---|---|
| Region | HC/HC | HC/25 | 19/HC | 19/25 | 25/HC | 25/25 |
| DRD | 19.33 ± 2.87 | 52.75 ± 8.25 ** | 25.00 ± 5.41 | 38.43 ± 7.09 | 18.71 ± 1.77 | 52.14 ± 7.63 ** |
| DRVL/VLPAG | 64.14 ± 9.93 | 105.37 ± 8.15 *** | 51.71 ± 6.56 | 100.62 ± 7.27 *** | 41.54 ± 8.75 | 109.00 ± 12.79 *** |
| DRI | 12.00 ± 2.78 | 27.22 ± 5.90 * | 10.80 ± 3.79 | 19.60 ± 4.93 | 23.00 ± 5.64 | 26.67 ± 3.04 |
All comparisons are versus Day 2 home cage (HC) control conditions under the same Day 1 condition
p < 0.05;
p < 0.01;
p < 0.001
3.3.2.1 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to HC control conditions on Day 1
Among rats exposed to HC control conditions on Day 1, rats exposed to 25 °C swim stress on Day 2 (HC/25) had increased numbers of c-Fos-ir/Tph− cells in the DRD (p < 0.01), DRVL/VLPAG (p < 0.01), and DRI (p < 0.05) when compared to rats exposed to HC control conditions (HC/HC) on Day 2 (Table 1).
3.3.2.2 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 19 °C swim stress on Day 1
Among rats exposed to 19 °C swim stress on Day 1, rats exposed to 25 °C swim stress on Day 2 (19/25) had increased numbers of c-Fos-ir/Tph− cells in the DRVL/VLPAG (p < 0.001) when compared to rats exposed to HC control conditions (19/HC) on Day 2 (Table 1). An effect of differential treatment on Day 2 was not found to affect the number of c-Fos-ir/Tph− neurons in the DRD and DRI (Table 1).
3.3.2.3 Effects of differential treatment (HC control conditions or 25 °C swim stress) on Day 2 among rats exposed to 25 °C swim stress on Day 1
Among rats exposed to 25 °C swim stress on Day 1, rats exposed to 25 °C swim stress on Day 2 (25/25) had increased numbers of c-Fos-ir/Tph− cells in the DRD (p < 0.01) and the DRVL/VLPAG (p < 0.001) when compared to rats exposed to HC control conditions (25/HC) on Day 2 (Table 1). An effect of differential treatment on Day 2 was not found to affect the number of c-Fos-ir/Tph− neurons in the DRI (Table 1).
3.3.2.4 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to HC control conditions on Day 2
Among rats exposed to HC control conditions on Day 2, differential treatment on Day 1 did not affect the number of c-Fos-ir/Tph− cells in the DRD, DRVL/VLPAG, or DRI (Table 1).
3.3.2.5 Effects of differential treatment (HC control conditions, 19 °C swim stress, or 25 °C swim stress) on Day 1 among rats exposed to 25 °C swim stress on Day 2
Among rats exposed to 25 °C swim stress on Day 2, differential treatment on Day 1 did not affect the number of c-Fos-ir/Tph− cells in the DRD, DRVL/VLPAG, or DRI (Table 1).
3.3.3 Tph-ir neurons in subdivisions of the DR
Repeated swim stress had no effects on the numbers of Tph-ir neurons in subregions of the DR (treatment × brain region interaction: F(10, 33.55) = 0.34, p = 0.964; treatment: F(5,35.22) = 0.47, p = 0.798) (Figure 5). As expected, different subregions of the DR had different numbers of Tph-ir neurons, independent of treatment (brain region: F(2,33.60) = 282.93, p < 0.001) (Figure 5).
4. Discussion
Among rats exposed to a 5 min, 25 °C swim on Day 2, prior exposure to a cold water (19 °C) swim, but not prior exposure to a 25 °C swim, relative to prior exposure to a HC control condition, altered physiological, behavioral and serotonergic responses to swim stress. Specifically, rats exposed to 19 °C swim stress, relative to HC control conditions, on Day 1 responded on Day 2 with increased immobility, decreased climbing, attenuated hypothermia, and attenuated c-Fos expression in serotonergic neurons within the DRD and DRI subregions of the DR. Among rats exposed to HC control conditions on Day 1, exposure to 25 °C swim stress, relative to HC control conditions on Day 2, increased c-Fos expression in serotonergic neurons within the DRD and DRVL/VLPAG regions, an effect that was attenuated in rats exposed to 19 °C swim stress, but not 25°C swim stress, on Day 1. These data suggest cold swim potently modulates subsequent physiological, behavioral, and neural responses to subsequent forced swim.
Prior cold swim, relative to prior HC conditions, decreased climbing and increased immobility during a 5 min 25 °C swim, whereas prior 25 °C swim, like prior cold swim, decreased climbing but had no effect on immobility. The lack of effect of prior swim at 25 °C on immobility in this modified forced swim test is inconsistent with numerous studies demonstrating that prior swim increases immobility in the original forced swim test, as designed by Porsolt and colleagues (15 cm of water, 25 °C) (Porsolt et al., 1978). This increase in immobility in the forced swim test following a 15 min swim 24 h earlier has been described as “behavioral despair” and promoted by some to be a model of depression. Other groups argue that the increased immobility is an adaptive behavioral response. Here we show that the increased immobility is not evident in the modified forced swim test designed by Lucki and colleagues (Lucki, 1997), where rats are unable to support their body weight by placing their tail on the bottom of the cylinder (30 cm of water, 25 °C). To our knowledge, this is the first demonstration of a lack of effect of prior swimming (at 25 °C) on immobility in the modified version of the forced swim test. Nevertheless, the modified forced swim test remains a very effective screening tool for potential antidepressant drug activity. Prior exposure to a 15 min 25 °C swim did, however, decrease climbing (similar to prior exposure to a 15 min 19 °C swim (see below), suggesting that prior swim exposure under these conditions did alter subsequent behavior, albeit not immobility.
In contrast, in the current study prior exposure to cold swim (15 min at 19 °C) did alter behavior in a 5 min swim at 25 °C, 24 h later. Specifically, prior cold swim increased immobility and decreased climbing, with no effect on swimming behavior 24 h later. The increased immobility is consistent with previous studies demonstrating that intermittent cold water swim stress (consisting of 80, 5 sec cold water swims at 15 °C in an 80 min session) increases immobility during swim testing on Day 2, using conditions similar to the modified forced swim test (29 cm of water, 23 °C) (Christianson and Drugan, 2005). These data suggest that either the cold water stimulus itself, or the heterotypic nature of the stressor on Day 2, is responsible for the increased immobility.
Although previous studies have found that a 15 min pretest followed, 24 h later, by a 5 min retest results in increased immobility (e.g., Jefferys and Funder, 1994), in our study this was not the case. It is plausible that the differences are due to methodological differences as previous studies have frequently used smaller diameter swim tanks (18 cm diameter, versus 30 cm diameter in our study), and more shallow water depth (20 cm, versus 30 cm in our study) (e.g., Jefferys and Funder, 1994). Indeed, previous work has shown that water depth affects immobility time on the second day of the forced swim test when performed at either 25 °C or 30 °C; shallower water depths result in increased immobility, in part due to the ability of the rat to support its body with its tail (Abel, 1994; Pinter et al., 2011). Our studies intentionally used a water depth that prevents the rat from supporting its body with its tail, as recommended by Detke and colleagues (Detke et al., 1995), and this fundamental difference may have influenced the way rats coped with the stressor during testing. Increased immobility upon retesting has been observed following either a 5 min or 30 min pretest followed by a 5 min or 30 min test, respectively, but these increases were modest, and observed using a smaller diameter cylinder (20 cm) and warmer water temperature (30 °C) (Jang et al., 2009). Consistent with our findings using either a 19 °C or 25 °C pretest and 25 °C retest, the same study found decreased climbing, but not decreased swimming, in a 5 min test under these conditions (Jang et al., 2009).
As climbing behavior in the modified forced swim test is thought to be dependent on noradrenergic signaling, while swimming behavior in the same test is thought to be dependent on serotonergic signaling (Detke et al., 1995), these data suggest that prior cold swim decreases noradrenergic signaling upon testing on Day 2 (Cryan et al., 2005). Consistent with this finding, prior studies have demonstrated that the behavioral effects of intermittent cold water swim can be reversed by noradrenergic reuptake inhibitors, but not serotonin reuptake inhibitors (Christianson et al., 2008; Drugan et al., 2010; Warner and Drugan, 2012).
The effects of a 15 min 19 °C swim and a 15 min 25 °C swim on Tb on Day 1 were similar to what we previously reported (Kelly et al., 2011), and to studies by Linthorst and colleagues (Linthorst et al., 2008). Prior cold swim, relative to prior HC conditions, attenuated hypothermia during a 5 min 25 °C swim, whereas prior 25 °C swim had no effect on the hypothermic response. The physiologic basis of the altered hypothermic response is not clear, but could involve a sensitization toward increased cold defense mechanisms such as peripheral cutaneous vasoconstriction, shivering, and brown adipose tissue thermogenesis, all of which are dependent on increased sympathetic outflow (Morrison and Nakamura, 2011). An alternative explanation, which cannot be excluded, is that increased immobility/decreased climbing in rats previously exposed to a 5 min 19 °C swim resulted in a deeper positioning of the telemetric transmitter, resulting in an artificially warmer telemetric recording. An argument against this alternative explanation is that rats exposed to a 15 min 25 °C swim on Day 1, followed by a 5 min 25 °C swim on Day 2, also responded with decreased climbing behavior relative to rats exposed to HC control conditions on Day 1, but did not respond with an attenuated hypothermia.
We cannot differentiate between the relative contributions of the psychological and physiological stress associated with forced swimming on Day 1 to subsequent stress reactivity, 24 h later. However, we have shown that there are no differences in swimming, climbing, or immobility during a 15 min forced swim at either 25 °C or 35 °C, a temperature that causes only a mild hypothermia (~1 °C) (Kelly et al., 2011). Likewise, there were no differences in behavioral responses to the forced swim test conducted at 25 °C or 30 °C, in previous studies (Jefferys and Funder, 1994; Pinter et al., 2011). In addition, there were no differences in plasma adrenocorticotropic hormone (ACTH) or corticosterone concentrations following exposure of rats to the forced swim test at 20 °C, 25 °C, or 30 °C, suggesting that the differences in water temperature within this range do not strongly affect the aversiveness of the test, as measured by hypothalamic-pituitary-adrenal axis activity. A definitive determination of the contributions of the psychological and physiological stress associated with forced swimming on Day 1 to subsequent stress reactivity, 24 h later, will require further study.
Among rats exposed to HC control conditions on Day 1, a 5 min 25 °C swim on Day 2, relative to exposure to HC control conditions on Day 2, increased c-Fos expression in the DRD and DRVL/VLPAG, while an effect in the DRI approached statistical significance (p = 0.056). These data are consistent with a previous study in which a longer swim (15 min) under the same conditions, increased c-Fos expression in serotonergic neurons in the DRD and (if cell counts from −8.00 and −8.18 mm bregma are summed, as done here) the DRVL/VLPAG (Kelly et al., unpublished). In the previous study, there were no effects in the rostrocaudal levels of the DRI studied here, but there was a significant effect in the caudal DRI (−8.72 mm bregma). Therefore, c-Fos responses in serotonergic neurons following a 5 min or 15 min swim at 25 °C in a water depth of 30 cm are remarkably consistent. The DRI serotonergic neurons project to the hippocampus (Imai et al., 1986; Köhler and Steinbusch, 1982), and therefore may contribute to swim stress-induced increases in hippocampal extracellular 5-HT concentrations (Linthorst et al., 2002; Linthorst et al., 2008). The data are also consistent with studies by Roche and colleagues showing that a small number of serotonergic neurons are c-Fos-positive following forced swim (although most cells in the DR affected by forced swim are GABAergic, not serotonergic (see below)). Among rats exposed to HC conditions on Day 1 or 25 °C swim on Day 1, the c-Fos responses in serotonergic neurons in response to 25 °C swim on Day 2 were identical (that is, increases in the DRD and DRVL/VLPAG, but not the DRI). However, in rats exposed to 19 °C swim on Day 1, c-Fos responses to 25 °C swim on Day 2 were attenuated in the DRD and DRI. An attenuated response on Day 2 would be expected if swim-induced c-Fos expression is dependent on noradrenergic signaling, and prior cold swim results in attenuated noradrenergic signaling on Day 2. Serotonergic neuronal firing rates are stimulated by noradrenergic signaling acting via the α1 adrenergic receptor (Vandermaelen and Aghajanian, 1983). Alternatively, the prior exposure of the cold swim may have changed the aversiveness of the subsequent ambient swim (i.e., a contrast effect in which the rat remembers the forced swimming at 19 °C and interprets it as less aversive than forced swimming at 25 °C). Caution is warranted, therefore, in extrapolating findings from this study, which uses a very similar behavioral paradigm both as the stressor and as the subsequent behavioral test, to other paradigms investigating physiological and behavioral consequences of inescapable stress. Importantly, our results do not address the issue of trans-situationality, an important component of learned helplessness following inescapable tailshock (Maier and Watkins, 2005); consequently, particular caution should be used when comparing the results in this study to the results of inescapable shock in a model of learned helplessness.
It is also worth noting what did not happen following the cold water swim in the present study. There was no potentiation of swim stress-induced c-Fos expression in serotonergic neurons on Day 2 following prior exposure to cold swim on Day 1. In a model of learned helplessness, exposure to inescapable shock leads to a functional desensitization of inhibitory 5-HT1A autoreceptor function, specifically in the DRD subregion of the DR (Rozeske et al., 2011), and increased stress-induced serotonergic activity in forebrain targets of DRD neurons, such as the basolateral amygdala, 24 h later (Amat et al., 2005; Christianson et al., 2010; Maier and Watkins, 1998; Maier and Watkins, 2005). There is no evidence for increased excitability of DRD serotonergic neurons 24 h following cold swim and this suggests a fundamental distinction between these 2 stressors.
Among rats exposed to HC conditions on Day 1, exposure to swim at 25 °C, relative to HC control conditions, on Day 2 increased c-Fos expression in non-serotonergic neurons within all subregions of the DR studied, the DRD, DRVL/VLPAG, and DRI. As reported previously, quantitatively, c-Fos expression in non-serotonergic neurons in the DR far outnumbers c-Fos expression in serotonergic neurons in the DR following forced swimming (Roche et al., 2003) (the majority of swim-activated non-serotonergic cells are GABAergic), and the greatest number of c-Fos-positive non-serotonergic neurons are in the DRVL/VLPAG, an area that contains large numbers of GABAergic neurons (Day et al., 2004). Swim exposure at 25 °C, relative to HC conditions, on Day 2 increased c-Fos expression in non-serotonergic neurons in the DRVL/VLPAG regardless of experience on Day 1. There were no differences in c-Fos expression in any subregion due to stress condition on Day 1, in either rats exposed to HC conditions or swim at 25 °C on Day 2.
Although measurement of c-Fos expression is a useful method of identifying functional cellular responses among large populations of neurons in the CNS, a reduction in cellular c-Fos induction does not necessarily imply a reduction of neuronal responses, or a reduction in serotonergic neurotransmission, in this case. Despite these limitations, this technique has been a useful tool in the functional mapping of neural circuitry mediating or modulating many stimuli, including stress-related stimuli (Herman et al., 1996; reviewed in Herman and Cullinan, 1997; Kovacs, 1998; Senba and Ueyama, 1997). We cannot exclude the possibility that, 24 h following a severe stressor, induction of c-Fos is somehow compromised, while serotonergic neurotransmission is not. Clarification of the effects of treatment on serotonin release in terminal regions of DRD serotonergic neurons would require additional studies using in vivo microdialysis, and additional functional endpoints.
Conclusions
These studies suggest that, unlike the model of learned helplessness, which is dependent on a functional desensitization of inhibitory 5-HT1A autoreceptors on serotonergic neurons in the DR, and consequently increased excitability of DRD serotonergic neurons 24 h following the stressor, behavioral responses to cold swim 24 h following the stressor are not dependent on sensitization of DR serotonergic neurons. In contrast, behavioral responses to a 25 °C swim 24 h following a cold swim exposure are associated with a decreased excitability of DR serotonergic neurons, perhaps due to decreased noradrenergic input to serotonergic neurons. Regardless, these studies support the conclusion that exposure to cold swim results in behavioral, physiological, and neural adaptations 24 h later (following a second stressor), including increased immobility in the modified forced swim test, attenuation of hypothermia, and attenuation of swim-induced c-Fos expression in serotonergic neurons.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine; serotonin
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRVL/VLPAG
dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray
- FST
forced swim test
- HC
home cage
- ir
immunoreactive
- PBS
phosphate-buffered saline
- SS
swim stress
- Tb
core body temperature (°C)
- Tph
tryptophan hydroxylase
Reference List
- Abel EL. Ontogeny of immobility and response to alarm substance in the forced swim test. Physiol Behav. 1993;54:713–716. doi: 10.1016/0031-9384(93)90081-p. [DOI] [PubMed] [Google Scholar]
- Abel EL. Behavioral and physiological effects of different water depths in the forced swim test. Physiol Behav. 1994;56:411–414. doi: 10.1016/0031-9384(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomical and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer S, Li TT, Evans AT, Britland ST, Morgan H. Cell reactions to dielectrophoretic manipulation. Biochem Biophys Res Commun. 1999;257:687–698. doi: 10.1006/bbrc.1999.0445. [DOI] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52:1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Drugan RC. Intermittent cold water swim stress increases immobility and interferes with escape performance in rat. Behav Brain Res. 2005;165:58–62. doi: 10.1016/j.bbr.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Rabbett S, Lyckland J, Drugan RC. The immobility produced by intermittent swim stress is not mediated by serotonin. Pharmacol Biochem Behav. 2008;89:412–423. doi: 10.1016/j.pbb.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neuroscience. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. C-Fos expression, behavioural, endocrine and autonomic responses to acute social stress in male rats after chronic restraint: modulation by serotonin. Neuroscience. 2000;95:453–463. doi: 10.1016/s0306-4522(99)00459-5. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Macomber H, Warner TA. Intermittent and continuous swim stress-induced behavioral depression: sensitivity to norepinephrine- and serotonin-selective antidepressants. Psychopharmacology (Berl) 2010;212:85–91. doi: 10.1007/s00213-010-1935-3. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HEW, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hale MW, Dady KF, Evans AK, Lowry CA. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp Neurol. 2011a;227:264–278. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A. Development by environment interactions controlling tryptophan hydroxylase expression. J Chem Neuroanat. 2011b;41:219–226. doi: 10.1016/j.jchemneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn F, Edwards E, Muneyyirci J. Animal models of depression. Clin Neurosci. 1993;1:152–156. [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CMF, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jang DP, Lee SH, Lee SY, Park CW, Cho ZH, Kim YB. Neural responses of rats in the forced swimming test: [F-18]FDG micro PET study. Behav Brain Res. 2009;203:43–47. doi: 10.1016/j.bbr.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jefferys D, Funder J. The effect of water temperature on immobility in the forced swimming test in rats. Eur J Pharmacol. 1994;253:91–94. doi: 10.1016/0014-2999(94)90761-7. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Donner NC, Hale MW, Lowry CA. Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience. 2011;197:251–268. doi: 10.1016/j.neuroscience.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, Buwalda B, van Reenen K. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70:218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- Korte SM, Meijer OC, De Kloet ER, Buwalda B, Keijser J, Sluyter F, van OG, Bohus B. Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res. 1996;736:338–343. doi: 10.1016/0006-8993(96)00723-8. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Reul JM. Water temperature determines neurochemical and behavioural responses to forced swim stress: An in vivo microdialysis and biotelemetry study in rats. Stress1. 2007 doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Reul JM. Water temperature determines neurochemical and behavioural responses to forced swim stress: an in vivo microdialysis and biotelemetry study in rats. Stress. 2008;11:88–100. doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Penalva RG, Flachskamm C, Holsboer F, Reul JM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci. 2002;16:2441–2452. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, de VA, Pan B, Brunet LR, Hunt JR, Paton JF, van KE, Knight DM, Evans AK, Rook GA, Lightman SL. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LF. Stressor controllability, anxiety, and serotonin. Cognitive Therapy Res. 1998;22:595–613. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res Bull. 2001;54:619–630. doi: 10.1016/s0361-9230(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Olivera-Lopez JI, Jaramillo MT. Intra-lateral septal infusions of folic acid alone or combined with various antidepressant drugs produce antidepressant-like actions in male Wistar rats forced to swim. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:78–84. doi: 10.1016/j.pnpbp.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46:1301–1308. doi: 10.1016/s0006-3223(99)00173-0. [DOI] [PubMed] [Google Scholar]
- Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011;104:272–282. doi: 10.1016/j.physbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Fourth Edition. San Diego: Academic Press. 1998 [Google Scholar]
- Petersen RG. Marcel Dekker, Inc; New York: 1985. Design and Analysis of Experiments. [Google Scholar]
- Pierce ET, Foote WE, Hobson JA. The efferent connection of the nucleus raphe dorsalis. Brain Res. 1976;107:137–144. doi: 10.1016/0006-8993(76)90102-5. [DOI] [PubMed] [Google Scholar]
- Pinter O, Domokos A, Mergl Z, Mikics E, Zelena D. Do stress hormones connect environmental effects with behavior in the forced swim test? Endocr J. 2011;58:395–407. doi: 10.1507/endocrj.k10e-375. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, But Not Controllable, Stress Desensitizes 5-HT1A Receptors in the Dorsal Raphe Nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Serrats J, Sawchenko PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol. 2006;495:236–254. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- Stone EA. Behavioral and neurochemical effects of acute swim stress are due to hypothermia. Life Sci I. 1970a;9:877–888. doi: 10.1016/0024-3205(70)90050-0. [DOI] [PubMed] [Google Scholar]
- Stone EA. Swim-stress-induced inactivity: relation to body temperature and brain norepinephrine, and effects of d-Amphetamine. Psychosom Med. 1970b;32:51–59. doi: 10.1097/00006842-197001000-00004. [DOI] [PubMed] [Google Scholar]
- Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 2011;31:9101–9110. doi: 10.1523/JNEUROSCI.0404-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vegt BJ, De Boer SF, Buwalda B, de Ruiter AJ, de Jong JG, Koolhaas JM. Enhanced sensitivity of postsynaptic serotonin-1A receptors in rats and mice with high trait aggression. Physiol Behav. 2001;74:205–211. doi: 10.1016/s0031-9384(01)00565-0. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Cremers TI, Jongsma ME, Steenbergen PJ, De Boer SF, Koolhaas JM. Differences in the effects of 5-HT(1A) receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology (Berl) 2005;178:151–160. doi: 10.1007/s00213-004-2005-5. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA. Learned helplessness in the rat: improvements in validity and reliability. Brain Res Brain Res Protoc. 2001;8:1–7. doi: 10.1016/s1385-299x(01)00067-8. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- Warner TA, Drugan RC. Morris water maze performance deficit produced by intermittent swim stress is partially mediated by norepinephrine. Pharmacol Biochem Behav. 2012;101:24–34. doi: 10.1016/j.pbb.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Research Reviews. 1981;3:167–205. [Google Scholar]
- Weiss JM, Cierpial MA, West CH. Selective breeding of rats for high and low motor activity in a swim test: toward a new animal model of depression. Pharmacol Biochem Behav. 1998;61:49–66. doi: 10.1016/s0091-3057(98)00075-6. [DOI] [PubMed] [Google Scholar]