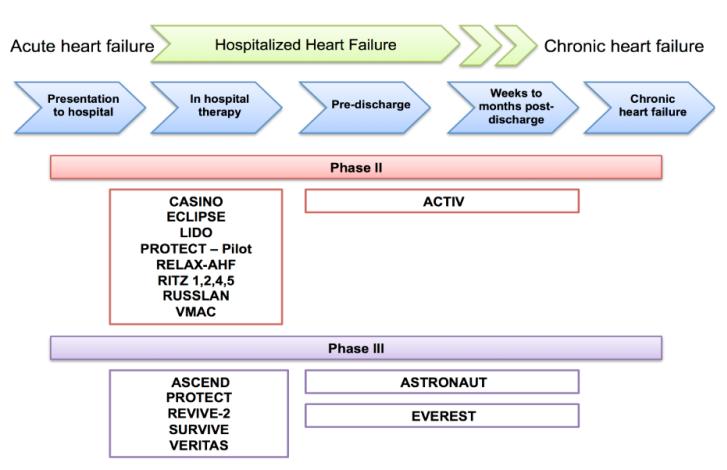
There are more than a million hospitalizations for heart failure (HHF) annually in the United States1 and over 80% of them occur in patients with known chronic HF.2, 3 These patients have a combined mortality and readmission rate of 35-40% within 90 days, a readmission rate of 25% within 30-days, and a mortality rate of 30% within a year post-discharge.1 Many trials have been conducted on these patients and yet there are no approved therapies for these patients with proven efficacy for reducing mortality or readmission.4-12 There are many reasons for this 13, including the lack of consensus on therapeutic targets and aims. Most trials have focused on short-term dyspnea, but the rapid response to conventional therapy may indicate that dyspnea relief is a largely a met need. These trials have generally intervened by infusing investigational drugs for 2-3 days early in the hospital course, signifying a philosophy that HHF represents an acute disease with distinct pathophysiology, like acute myocardial infarction, where short-term interventions can improve outcomes.13 (Figure 1) Myocardial infarction, however, represents a truly acute onset of a distinct pathophysiology, i.e. plaque rupture and thrombus formation. Whether HHF is an acute disease representing a distinct pathophysiology is uncertain. The selection of timing and duration of novel intervention among HHF patients is rooted on fundamental concepts regarding the acuity of the disease process, its pathophysiology, the timing of adverse events, and past experience.
Figure 1. Clinical trials in patients hospitalized for heart failure: Most trials initiated and terminated novel therapies during the acute stages of management.
CASINO=Calcium Sensitizer or Inotrope or None in Low-Output Heart Failure; ECLIPSE=Effect of Tolvaptan on HemodynamIc Parameters in Subjects with Heart failure; LIDO=Efficacy and Safety of Intravenous Levosimendan Compared with Dobutamine in Severe Low-Output Heart Failure; PROTECT=Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function; RELAX-AHF= Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure; RITZ= Randomized Intravenous Tezosentan; RUSSLAN=Randomized Study on Safety and Effectiveness of Levosimendan in Patients with Left Ventricular Failure after an Acute Myocardial Infarct; VMAC=Vasodilation in the Management of Acute Congestive Heart Failure; ACTIV=Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure; ASCEND=Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure; REVIVE=Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy Versus Placebo in the Short-Term Treatment of Decompensated Heart Failure; SURVIVE=Survival of Patients with Acute Heart Failure in Need of Intravenous Inotropic Support; VERITAS=Value of Endothelin Receptor Inhibition with Tezosentan in Acute Heart Failure Studies; ASTRONAUT=Aliskiren Therapy on Top of Standard Therapy, on Morbidity and Mortality in Patients With Acute Decompensated Heart Failure; EVEREST=Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart Failure.
SUB-ACUTE SYMPTOM ONSET
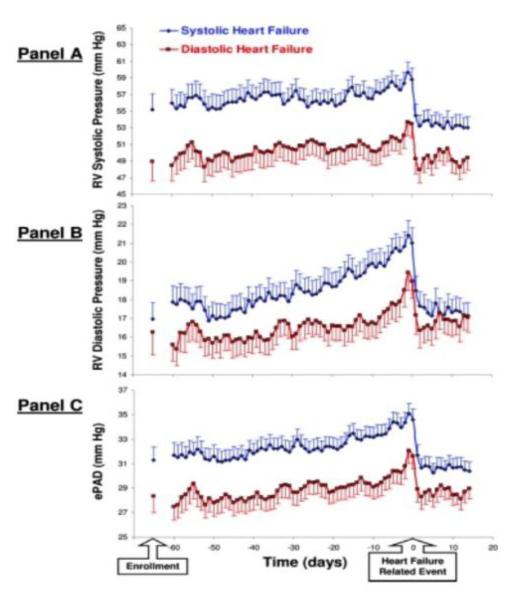
Some episodes of HHF are truly acute, however, many of these are related to secondary conditions e.g. acute coronary syndrome, rapid atrial fibrillation, or infections. The majority of HHF reflects sub-acute, progressively worsening congestion.1, 13 Changes occur 12.4±1.4 days before admission for edema, 11.3±1.6 days for weight gain, and 8.4±0.9 days for dyspnea.14 Changes in weight frequently begin at least a week before admission.15, 16 Defibrillators and cardiac resynchronization devices can assess thoracic impedance that may correlate with fluid status and predict worsening HF before symptom onset. Ninety percent of the detected alerts for changes in impedance were followed by a hospital evaluation for HF within 30 days in one study.17 In another, 53 alert and HF deteriorations were identified in 43 subjects; 83% showed evidence of fluid accumulation several days prior to the event.18 In one study with ambulatory hemodynamic monitoring, elevated pulmonary artery diastolic pressure correlating with HF events began rising on average 29±22 days before the event.19 (Figure 2) Data with implanted sensors demonstrate that left atrial pressures are most commonly elevated during the 30 days before HF events.20 Many patients do not have acute symptoms when admitted but present based on availability of caregiver, lack of ad hoc outpatient appointment, reaching a threshold of slowly worsening symptoms, or other social issues; some are hospitalized for worsening HF detected during routine clinic visits.
Figure 2. Daily implantable hemodynamic monitor-derived pressures in heart failure patients.
Trends in daily median implantable monitor-derived pressures are shown beginning 60 days before a hypervolemic HF-related event and continuing for 14 days after. Open blue circles represent low ejection fraction and closed red squares preserved ejection fraction HF patients. Right ventricular systolic pressure (A) and diastolic pressure (B), and an estimate of pulmonary artery diastolic pressure (C), are shown. In both group of patients, an increase was found preceding the event in all 3 pressures, which returned to baseline after treatment.
With permission, Zile et al. Circulation. 2008;118:1433-1441
NO DISTINCT PATHOPHYSIOLOGY
The abnormalities that have been described in HHF patients, though exacerbated, mirror those seen in chronic stable HF patients (Table). If indeed a “ruptured plaque” HHF pathophysiology is identified, it will forestall a new era of intervention development; however no such insights have been gained to date. HHF patients have a cardiac structure and function that is similar to chronic HF with varying left 21, 22 and right ventricular function,23 left atrial enlargement, mitral regurgitation, and other abnormalities.24 Both HHF and stable patients show a wide range of hemodynamics ranging from altered filling pressures, afterload, and cardiac output, however these tend to be worse during hospitalization.25 Neurohormonal activation (plasma renin activity, norepinephrine, vasopressin, copeptin, and adrenomedullin);26-29 myocyte damage (troponin);30, 31 inflammation (tumor necrosis factor,32 C-reactive protein,33 interleukin-6,34 and ST-2);35 oxidative stress (isoprostane, aminothiols, uric acid, myeloperoxidases);36-39 extracellular matrix (matrix metalloproteinases and their inhibitor, galactin-3);40, 41 myocardial stress (natriuretic peptides)42, 43 and renal function (blood urea nitrogen, creatinine, Cystatin C, neutrophil gelatinase–associated lipocalin, kidney injury molecule)44-48 alterations have all been shown in both HHF and chronic stable HF patients.
Table.
Reported Pathophysiologic Abnormalities in Chronic and Hospitalized Heart Failure Patients
| Pathophysiology | Chronic Heart Failure | Heart Failure Hospitalization |
|---|---|---|
|
| ||
| Hemodynamics | ||
| Central venous pressure, Pulmonary artery pressure | ↑ | ↔ ↑ ↑ ↑ |
| Pulmonary capillary wedge pressure, Systemic vascular resistance | ↑ | ↔ ↑ ↑ ↑ |
| Cardiac Output | ↔ ↓ | ↔ ↓ |
|
Neurohormonal Activation
| ||
| Sympathetic Nervous System | ↔ ↑ | ↔ ↑ ↑ ↑ |
| Renin-Angiotensin-Aldosterone System | ↔ ↑ | ↔ ↑ ↑ ↑ |
| Arginine Vasopressin | ↔ ↑ | ↔ ↑ ↑ ↑ |
| Endothelin | ↔ ↑ | ↔ ↑ ↑ ↑ |
| Adrenomedullin | ↔ ↑ | ↔ ↑ ↑ ↑ |
|
Myocyte Damage
| ||
| Troponin | ↔ ↑ | ↔ ↑ ↑ |
|
Inflammation
| ||
| C-reactive protein, Interleukin-6, Tumor necrosis factor, ST-2 | ↔ ↑ | ↔ ↑ ↑ ↑ |
|
Oxidative Stress
| ||
| Myeloperoxidases, Uric acid | ↔ ↑ | ↔ ↑ ↑ |
|
Extracellular Matrix Regulation
| ||
| Matrix metalloproteinase, Tissue inhibitor of metalloproteinase, Galactin-3 | ↔ ↑ | ↔ ↑ ↑ ↑ |
|
Natriuretic Peptide
| ||
| B-type natriuretic peptide, N-terminal prohormone of B-type natriuretic peptide | ↔ ↑ | ↔ ↑ ↑ ↑ |
|
Renal Function and Injury
| ||
| Blood urea nitrogen and Creatinine | ↔ ↑ | ↔ ↑ ↑ |
| Cystatin-C | ↔ ↑ | ↔ ↑ ↑ |
| Neutrophil gelatinase-associated lipocalin, Kidney Injury Molecule-1 | ↔ ↓ | ↔ ↓ ↓ |
|
Endothelial Function
| ||
POST-DISCHARGE RISK
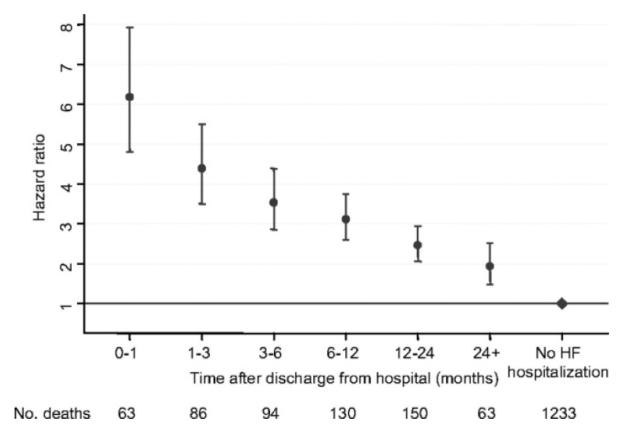
Current data suggest that HHF patients have lower in-hospital but higher post-discharge risk for adverse events compared to acute myocardial infarction patients. The HHF patients are at a substantially higher risk for death and readmissions compared to stable outpatients with a recent HHF being one of the strongest and most consistent predictors of poor outcomes. Each successive readmission is associated with incrementally higher risk of mortality. The risk for death or readmission is highest within 30 days and the observed risk decreases significantly within 3-6 months. In the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trial, odds for mortality after discharge following HHF declined from 6-fold in the first month after discharge to 2-fold over time (Figure 3).49 Similar data from registries showed a period of increased risk within the first 6 months after discharge.50, 51 Whether HHF identifies patients at higher risk (a marker) or there are discrete pathophysiologic processes in HHF patients that contribute the adverse outcomes (a mediator), has not been fully elucidated.
Figure 3. Changes in risk profile after hospitalization.
Hazard ratios and 95% confidance intervals of all-cause mortality after discharge from hospital for first hospitalization for HF at various time intervals after discharge adjusted for other baseline predictors.
With permission, Solomon et al. Circulation. 2007;116:1482-1487
IMPACT OF SHORT-TERM INTERVENTIONS
The current standard of HHF care, i.e. intravenous diuretics, nitrates and other vasodilators, and in select cases, inotropes are all short-term interventions to improve symptoms and signs, but none have been shown to improve outcomes post-discharge. Most HHF clinical trials have also focused on short-term intravenous infusions and none have improved post-discharge outcomes, barring one trial. Seralaxin in the Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure (RELAX-AHF) trial showed improved 6-month mortality but not the readmission rate or the composite endpoint of cardiovascular death or readmission to the hospital for HF or renal failure. The results of RELAX-AHF are promising but require confirmation. Therapies that improve post-discharge HHF outcomes are those that affect HF with reduced ejection fraction pathophysiology, and using HHF as an opportunity to optimize care, are initiated in-hospital and continued post-discharge, e.g. ACE inhibitors or beta-blockers.52-55 Drugs targeting dyspnea and hemodynamics are usually given intravenously at doses aiming to reverse pulmonary pressures rapidly. Such doses may not be needed for disease modification over long term and may lead to adverse effects e.g. ACE inhibitor was related to hypotension when given acutely intravenously for patients with acute myocardial infarction, whereas chronic oral use improves outcomes.56
APPROACHES TO FUTURE CLINICAL TRIALS
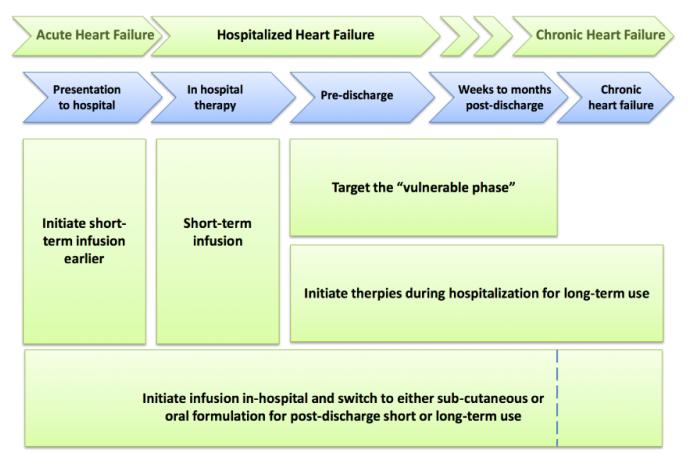
If HHF mostly represents worsening chronic HF with no entirely distinct pathophysiologic targets beyond those operative in chronic HF known, and that the highest risk for adverse events are post-discharge among these patients, these facts then have important implications for trial design in HHF. Moving forward, based on the current pathophysiologic understanding and the past experiences with clinical trials, there are several possibilities for study design for HHF patients, Figure 4.
Figure 4.
Options for targeting therapy at various stages during hospitalization
1. In-Hospital Short-Term Infusions
This most commonly applied approach has failed with many drugs, raising the possibility that short term infusions that do not affect the fundamental disease pathway but affects its secondary manifestation, will not be successful. However, pulmonary pressures are associated with outcomes in HHF and it is also possible this approach might be effective with a given particular drug and/or if this approach is matched with the most appropriate patient population. In addition, if a short-term infusion facilitated improved initiation, continuation, and titration of guideline-directed medical therapy post-discharge, outcomes could be benefited. A short-term infusion of therapy if efficacious for reducing mortality and readmission has the distinct advantage of limiting duration of drug exposure and potentially side effects as well as costs. Seralaxin has revived interest in this respect, but is an early experience needing validation. It is humbling to note that multiple prior attempts with drugs tested on the basis of sound physiologic promise and positive preliminary data failed to improve clinical outcomes or were harmful with this approach.
2. In-Hospital Short Term Infusions but Initiate Earlier
It is plausible that the strategy empolying short-term infusion will improve outcomes if initiated earlier. It is argued that delay in initiating therapies leads to worsening ongoing myocardial and renal damage. Some observational studies have suggested that earlier initiation of intravenous diuretic and vasoactive medications is associated with better outcomes whereas other studies have not.57, 58 Secondary analysis of the RELAX-AHF data showed that seralaxin infusion was associated with improved natriuretic peptide, liver function, and cardiac troponin levels at 72 hours. Interestingly, RELAX-AHF trial started infusion at about 12 hours after hospital presentation, which is earlier than other HHF trials. The ongoing Trial to Evaluate the Efficacy and Safety of Ularitide Intravenous Infusion in Patients Suffering From Acute Decompensated Heart Failure (TRUE-AHF) is planning to enroll patients within 6 hours.59 It has also argued that intravenous diuretics worsens neurohormonal activation and earlier investigational therapy may prevent iatrogenic complications of loop diuretics. However, it has been demonstrated that adverse physiologic changes in HHF patients occur days to weeks before admission. It is not clear that a few hours difference in initiation of novel therapies in patients already receiving otherwise medications for congestion relieve can have a pronounced impact on long term outcomes. However, it is conceivable that if an early infusion can avoid cardio-renal or other end-organ damage, or limit its extent, then this approach can be beneficial. The degree to which decompensated HF leads to permanent organ damage however is not clear.
3. Target the Immediate Post-Discharge Phase
Since the post-discharge risk among HHF patients appears to be primarily within the first few weeks to months post-discharge, one options is to target this “vulnerable phase” when patients are at the highest risk. Use of sub-cutaneous natriuretic peptide in HHF patients post-discharge for 8 weeks has been showed to improve cardiac function and quality of life scores.60 Another trial with this strategy that is currently enrolling patients is the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) trial testing three months of subcutaneous GLP-1 agonist initiated at the time of discharge among HHF patients. Targeting the vulnerable phase post discharge makes intuitive sense, however, the vulnerable phase hypothesis has not been proven. An alternate explanation to the observed highest risk in the immediate post-discharge phase that appears to decrease over time is that the highest risk patients die earlier post discharge leaving behind a population of patients with lower absolute risk translating into changes in observed risk over time. Giving long-term injections is challenging and even with oral drugs, limiting effective therapy to a short time assuming that the targeted abnormality is normalized, is unlikely. Indeed some of these abnormalities may improve over time, but without fundamentally changing the HF state, they are unlikely to completely reverse, and limiting therapy rather than long term continuation, waiting for another exacerbation to use them, is less appealing. However, if this approach allows patients to be bridged to achieve optimal initiation and titration of guideline-directed medical and device therapy as indicated over the ensuing months post-discharge, this may be an attractive and ultimately successful approach.
4. Initiate Therapies During Hospitalization For Long-Term Use
Another approach is to consider HHF as a marker of high risk HF patients and using admission as an opportunity to initiate effective long-term therapies. Indeed initiation of ACE-inhibitors or beta-blockers during HHF has been associated with improved outcomes in patients with HF and reduced ejection faction.52-55 Any pathway that is effectively targeted in HHF patients is unlikely to be completely reversed in any short time frame to allow for safe discontinuation of therapy. This approach permits long term exposure of effective therapies, possibly at lower doses that may be safer and still effective, without the intent of using higher intravenous doses to acutely reverse hemodynamic abnormalities. Although promising, two trials using this approach did not improve post-discharge outcomes, including the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST)6 and Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT)61. Whether this failure to demonstrate efficacy was related to the drugs that were tested or the patient population chosen can be debated. However, both EVEREST and ASTRONAUT were successful in enrolling patients with sufficient event risk where the effect on primary outcomes could be readily determined, which is a significant additional benefit of this design.
5. In-hospital Infusion Followed by Post-Discharge Administration
Potentially one can combine several options listed above. For example, a novel therapy may be available in both intravenous infusion form for early in-hospital use but may be continued post-discharge for short or long-term in the form of sub-cutaneous injections or an oral formulation. One example of such an approach potentially under current development is omecamtiv mecarbil, with which a phase II short term intravenous use trial just completed enrollment, the Study to Evaluate the Safety and Efficacy of IV Infusion Treatment With Omecamtiv Mecarbil in Subjects With Left Ventricular Systolic Dysfunction Hospitalized for Acute Heart Failure (ATOMIC-AHF), while another trial with an oral formulation, the Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF), is under way. If any particular drug does show benefit with this approach, it has the obvious significant benefit of bridging the acute early phase to more chronic use with an effective agent.
CONCLUSION
The HHF population is currently a significant health and healthcare burden to the society. Even if the hospitalization rates fall, with the growing elderly population and the increasing prevalence of HF, the actual numbers of HHF are projected to grow. There are no targeted therapies for these patients that can reduce the risk of mortality or readmissions post-discharge. Many trials have attempted to alter the outcomes of these patients with novel therapies, but the results have been disappointing. It is possible that this lack of success may partially be related to trial design and execution. In this respect, there are multiple options for testing the timing and duration for new drugs for HHF patients, but all have their pros and cons. Clinical trials should consider these opportunities and challenges carefully, as besides the pharmacologic properties of the drugs, these factors may well determine the fate of the intervention. These options should also be taken into the context of the clinical scenario and the patient substrate, e.g. there may be a differential benefit of earlier treatment if it is instituted prior to a possible cardio-renal injury, as opposed to once the injury has taken place. Future research leading to better understanding of HHF triggers and pathophysiology may make the selection from the possible choices more obvious.
Acknowledgments
Sources of Funding This work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the optimize-hf registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the united states: Rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (adhere) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Cuffe MS, Califf RM, Adams KF, Jr., Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Konstam MA, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The everest clinical status trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 6.Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: The veritas randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 8.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The survive randomized trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 9.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: A pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 10.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 11.Packer M. Current perspectives on the design of phase ii trials of new drugs for the treatment of heart failure. Am Heart J. 2000;139:S202–206. doi: 10.1016/s0002-8703(00)90074-7. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Pang PS, O’Connor CM, Prasad K, McMurray J, Teerlink JR, Fiuzat M, Sabbah H, Komajda M. Clinical development of pharmacologic agents for acute heart failure syndromes: A proposal for a mechanistic translational phase. American Heart Journal. 2011;161:224–232. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: Current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 14.Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: Symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114:625–630. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549–1554. doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamokoski LM, Haas GJ, Gans B, Abraham WT. Optivol fluid status monitoring with an implantable cardiac device: A heart failure management system. Expert review of medical devices. 2007;4:775–780. doi: 10.1586/17434440.4.6.775. [DOI] [PubMed] [Google Scholar]
- 17.Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: The sense-hf trial. European Heart Journal. 2011;32:2266–2273. doi: 10.1093/eurheartj/ehr050. [DOI] [PubMed] [Google Scholar]
- 18.Vollmann D, Nagele H, Schauerte P, Wiegand U, Butter C, Zanotto G, Quesada A, Guthmann A, Hill MR, Lamp B. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28:1835–1840. doi: 10.1093/eurheartj/ehl506. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr., Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 20.Ritzema J, Troughton R, Melton I, Crozier I, Doughty R, Krum H, Walton A, Adamson P, Kar S, Shah PK, Richards M, Eigler NL, Whiting JS, Haas GJ, Heywood JT, Frampton CM, Abraham WT. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086–1095. doi: 10.1161/CIRCULATIONAHA.108.800490. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 22.Tribouilloy C, Rusinaru D, Leborgne L, Mahjoub H, Szymanski C, Houpe D, Beguin M, Peltier M. In-hospital mortality and prognostic factors in patients admitted for new-onset heart failure with preserved or reduced ejection fraction: A prospective observational study. Arch Cardiovasc Dis. 2008;101:226–234. doi: 10.1016/s1875-2136(08)73697-0. [DOI] [PubMed] [Google Scholar]
- 23.Verhaert D, Mullens W, Borowski A, Popovic ZB, Curtin RJ, Thomas JD, Tang WH. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circulation. Heart failure. 2010;3:340–346. doi: 10.1161/CIRCHEARTFAILURE.109.900134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. Euroheart failure survey ii (ehfs ii): A survey on hospitalized acute heart failure patients: Description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 25.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The escape trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 26.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. The New England journal of medicine. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 27.Aronson D, Burger AJ. Neurohormonal prediction of mortality following admission for decompensated heart failure. Am J Cardiol. 2003;91:245–248. doi: 10.1016/s0002-9149(02)03119-3. [DOI] [PubMed] [Google Scholar]
- 28.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Potocki M, Breidthardt T, Reichlin T, Morgenthaler NG, Bergmann A, Noveanu M, Schaub N, Uthoff H, Freidank H, Buser L, Bingisser R, Christ M, Mebazaa A, Mueller C. Midregional pro adrenomedullin in addition to b-type natriuretic peptides in the risk stratification of patients with acute dyspnea: An observational study. Crit Care. 2009;13:R122. doi: 10.1186/cc7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin i is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 31.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 32.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (solvd) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 33.Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca HP, Perruchoud AP. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006;151:845–850. doi: 10.1016/j.ahj.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, Matsumori A. Serial circulating concentrations of c-reactive protein, interleukin (il)-4, and il-6 in patients with acute left heart decompensation. Clinical cardiology. 1999;22:811–813. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker st2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, Jasper S, Hazen SL, Klein AL. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 38.Tamariz L, Harzand A, Palacio A, Verma S, Jones J, Hare J. Uric acid as a predictor of all-cause mortality in heart failure: A meta-analysis. Congestive heart failure. 2011;17:25–30. doi: 10.1111/j.1751-7133.2011.00200.x. [DOI] [PubMed] [Google Scholar]
- 39.Pascual-Figal DA, Hurtado-Martinez JA, Redondo B, Antolinos MJ, Ruiperez JA, Valdes M. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. European journal of heart failure. 2007;9:518–524. doi: 10.1016/j.ejheart.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008;14:467–474. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Shirakabe A, Asai K, Hata N, Yokoyama S, Shinada T, Kobayashi N, Mizuno K. Clinical significance of matrix metalloproteinase (mmp)-2 in patients with acute heart failure. International heart journal. 2010;51:404–410. doi: 10.1536/ihj.51.404. [DOI] [PubMed] [Google Scholar]
- 42.Fonarow GC, Peacock WF, Horwich TB, Phillips CO, Givertz MM, Lopatin M, Wynne J. Usefulness of b-type natriuretic peptide and cardiac troponin levels to predict in-hospital mortality from adhere. Am J Cardiol. 2008;101:231–237. doi: 10.1016/j.amjcard.2007.07.066. [DOI] [PubMed] [Google Scholar]
- 43.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: Primary results of the registry to improve the use of evidence-based heart failure therapies in the outpatient setting (improve hf) Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 44.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA : the journal of the American Medical Association. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 45.Fonarow GC, Adams KFJ, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 46.Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Mehra MR, O’Connor CM, Reynolds D, Walsh MN. Heart failure care in the outpatient cardiology practice setting: Findings from improve hf. Circ Heart Fail. 2008;1:98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 47.Manzano-Fernandez S, Januzzi JL, Jr., Boronat-Garcia M, Bonaque-Gonzalez JC, Truong QA, Pastor-Perez FJ, Munoz-Esparza C, Pastor P, Albaladejo-Oton MD, Casas T, Valdes M, Pascual-Figal DA. Beta-trace protein and cystatin c as predictors of long-term outcomes in patients with acute heart failure. Journal of the American College of Cardiology. 2011;57:849–858. doi: 10.1016/j.jacc.2010.08.644. [DOI] [PubMed] [Google Scholar]
- 48.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (ngal) in predicting worsening renal function in acute decompensated heart failure. Journal of Cardiac Failure. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 50.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. American heart journal. 2007;154:260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 51.Kaul P, McAlister FA, Ezekowitz JA, Grover VK, Quan H. Ethnic differences in 1-year mortality among patients hospitalised with heart failure. Heart. 2011;97:1048–1053. doi: 10.1136/hrt.2010.217869. [DOI] [PubMed] [Google Scholar]
- 52.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy C, Young JB. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 53.Krantz MJ, Havranek EP, Haynes DK, Smith I, Bucher-Bartelson B, Long CS. Inpatient initiation of beta-blockade plus nurse management in vulnerable heart failure patients: A randomized study. J Card Fail. 2008;14:303–309. doi: 10.1016/j.cardfail.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: Results of the initiation management predischarge: Process for assessment of carvedilol therapy in heart failure (impact-hf) trial. J Am Coll Cardiol. 2004;43:1534–1541. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 55.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy C, Young JB. Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: An analysis from organized program to initiate lifesaving treatment in hospitalized patients with heart failure (optimize-hf) Am Heart J. 2007;153(82):e81–11. doi: 10.1016/j.ahj.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Swedberg K, Held P, Kjekshus J, Rasmussen K, Ryden L, Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the cooperative new scandinavian enalapril survival study ii (consensus ii) N Engl J Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 57.Peacock WFt, Fonarow GC, Emerman CL, Mills RM, Wynne J. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in adhere. Cardiology. 2007;107:44–51. doi: 10.1159/000093612. [DOI] [PubMed] [Google Scholar]
- 58.Wong Y, Fonarow G, Mi X, Mills R, Peacock F, Curtis L, Qualls L, Klaskala W, Hernandez AF. Time to intravenous therapy and clinical outcomes in older patients presenting with acute decompensated heart failure: An analysis of the adhere registry emergency molule. J Am Coll Cardiol. 2013;61:E622. [Google Scholar]
- 59.Mitrovic V, Luss H, Nitsche K, Forssmann K, Maronde E, Fricke K, Forssmann WG, Meyer M. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: A double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005;150:1239. doi: 10.1016/j.ahj.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 60.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure: Chronic subcutaneous b-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled aliskiren trial on acute heart failure outcomes (astronaut) Eur J Heart Fail. 2011;13:100–106. doi: 10.1093/eurjhf/hfq209. [DOI] [PubMed] [Google Scholar]