Abstract
Objective
We sought to determine whether recurrent AOM (rAOM) occurring within 30 days of amoxicillin/clavulanate treatment was caused by bacterial relapse or new pathogens.
Methods
Pneumococcal conjugate vaccinated children, age 6–36 months, enrolled in a prospective, longitudinal study experiencing rAOM < 1 month after completing amoxicillin/clavulanate therapy were studied. AOM episodes occurred between June 2006–Nov 2012. Multi locus sequence typing was used to genotype isolates.
Results
66 children were in the study cohort; 63 otopathogens were recovered from middle ear fluid after tympanocentesis. Nontypeable H. influenzae (NTHi) accounted for 47% of initial AOMs vs. 15% by S. pneumoniae (Spn), p<0.0001. NTHi accounted for 42% of rAOM vs. 24% by Spn (p-value =0.04). NTHi was the main otopathogen that caused true bacteriologic relapses (77%). Beta lactamase-producing NTHi and penicillin nonsusceptible Spn were not more common in rAOM than initial AOM infections. Among 21 paired (initial and rAOM events) NTHi isolates genotyped, 13 (61.9%) were the same organism; 1 of 9 (11.1%) of paired Spn isolates was the same (p-value =0.017). rAOM occurring within a week of stopping amoxicillin/clavulanate was a different pathogen in 21% of cases, 8–14 days later in 33%, 15–21 days in 41% and 22–30 days in 57% (p =0.04).
Conclusions
In amoxicillin/clavulanate treated children, NTHi was the main otopathogen that caused true bacteriologic relapses. New pathogens causing rAOM vs. persistence of the initial pathogen significantly increased week to week. Neither relapses nor new infections were caused more frequently by beta lactamase producing NTHi or penicillin nonsusceptible Spn.
Keywords: Recurrent AOM, Non-typeable Haemophilus influenzae, Streptococcus pneumoniae, Bacterial Relapse
Introduction
Acute otitis media (AOM) is the most commonly diagnosed infectious disease in children associated with appropriate antibiotic prescriptions. About 30% of children develop recurrent AOM (rAOM) and are labeled otitis prone (OP) because they experience 3 AOM within 6 months or 4 episodes of AOM within 12 months.[1, 2] The bacteria causing rAOM have been investigated in the past. Nontypeable Haemophilus influenzae (NTHi) and Streptococcus pneumoniae (Spn) are mainly responsible for rAOM episodes but the relative proportion and antibiotic susceptibility varies with NTHi and antibiotic resistant strains occurring more frequently in rAOM following amoxicillin therapy.[3, 4]
The 2013 American Academy of Pediatrics AOM guideline recommends amoxicillin in high dose for AOM and high dose amoxicillin/clavulanate for rAOM.[5] A recurrence is defined according to a time interval of 30 days between completion of antibiotic therapy for an initial AOM and rAOM. One prior study by Leibovitz et al[6] in Israel in the pre-pneumococcal conjugate vaccine era showed that most rAOM were caused by NTHi and were true bacteriologic relapses if they occurred within 2 weeks of initial infection. However, a recurrent episode more than 2 weeks after the initial infection was most frequently caused by a new pathogen.[7] The purpose of this study was to determine whether rAOM that occurs in children in the U.S. within 30 days of initial treatment with amoxicillin/clavulanate is caused by a bacterial relapse or a new pathogen. Our study occurred during the pneumococcal conjugate vaccine era and the use of molecular diagnostics was included to make specific organism determinations.
Materials and Methods
Children Population and study design
The population and study design from our AOM research center has been previously described in detail.[8, 9] For this study, all children with clinical recurrence of AOM occurring within one month of completion of amoxicillin/clavulanate therapy for an initial AOM event were included from June 2006 to Nov 2012. Middle ear fluid was obtained by tympanocentesis for all AOM events as previously described.[10] The study was approved by the University of Rochester and subsequently by the Rochester General Hospital IRB and written informed consent was obtained from parents.
Definition of AOM
AOM was diagnosed by validated otoscopists[11], when children with acute onset of otalgia have tympanic membranes (TMs) that were: (1) mild, moderate or severe bulging; and (2) a cloudy or purulent effusion was observed, or the TM was completely opacified; and (3) TM mobility was reduced or absent, consistent with the AAP 2013 guidelines.[12] Children with spontaneous tympanic membrane perforation and tympanostomy tubes were excluded from the study. After tympanocentesis the children received high dose amoxicillin/clavulanate; all children received the antibiotic for 5 days regardless of the child’s age consistent with our earlier research.[13] We use amoxicillin/clavulanate to treat our patients because we have shown that high dose amoxicillin will eradicate only about 30% of the otopathogens isolated from MEF.[14, 15] Children allergic to amoxicillin, receiving a cephalosporin instead were excluded from the study cohort (n=3). Antibiotic therapy for the initial AOM event was considered successful if no clinical symptoms of AOM were observed after 48 hrs of therapy and on follow up examination 3 weeks later the tympanic membrane was in the neutral or retracted position.
For this study a second tympanocentesis was performed in children who developed a clinical recurrence of symptoms of AOM after completion of therapy and again the examination met the AAP criteria for AOM.[16] Children with antibiotic treatment failure, who persisted with symptoms for >48 hours and had persistent tympanic membrane bulging have been described elsewhere[15, 17, 18] and were not included in this analysis.
Definitions of relapse and new infections
True bacteriologic relapse was defined as the presence in MEF of an organism in a second AOM event that was identical to the organism isolated from the first AOM event, confirmed by serotype and Multi-locus sequence typing (MLST) for S. pneumoniae and by MLST for NTHi.[19, 20] When a mixed initial infection was followed by a relapse caused by a single pathogen identical with one of the initial pathogens, the case was considered a true bacteriologic relapse. New infections were defined as any recurrent AOM caused by pathogens that were different in the second MEF sample than the isolates from the MEF at the initial AOM event.
Microbiology
MEF samples were cultured as previously described.[21] The antibiotic susceptibility of S. pneumoniae isolates was determined with the VITEK 2 Gram Positive Susceptibility Card-AST-GP68 (BioMerieux, Inc) using VITEK2 system.[15]
Multi-Locus Sequence Typing (MLST)
Bacterial genomic DNA was extracted from pure cultures of Spn and NTHi isolated from MEF samples of children with AOM and sequence types (STs) of S. pneumoniae and NTHi isolates were determined as described previously.[22–24]
Statistics
Logistic regression model was used to calculate significant differences between new pathogens and true bacteriologic relapse. Fisher’s exact test was used to calculate the difference in proportions of NTHi and Spn between rAOM after 1–15days and 16–30 days of completion of antibiotic therapy. A p-value of ≤ 0.05 was considered significant.
Results
Sixty-six children experienced a rAOM infection within one month of completion of antibiotic therapy (Table 1). A total of 63 otopathogens were recovered from MEF at the initial AOM infection; 18% of the MEFs were culture negative. 60 otopathogens were recovered from MEF at a rAOM infection from the same child. 17% of MEFs were culture negative at the second AOM infection. NTHi accounted for 46.9% of initial AOMs vs. 15% by Spn, p <0.0001. NTHi accounted for 42.4% of rAOM vs. 24.2% by Spn (p =0.04). NTHi and Spn beta lactamase production and penicillin susceptibility are shown in Table 1; There was no difference in percentage of isolates comparing intial and rAOM.
Table 1.
AOM pathogens recovered from middle ear fluid (MEF) at initial episodes of AOM vs. pathogens recovered at rAOM within one month of completion of amoxicillin/clavulanate in 66 children.
| Comparison of otopathogens in initial and rAOM (n=66 children) | ||||
|---|---|---|---|---|
| Pathogens | Initial Episode of AOM | rAOM | ||
| Number (%) | # (%) of Antibiotic Resistance* | Number (%) | # (%) of Antibiotic Resistance* | |
| NTHi | 31 (46.9) | 16 (51.6) | 28 (42.4) | 14 (50.0) |
| Spn | 10 (15.2) | 4 (40.0) | 16 (24.2) | 8 (50) |
| Mcat | 5 (7.6) | 5 (100) | 6 (9.1) | 6 (100) |
| Spn + Mcat | 3 (4.6) | 0 (0) + 3 (100) | 2 (3.0) | 0 (0) + 2 (100) |
| NTHi + Mcat | 0 (0) | NA | 1 (1.5) | 1 (100) |
| Spn+NTHi | 4 (6.1) | 1 (25) + 1 (25) | 2 (3.0) | 0 (0) + 0 (0) |
| Spn+ NTHi + Mcat | 1 (1.5) | 0 (0) + 0 (0) + 1 (100) | 0 (0) | NA |
| No AOM Pathogens | 12 (18.2) | NA | 11 (16.7) | NA |
: NTHi and Mcat resistance refers to β-lactamase non-susceptibility.
Spn resistance refers to penicillin non-susceptibility using Oxacillin disc test.
No significant difference in the antibiotic resistance was found in initial vs. rAOM whether a relapse or new infection.
Out of 5 Resistant Spn isolates at initial episodes of AOM, 5 had an MIC >2 to penicillin also using the Vitek susceptibility test; for ceftriaxone, 2 were resistant, 2 Intermediate and 1sensitive using Vitek. Out of 8 resistant Spn isolates at rAOM episodes, 8 were tested using the Vitek system and MIC was >2 to penicillin for all 8; for ceftriaxone, 3 were resistant and 5 intermediate using the Vitek system. All the oxacillin sensitive Spn isolates were sensitive to both penicillin and ceftriaxone in both episodes in the Vitek system.
More than 2/3 of the time more than one otopathogen was cultured in the nasopharynx at onset of initial and rAOM but the etiology of AOM as shown by culture of MEF was caused a single pathogen (data not shown). We did detect more than one otopathogens in 12.1% of children at an initial AOM compared to 7.6% at a rAOM infection in MEF.
We analyzed 21 paired (initial and rAOM infections) NTHi isolates and found that 13 of 21(61.9%) were genotypically the same while 8 of 21 (38.1%) were genotypically different, representing new infections. We analyzed 9 paired S. pneumoniae isolates and found only one case (11%) where same MLST sequence type was found at initial and recurrent AOM events (p=0.2 comparing the proportion of genotypically identical NTHi and Spn in initial and rAOM infections).
Based on culture and molecular typing, among 66 rAOM infections occurring within one month of completion of amoxicillin/clavulanate therapy, 17(25.7%) were true bacteriologic relapses; 13(19.7%) of elapses were due to NTHi and 4 (6.1%) were due to Spn. NTHi was the main otopathogen that caused most bacteriologic relapses (77%) as shown in Figure 1. Of 66 patients we identified 25 cases (37.9%) where rAOM was caused by a different pathogen than the initial pathogen. In 7 (28%) of the 25 new AOM infections, NTHi was cultured in both episodes but we proved that strain was different based on MLST characterization. Comparing 42 children out of 66 where bacteria were recovered from culture and genotyped in both initial and rAOM infections, 40% were true bacteriologic relapses and 60% were new infections (Figure 1).
Figure 1.
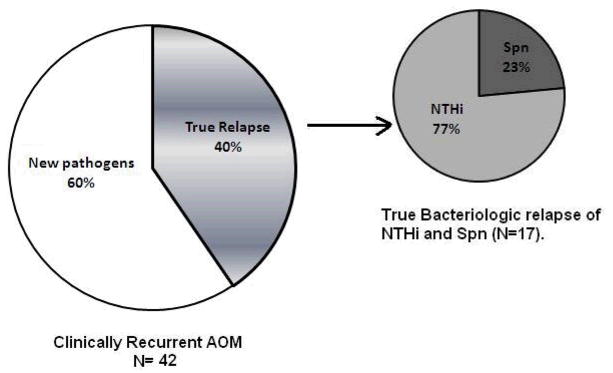
Proportions of True bacteriologic relapse and new pathogens in 42 children within one month of completion of amoxicillin/clavulanate therapy of initial AOM. 24 cases of rAOM were undetermined (negative cultures).
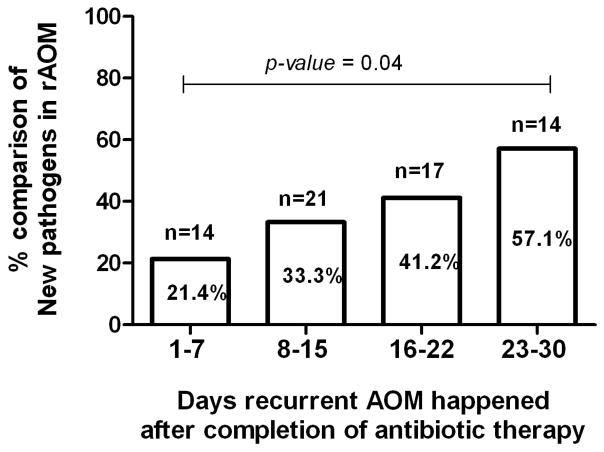
Ten children had culture negative MEF at the initial AOM event, 9 had culture negative MEF at the rAOM event and 2 children had culture negative MEF at both the initial and rAOM event. Those 21 children were classified in this study as undetermined for rAOM. In addition 3 of the children had Mcat isolated from MEF in both AOM episodes within one month but because we did not perform molecular characterization we were obliged to classify them as undetermined. Logistic regression was performed to determine the impact of time between initial AOM and rAOM within the 30 day window. The percentage of new pathogens as causative agents of rAOM significantly increased weekly (p-value=0.04; figure 2). Using the 30-day window employed by the AAP AOM guideline[25], the likelihood of detecting the same pathogen was not significant (p-value=0.29).
Figure 2.
Percentage of rAOM cases causing new infection according to days after completion of amoxicillin/clavulanate therapy for initial AOM event.
To determine whether true bacteriologic relapses in rAOM were more frequently caused by NTHi or Spn we compared rAOM episodes within 15 days and 16–30 days of the completion of amoxicillin/clavulanate therapy for initial AOM. Out of 10 bacteriologic relapses within 15 days of completion of therapy for initial AOM, NTHi caused 9 and 1 (10%) was caused by Spn (p-value=0.017). Out of 7 bacteriologic relapses within 16–30 days of the completion of therapy for initial AOM, 4 (57.1%) were caused by NTHi and 3 (42.9%) were caused by Spn.
Discussion
In this study we examined the question of otopathogen mix and antibiotic susceptibility in pneumococcal conjugate vaccinated children when a relapse of AOM occurs within 30 days of high dose amoxicillin combined with the β-lactamase inhibitor clavulanate, anticipated to be efficacious against both penicillin nonsusceptible S. pneumoniae and beta lactamase resistant H. influenzae. The major findings were: (1) NTHi was the main otopathogen that caused true bacteriologic relapses (77%); (2) NTHi caused initial AOMs 3 fold more often than Spn and rAOM 2-fold more often than Spn; (3) Neither relapses nor new infections were caused more frequently by beta lactamase producing NTHi or penicillin nonsusceptible Spn; (4) Two thirds of rAOM caused by NTHi were due to the same organism whereas only about 10% of rAOM caused by Spn were due to the same organism; (5) A rAOM occurring within a week of stopping antibiotic therapy was likely to be a different pathogen as at the onset of AOM in 21% of cases, 8–14 days later in 33% of cases, 15–21 days in 41% of cases and 22–30 days in 57% of cases.
Prior studies conducted by Schwartz et al[26], Barenkamp et al[27], Harrison et al[28], and Carlin et al[29] in the mid-1980s, Pichichero &Pichichero[30], Brook & Yocum[31], Pichichero et al[32], Gehanno et al [33] and Del Castillo et al[34] in the 1990s and Leibovitz et al[35] in 2003 established that about one-third of children with clinical rAOM have no bacteria isolated on tympanocentesis. The clinical examination reflects ongoing inflammation in the middle ear space likely caused by residual fragments of ingested bacteria but the organisms were no longer alive. We considered performing PCR to detect DNA in the culture negative samples and we have been performed MLST on culture negative samples in the past.[36–38] However with such close spacing between initial and rAOM events it would not have been possible to distinguish true persistence bacterial DNA from new infections. There is also possibility that at least part of the undetermined cases had a viral etiology. Studies by ChonmaitreeT et al[39]and Ruohola A et al[40] had shown ~4–10% of viruses in the middle ear of AOM cases.
Among the two-thirds of children who grow an otopathogen on repeat tympanocentesis within a month, the organism was new, representing a new infection about 75% of the time. So pediatricians should not assume that rAOMs within a month of initial infection are necessarily the result of initial treatment failure. To the contrary, more often they are new infections.
In the current study we show again that NTHi is the predominant otopathogen in pneumococcal conjugate vaccinated children and most NTHi causing AOM produce β-lactamase. In our AOM research center and private practice we don’t consider it ethical to prescribe amoxicillin as first line therapy for AOM because we have shown that only 30% of the bacteria we isolate from tympanocentesis in initial AOMs or rAOM are susceptible to amoxicillin.[15, 41] The 2013 AOM guidelines by the AAP recommend high dose amoxicillin/clavulanate or a preferred cephalosporin (cefdinir, cefuroxime, cefpodoxime or ceftriaxone) as a second line antibiotic for children who have taken high dose amoxicillin in the previous 30 days.[42] We have published results in Journal Drugs showing amoxicillin/clavulanate is superior in efficacy to cefdinir and has participated in single and double tap studies.[43] Our findings support use of amoxicillin/clavulanate as the first line antibiotic for AOM in pneumococcal conjugate vaccinated children.
There is ample evidence that rAOM occurring within one month of an initial AOM treated with amoxicillin will more often involve penicillin nonsusceptible S. pneumoniae and β-lactamase resistant H. influenzae.[44–51] In those studies the bacteria isolated in rAOM were more often amoxicillin resistant but about half of the isolated organisms from MEF during rAOM were susceptible in vitro to the agent used for the initial infection. In the current study no increase in penicillin nonsusceptible S. pneumoniae and β-lactamase resistant H. influenzae was observed in rAOM and all the organisms were susceptible to amoxicillin/clavulanate. In non-study situations is may be due to non-compliance with the antibiotic but in study situations drug compliance is high and the explanation is more likely poor absorption of amoxicillin and/or poor penetration to the middle ear space.[52, 53] Since our treatment protocol used amoxicillin/clavulanate as first line in the first episode, results might have been different if amoxicillin had been used.
Leibovitz et al[54] were the first to show that rAOM occurring within 1–2 weeks of an initial AOM more likely is caused by the same otopathogen whereas in the 3rd and 4th week after an initial AOM a rAOM is more likely to be caused by a new organism. Our results agree with those observations and call into question whether the definition of rAOM should be an AOM occurring within 2 weeks on an initial AOM (a definition our group has adopted), rather than an rAOM occurring 3–4 weeks after an initial AOM.
In conclusion, as shown in our prospective studies of otopathogens causing AOM detected by tympanocentesis, the dominant organism is NTHi.[15, 55] Most NTHi causing AOM produce β-lactamase, more so in rAOM when amoxicillin is the treatment used.[15, 56–62] PCV7 and PCV13 have reduced the frequency of Spn causing AOM, especially penicillin nonsusceptible strains in the PCV13 era.[63] As a result we used amoxicillin/clavulanate as first line empiric treatment of AOM and even so NTHi caused more than three-fourths of the rAOM infections. We use a third generation cephalosporin to treat rAOM, reasoning that the most likely cause for bacterial failure is poor absorption of amoxicillin.[52, 53] Success is achieved in most children but not all and points to the need for an NTHi vaccine to prevent AOM.
Acknowledgments
This study was supported by NIH NIDCD RO1 08671. We thank Katerina Czup, Arthur Chang, Jill Mangiafesto and Victoria Friedel at Rochester General Hospital Research Institute for their help in Microbiology and MLST characterization. We also thank the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this study.
Reference List
- 1.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119(4):707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 2.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichichero ME, Pichichero CL. Persistent acute otitis media: I. Causative pathogens. Pediatr Infect Dis J. 1995;14(3):178–83. doi: 10.1097/00006454-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 6.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 7.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 8.Pichichero ME, Casey JR, Almudevar A. Recurrent Acute Otitis Media Despite individualized Care: A New Subpopulation of Otitis Prone Children. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0b013e3182862b57. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaleida PH, Stool SE. Assessment of otoscopists’ accuracy regarding middle-ear effusion. Otoscopic validation. Am J Dis Child. 1992;146(4):433–5. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 12.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 13.Pichichero ME, Marsocci SM, Murphy ML, Hoeger W, Francis AB, Green JL. A prospective observational study of 5-, 7-, and 10-day antibiotic treatment for acute otitis media. Otolaryngol. Head Neck Surg. 2001;124(4):381–7. doi: 10.1067/mhn.2001.114311. [DOI] [PubMed] [Google Scholar]
- 14.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute Otitis Media Otopathogens During 2008–2010 in Rochester NY. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0b013e31828d9acc. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 17.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichichero ME, Casey JR, Hoberman A, Schwartz R. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003–2006. Clin Pediatr (Phila) 2008;47(9):901–6. doi: 10.1177/0009922808319966. [DOI] [PubMed] [Google Scholar]
- 19.Kaur R, Chang A, Xu Q, Casey JR, Pichichero ME. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J Med Microbiol. 2011;60(Pt 12):1841–8. doi: 10.1099/jmm.0.034041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Kaur R, Casey JR, Adlowitz DG, Pichichero ME, Zeng M. Identification of Streptococcus pneumoniae and Haemophilus influenzae in culture-negative middle ear fluids from children with acute otitis media by combination of multiplex PCR and multi-locus sequencing typing. Int J Pediatr Otorhinolaryngol. 2011;75(2):239–44. doi: 10.1016/j.ijporl.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur R, Chang A, Xu Q, Casey JR, Pichichero ME. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J Med Microbiol. 2011;60(Pt 12):1841–8. doi: 10.1099/jmm.0.034041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Kaur R, Casey JR, Adlowitz DG, Pichichero ME, Zeng M. Identification of Streptococcus pneumoniae and Haemophilus influenzae in culture-negative middle ear fluids from children with acute otitis media by combination of multiplex PCR and multi-locus sequencing typing. Int J Pediatr Otorhinolaryngol. 2011;75(2):239–44. doi: 10.1016/j.ijporl.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH, Rodriguez WJ, Khan WN. Persistent purulent otitis media. Clin Pediatr (Phila) 1981;20(7):445–7. doi: 10.1177/000992288102000703. [DOI] [PubMed] [Google Scholar]
- 27.Barenkamp SJ, Shurin PA, Marchant CD, et al. Do children with recurrent Haemophilus influenzae otitis media become infected with a new organism or reacquire the original strain? J Pediatr. 1984;105(4):533–7. doi: 10.1016/s0022-3476(84)80415-1. [DOI] [PubMed] [Google Scholar]
- 28.Harrison CJ, Marks MI, Welch DF. Microbiology of recently treated acute otitis media compared with previously untreated acute otitis media. Pediatr Infect Dis. 1985;4(6):641–6. doi: 10.1097/00006454-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Carlin SA, Marchant CD, Shurin PA, Johnson CE, Murdell-Panek D, Barenkamp SJ. Early recurrences of otitis media: reinfection or relapse? J Pediatr. 1987;110(1):20–5. doi: 10.1016/s0022-3476(87)80281-0. [DOI] [PubMed] [Google Scholar]
- 30.Pichichero ME, Pichichero CL. Persistent acute otitis media: I. Causative pathogens. Pediatr Infect Dis J. 1995;14(3):178–83. doi: 10.1097/00006454-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Brook I, Yocum P. Bacteriology and beta-lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr Infect Dis J. 1995;14(9):805–8. [PubMed] [Google Scholar]
- 32.Pichichero ME, McLinn S, Aronovitz G, et al. Cefprozil treatment of persistent and recurrent acute otitis media. Pediatr Infect Dis J. 1997;16(5):471–8. doi: 10.1097/00006454-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Gehanno P, N’Guyen L, Derriennic M, Pichon F, Goehrs JM, Berche P. Pathogens isolated during treatment failures in otitis. Pediatr Infect Dis J. 1998;17(10):885–90. doi: 10.1097/00006454-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 34.del Castillo F, Baquero-Artigao F, Garcia-Perea A. Influence of recent antibiotic therapy on antimicrobial resistance of Streptococcus pneumoniae in children with acute otitis media in Spain. Pediatr Infect Dis J. 1998;17(2):94–7. doi: 10.1097/00006454-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 36.Kaur R, Chang A, Xu Q, Casey JR, Pichichero ME. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J Med Microbiol. 2011;60(Pt 12):1841–8. doi: 10.1099/jmm.0.034041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q, Kaur R, Casey JR, Adlowitz DG, Pichichero ME, Zeng M. Identification of Streptococcus pneumoniae and Haemophilus influenzae in culture-negative middle ear fluids from children with acute otitis media by combination of multiplex PCR and multi-locus sequencing typing. Int J Pediatr Otorhinolaryngol. 2011;75(2):239–44. doi: 10.1016/j.ijporl.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur R, Adlowitz DG, Casey JR, Zeng M, Pichichero ME. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010;29(8):741–5. doi: 10.1097/INF.0b013e3181d9e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear: acute otitis media pathogen or bystander? Pediatr Infect Dis J. 2012;31(4):325–30. doi: 10.1097/INF.0b013e318241afe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruohola A, Meurman O, Nikkari S, et al. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin Infect Dis. 2006;43(11):1417–22. doi: 10.1086/509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 43.Casey JR, Block SL, Hedrick J, Almudevar A, Pichichero ME. Comparison of amoxicillin/clavulanic acid high dose with cefdinir in the treatment of acute otitis media. Drugs. 2012;72(15):1991–7. doi: 10.2165/11590320-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brook I, Yocum P. Bacteriology and beta-lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr Infect Dis J. 1995;14(9):805–8. [PubMed] [Google Scholar]
- 45.del Castillo F, Baquero-Artigao F, Garcia-Perea A. Influence of recent antibiotic therapy on antimicrobial resistance of Streptococcus pneumoniae in children with acute otitis media in Spain. Pediatr Infect Dis J. 1998;17(2):94–7. doi: 10.1097/00006454-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Gehanno P, N’Guyen L, Derriennic M, Pichon F, Goehrs JM, Berche P. Pathogens isolated during treatment failures in otitis. Pediatr Infect Dis J. 1998;17(10):885–90. doi: 10.1097/00006454-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Harrison CJ, Marks MI, Welch DF. Microbiology of recently treated acute otitis media compared with previously untreated acute otitis media. Pediatr Infect Dis. 1985;4(6):641–6. doi: 10.1097/00006454-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 49.Pichichero ME, McLinn S, Aronovitz G, et al. Cefprozil treatment of persistent and recurrent acute otitis media. Pediatr Infect Dis J. 1997;16(5):471–8. doi: 10.1097/00006454-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pichichero ME, Pichichero CL. Persistent acute otitis media: I. Causative pathogens. Pediatr Infect Dis J. 1995;14(3):178–83. doi: 10.1097/00006454-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Pichichero ME, Doern GV, Kuti JL, Nicolau DP. Probability of achieving requisite pharmacodynamic exposure for oral beta-lactam regimens against Haemophilus influenzae in children. Paediatr Drugs. 2008;10(6):391–7. doi: 10.2165/0148581-200810060-00006. [DOI] [PubMed] [Google Scholar]
- 53.Pichichero ME, Reed MD. Variations in amoxicillin pharmacokinetic/pharmacodynamic parameters may explain treatment failures in acute otitis media. Paediatr Drugs. 2009;11(4):243–9. doi: 10.2165/00148581-200911040-00003. [DOI] [PubMed] [Google Scholar]
- 54.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 55.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brook I, Yocum P. Bacteriology and beta-lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr Infect Dis J. 1995;14(9):805–8. [PubMed] [Google Scholar]
- 57.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.del Castillo F, Baquero-Artigao F, Garcia-Perea A. Influence of recent antibiotic therapy on antimicrobial resistance of Streptococcus pneumoniae in children with acute otitis media in Spain. Pediatr Infect Dis J. 1998;17(2):94–7. doi: 10.1097/00006454-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Gehanno P, N’Guyen L, Derriennic M, Pichon F, Goehrs JM, Berche P. Pathogens isolated during treatment failures in otitis. Pediatr Infect Dis J. 1998;17(10):885–90. doi: 10.1097/00006454-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Leibovitz E, Greenberg D, Piglansky L, et al. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr Infect Dis J. 2003;22(3):209–16. doi: 10.1097/01.inf.0000066798.69778.07. [DOI] [PubMed] [Google Scholar]
- 61.Pichichero ME, Pichichero CL. Persistent acute otitis media: I. Causative pathogens. Pediatr Infect Dis J. 1995;14(3):178–83. doi: 10.1097/00006454-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Pichichero ME, McLinn S, Aronovitz G, et al. Cefprozil treatment of persistent and recurrent acute otitis media. Pediatr Infect Dis J. 1997;16(5):471–8. doi: 10.1097/00006454-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Efficacy of PCV13 in Prevention of AOM and NP Colonization in Children: First Year of Data from U.S. Iguaza Falls. Brazil. 8th International Symposium of Pneumococci and Pneumococcal Diseases; 2012. [Google Scholar]