Summary
Individuals with major depressive disorder show blunted cortisol responses to psychosocial stressors, but the extent to which this pattern of dampened responding characterizes individuals experiencing sub-clinical levels of depressive symptoms is unknown. This study investigated whether self-reports of depressive and anxious symptoms over the previous two weeks were associated with cortisol responses to a laboratory social stress task. In addition, we tested whether these associations were mediated by baseline cortisol, subjective responses to the task, or health behaviors. Healthy adults (N = 76) completed the Mood and Anxiety Symptom Questionnaire prior to engaging in the Trier Social Stress Task. Salivary cortisol was measured at 8 points before and after the task to assess cortisol responding. Linear regressions revealed that men reporting more distress and somatic symptoms had smaller cortisol responses, but anhedonic symptoms were not related to cortisol. Distress was associated with lower baseline cortisol, which in turn statistically mediated the relationship between distress and cortisol response. These results demonstrate that the recent experience of depressive and anxious symptoms is associated with smaller cortisol responses to a psychosocial stressor in a nonclinical population.
Keywords: cortisol, stress, negative emotion, men, depression, anxiety
Recent depressive and anxious symptoms predict cortisol responses to stress in men
Major depressive disorder is associated with alterations in hypothalamic-pituitary-adrenal (HPA) responses to stress. While healthy controls exhibit an acute elevation in cortisol following exposure to a psychosocial stressor, followed by a return to baseline levels, depressed patients show smaller elevations and more sluggish recovery (see Burke et al., 2005a, for a review). This pattern of blunted responding has been observed among depressed inpatients (Trestman et al., 1991; Croes et al., 1993), outpatients (Ravindran et al.,1996), and remitted patients (Ahrens et al., 2008), both in response to laboratory stressors and to negative events in daily life (Peeters et al., 2003). The biological mechanisms that explain how depression alters HPA axis function are not clear, but may include dysfunction in the negative feedback circuitry of the HPA axis, as administration of dexamethasone, a synthetic glucocorticoid, does not typically suppress cortisol production among most depressed patients (Carroll et al., 1981). In addition, depression may be associated with alterations in the number and function of glucocorticoid receptors, thereby altering the functional effects of cortisol (Raison & Miller, 2003).
Although prolonged elevations in cortisol are deleterious (Sapolsky, 1996; McEwen, 1998), brief elevations are considered adaptive. Once released into the bloodstream, cortisol enacts a series of physiological changes that help the body respond to environmental demands (Sapolsky et al., 2000) and restrain other components of the stress response from becoming overly active. Thus, the failure to mount a sufficient cortisol reponse to stressors may indicate underlying HPA axis dysregulation, and may ultimately contribute to the development of disease, as insufficient cortisol signaling has been implicated in numerous stress-related disease states (Raison & Miller, 2003).
Historically, clinical depression was viewed as a discrete condition, qualitatively distinct from the experience of negative affect in non-depressed individuals (e.g. Robins & Helzer, 1986), but there is increasing evidence from large clinical and non-clinical populations that depressive symptoms exist on a continuum (Flett et al., 1997, Ruscio & Ruscio, 2000). If depression represents the extreme end of a continuum of affective symptoms, then the clinical depression literature can inform hypotheses about physiological correlates of negative affect in non-depressed individuals. Indeed, depressive symptoms may be associated with cortisol responses to stress in non-clinical populations. In a study of 1,109 women, those women high in depressive symptoms failed to show a cortisol response to an unexpected home visit by a team of experimenters, which was considered a naturalistic stressor (Burke et al., 2005b). In a meta-analysis of 8 studies assessing depression and cortisol responses to stress, the degree of depressive symptom severity was marginally associated with the degree of cortisol response blunting (Burke et al., 2005a), suggesting that the degree of affective disturbance may be related to cortisol responses to acute stress in individuals with reporting depressive symptoms within a normal or sub-clinical range.
The association between sub-clinical depressive symptoms and cortisol may extend to sub-clinical symptoms of anxiety. Anxiety disorders and depression are highly co-morbid and share common symptoms (Mineka et al., 1998). In their tripartite model, Clark and Watson (1991) suggested that depression and anxiety share a cluster of general distress symptoms, which includes symptoms like irritability and poor concentration. In addition to these shared symptoms, depression is characterized by anhedonic symptoms indicating lack of positive affect such as feelings of disinterest and apathy, and anxiety tends to be characterized by symptoms of physiological hyperarousal such as shortness of breath and feeling dizzy (1991). There is evidence of HPA dysregulation in anxiety disorders, but the specific nature of these alterations varies across studies and across disorders (for review, see Mantella et al., 2008). For example, post-traumatic stress disorder has been associated with lower basal cortisol and flatter diurnal rhythms (Yehuda, 2006), while general anxiety disorder has been associated with elevations in basal cortisol (Mantella et al., 2008). The extent to which sub-clinical levels of anxious symptoms are associated with altered cortisol responses to stress has not been explicitly evaluated.
Determining whether HPA axis alterations observed in major depression extend to sub-clinical levels of depressive and anxious symptoms can improve our understanding of the physiological correlates of negative affective experience. In addition, studying physiological responses to stress in individuals experiencing a non-clinical range of affective symptoms sheds light on the pathogenesis of affective disorders. Cortisol has been researched extensively in the context of depression, anxiety, and negative affect more generally, but most studies focus on diurnal patterns of cortisol release or cortisol responses to pharmacological challenge, rather than cortisol responses to acute psychosocial stressors that elicit negative affect. Observing how cortisol secretion changes in response to environmental challenges provides important information about the flexibility and efficiency of the HPA axis, and is relevant to understanding how stress ultimately contributes to neuroendocrine and affective disturbance. This study uses a brief psychological stressor that approximates social-evaluative stressors that individuals experience in their daily lives.
Potential Mediators
Individuals experiencing sub-clinical levels of depressive or anxious symptoms may exhibit smaller cortisol responses for a number of reasons. First, recent depressive and anxious symptoms may contribute to altered basal cortisol function. Negative affective episodes tend to be accompanied by the release of cortisol (Peeters et al., 2003; Smyth et al., 1998; Steptoe & Wardle, 2005; Adam, 2006), and individuals experiencing higher levels of depressive and anxious symptoms may experience more frequent HPA activations and greater daily cortisol production. A recent meta-analysis found that higher subjective reports of distress were related to greater total daily cortisol output (Miller et al., 2007), suggesting a linear relationship between negative affect and cortisol output. Elevated daily output may subsequently lead to diminished magnitude of cortisol responses to subsequent stressors. Mechanisms involved in this process could include inhibitory effects of cortisol on the hypothalamus and down-regulation of glucocorticoid receptors (Sapolsky et al., 2000; Raison & Miller, 2003).
Alternatively, depressive or anxious symptoms could influence cortisol responses by altering subjective responses to stressors. Depression is characterized by decreased emotional reactivity to both positive and negative stimuli (see Bylsma et al., 2008, for review). Individuals experiencing more depressive or anxious symptoms might perceive potentially stressful situations as less threatening and experience dampened emotional reactions to stressors. Perceptions of threat and uncontrollability (Dickerson & Kemeny, 2004) and the acute experience of negative emotion (Smyth et al., 1998) are related to cortisol production; thus, individuals who perceive stressors as less threatening and experience fewer negative emotions may show smaller cortisol responses.
Finally, individuals experiencing depressive or anxious symptoms may engage in health behaviors that alter cortisol responses to stressors. Distressed individuals are more likely to have risky health habits such as excessive alcohol consumption, smoking, inadequate exercise, poor nutrition, and poor sleep hygiene, and these behaviors are known to alter neuroendocrine, immune, and cardiovascular parameters (Kiecolt-Glaser & Glaser, 1988). In particular, sleep habits influence basal cortisol function, such that one night of poor sleep results in elevated evening cortisol the subsequent day (Leproult et al.,1997).
Aims and Hypotheses
This study tested whether recent depressive and anxious symptoms predict cortisol responses to a laboratory social stress task in a non-clinical population. We predicted that higher levels of recent depressive and anxious symptoms would be associated with smaller cortisol responses to acute stress. We also investigated three potential mediators of this association: baseline cortisol, stress appraisals, and health behaviors.
Method
Participants
Healthy individuals ages 18 to 44 were recruited from the local community surrounding a large Midwest university using fliers posted in public places. Individuals with medical conditions or taking medications known to affect cortisol levels were excluded. These exclusion criteria included: current pregnancy; medical conditions or medications with obvious immunological, dermatological, or endocrinological consequences; allergies to tape or other adhesives; smoking; and excessive caffeine or alcohol use. Individuals taking hormone-based contraception were excluded because of effects on cortisol responses to stress (Kirschbaum et al., 1999). Female participants were not scheduled around their menstrual cycle stage. A total of 99 individuals participated in the study, of which 12 women did not report taking hormonal-based contraception on our initial screening form, but reported it during the experimental session. Two individuals were unable to complete the entire protocol. In addition, 7 participants did not provide sufficient saliva samples for computing cortisol production and 2 participants did not complete the affective symptom measures. Therefore, data from those 23 individuals were not included in the data analyses that follow, leaving a sample of 76 individuals for the data analyses. The final sample included 41 female and 35 male participants, 65% white, 21% Asian, 10% black, and 4% of other ethnicities. Most participants (65%) had some college education; the remaining participants had either completed college (28%) or completed high school (8%).
Procedure
All participants were run individually during a single 3.5 h session at The Ohio State University General Clinical Research Center (GCRC) beginning at 1:30 PM. Data collection took place between October 2003 and July 2005. The data reported in this paper were collected as part of a study on stress, social support, and skin healing, described in detail elsewhere (Robles, 2007). Participants refrained from eating, vigorous exercise, and smoking for 1 h prior to the appointment. After providing informed consent, participants completed self-report measures assessing depressive and anxious symptoms, health behaviors, and provided a baseline saliva sample. As part of the study participants were fitted with a blood pressure cuff and received a minor skin irritation (described in Robles, 2007). After these procedures, participants were randomly assigned to a one of three groups (No Stress, Stress, Stress + Support) described below. Each group received task instructions, prepared for the task for 10 min, and then performed the 10 min task. All participants were seated throughout the tasks.
Participants in the No Stress group were instructed to read an article silently and alone during the preparation period, followed by reading the article and an additional article out loud into a tape recorder. Participants in the No Stress group were told that they were not being evaluated, and they performed the task alone. Video recording equipment was present, but the video camera was turned off and pointed towards a wall. Participants in the Stress group were provided instructions for the Trier Social Stress Test (TSST), a 5 min speech and 5 min mental arithmetic task (Kirschbaum et al.,1993). Participants in the Stress + Support group completed the TSST, and during the preparation period, received social support from a confederate. By comparison, the Stress group prepared for the task alone. In previous work (Robles, 2007), we showed that this social support manipulation did not have an effect on psychological or physiological responses to the task, and thus we collapsed across the Stress and Stress + Support groups for all analyses in this paper and will be referring to the “Stress group” throughout the paper. After a ten minute preparation period, the Stress group began the TSST in front of an evaluative and harassing audience and were videotaped throughout. After the tasks, saliva measures were collected over the next two hours (see below). Participants were debriefed by the experimenter at the end of the session. All procedures were approved by The Ohio State University Biomedical Sciences Institutional Review Board.
Measures
Recent Depressive and Anxious Symptoms
Depressive and anxious symptoms over the previous two weeks were assessed using the Mood and Anxiety Symptom Questionnaire – Short Form (MASQ-S), a 62 item scale based on Watson and Clark’s tripartite model of anxiety and depression (Clark & Watson, 1991). Though the MASQ-S is not a diagnostic instrument, it identifies the presence of symptoms that commonly occur in depression and anxiety and has been well validated (Watson et al., 1995). We generated three scales: distress symptoms (23 items), which refer to general symptoms common to both depression and anxiety (e.g. “felt like crying” or “felt tense”), anhedonic symptoms (22 items), which refer to the absence of positive emotion (e.g. “felt like nothing was very enjoyable” and reverse-keyed items like “felt hopeful about the future”), and somatic symptoms (17 items), which refer to symptoms of physiological hyperarousal (e.g. “was short of breath” and “was trembling or shaking”).
Participants completed the MASQ-S in the laboratory at the beginning of the session, prior to completing the stressor task. For each item, participants were asked to indicate how much that item describes how they felt over the past two weeks using a five point Likert-type scale, where 1 indicates “not at all” and 5 indicates “extremely.” The mean scores and standard deviations for the subscales were comparable with previous reports of non-clinical populations (Watson et al., 1995) and are presented in Table 1, along with descriptive statistics for other key independent and dependent variables. The subscales were moderately correlated, and the full correlation matrix is presented in Table 2.
Table 1.
Demographic Variables, Salivary Cortisol, Depressive and Anxious Symptoms, Task Ratings, and Emotional Responses to the Task, by Condition
| Variable | No Stress (n = 23) |
Stress (n = 53) |
|---|---|---|
| Age | 23.35 (5.48) | 22.33 (3.69) |
| Salivary Cortisol (µg/dL) relative to task onset | ||
| 1 h 20 min pre | 0.17 (0.73) | 0.22 (0.29) |
| 20 min pre (baseline) | 0.17 (0.15) | 0.16 (0.16) |
| 10 min post† | 0.14 (0.10) | 0.21 (0.15) |
| 30 min post* | 0.12 (0.10) | 0.21 (0.14) |
| 45 min post† | 0.13 (0.08) | 0.18 (0.13) |
| 60 min post | 0.14 (0.11) | 0.16 (0.10) |
| 75 min post | 0.13 (0.09) | 0.16 (0.11) |
| 90 min post | 0.14 (0.07) | 0.13 (0.08) |
| Cortisol response (AUCG) | 14.58 (9.76) | 20.60 (13.92) |
| Depressive and Anxious Symptoms | ||
| Distress Symptoms | 40.73 (14.49) | 47.02 (16.98) |
| Somatic Symptoms | 28.41 (6.73) | 29.13 (6.84) |
| Anhedonic Symptoms | 39.27 (9.71) | 39.09 (10.70) |
| Pre-task Ratings (1–7) | ||
| Perceived threat*** | 1.43 (.73) | 3.79 (1.67) |
| Perceived ability to cope *** | 6.70 (.56) | 5.02 (1.46) |
| Post-task Ratings (1–7) | ||
| Stress *** | 1.64 (1.33) | 4.47 (1.60) |
| Control *** | 6.32 (1.32) | 4.55 (1.64) |
| Helplessness *** | 1.27 (.88) | 3.74 (1.67) |
| Performance Satisfaction*** | 5.68 (1.17) | 4.06 (1.61) |
| Emotional Responses (Change from pre to post task) | ||
| Negative emotion† | −0.30 (2.23) | −2.08 (5.30) |
| Positive emotion | 0.18 (5.06) | 0.60 (4.43) |
| Anxiety** | 0.62 (5.57) | 5.25 (8.07) |
Note. Values are means with SDs in parentheses. Sample size for cortisol levels varied at each timepoint due to missing or undetectable levels, described as follows (No Stress n/Stress n): 1 h 20 min pre (22/50), 20 min pre (23/53), 10 min post (21/50), 30 min post (22/51), 45 min post (22/52), 60 min post (21/48), 75 min post (21/49), 90 min post (22/48).
Main effect of condition,
p <.10,
p < .05,
p < .01,
p < .001
AUCG = area under the curve with respect to ground; SD = standard deviation
Table 2.
Correlation Matrix for MASQ-S Symptom Subscales (N = 75)
| Distress | Somatic | Anhedonic | |
|---|---|---|---|
| Distress | 1.00 | .71** | .28* |
| Somatic | .71** | 1.00 | .08 |
| Anhedonic | .27* | .08 | 1.00 |
Subjective Responses to Stress
Cognitive appraisals, including perceptions of threat and coping, were measured after the task instructions, and again after the preparation period (Tomaka et al., 1993). Perceived stress, control and helplessness (Brier et al., 1987) experienced during the task were assessed with single items after the task. Emotional responses to the task were assessed by administering the Positive and Negative Affect Schedule (Watson et al., 1988) and Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1983) before and immediately after the task.
Health Behaviors
A background questionnaire collected a variety of demographic and health information including age, weight, height, and medical comorbidity. Health-related behaviors assessed included medication use (number of aspirin, extra strength aspirin, and ibuprofen tablets taken in the previous 72 hours), alcohol use (number of drinks consumed in the previous week), and caffeine use (amount consumed in previous 24 hours). As sleep is closely related to cortisol, sleep quality over the previous month was assessed using the Pittsburgh Sleep Quality Index (Buysse et al.,1989). This measure provides an overall sleep quality score, which accounts for a variety of factors including sleep latency, sleep duration, sleep disturbances, and daytime dysfunction.
Salivary cortisol
Eight saliva samples were obtained: 30 min after arriving at the laboratory (1 h 20 min before the task), before receiving instructions for the task (20 min before the task), and 10, 30, 45, 60, 75, and 90 min after the beginning of the task using Salivettes (Sarstedt 1534, Sarstedt Inc., Newton, NC). Mean cortisol values for each time point are presented in Table 1 by group (see Table 1). The assays were performed in the GCRC laboratory, using chemiluminescent techniques (Glaser et al., 1999). Raw cortisol values were used as they did not demonstrate significant skew.
As the novelty of arriving at the laboratory can elicit a cortisol reponse, the second saliva sample, obtained 1.5 hours into the protocol, was used as a baseline measure. This sample was collected prior to group randomization and task instructions, and thus was not influenced by experimental group or anticipation of the task. The last seven cortisol values were integrated into a single cortisol response measure by computing area under the curve with respect to the ground (AUCG), which was used in subsequent analyses (Pruessner et al., 2003). Using log-transformed values did not significantly alter the results, so for clarity and ease of interpretation raw values are used in all analyses.
Data analysis
Throughout the data analyses, SPSS 15 (SPSS, 2006) was used for all descriptive statistics and group comparisons. In order to determine whether the manipulation succeeded in producing a subjectively stressful experience, we conducted a one-way ANOVA with group (stress vs. no stress) as the independent variable and subjective ratings of the task and emotional responses as dependent variables. To examine how the stress manipulation influenced cortisol production, we regressed group, gender, and the group X gender interaction on cortisol. Gender was included as a predictor based on previous reports of gender differences in cortisol responses to laboratory stressors (Kajantie & Phillips, 2006). To test our hypotheses that higher levels of depressive and anxious symptoms would be associated with smaller cortisol responses, we added symptoms as a predictor in this regression equation, performing separate tests for each symptom score (distress, anhedonic symptoms, and somatic symptoms), including all two and three way interactions in these tests. Finally, to test for mediation, we performed a series of linear regressions based on previous recommendations (Baron & Kenny, 1986).
Results
The Stress and No Stress groups did not significantly differ on any demographic variables, health behaviors, baseline salivary cortisol, or depressive and anxious symptoms over the previous two weeks.
Manipulation Check
Descriptive statistics by condition are presented in Table 1. Prior to engaging in the task, participants in the Stress condition appraised the task as more threatening and appraised themselves as less able to cope than participants in the No Stress condition. After the task, participants in the Stress condition rated the task as more stressful, felt more helpless, were less satisfied with their performance, and felt less in control. Participants in the Stress condition reported significantly greater increases in anxiety and marginally greater increases in negative emotion (p = .06). A paired samples t-test confirmed that self reported levels of negative emotion and anxiety increased from baseline to post-task in the stress condition (all p’s < .01), but did not change in the No Stress condition. There were no gender differences in subjective responses to the task.
Cortisol Responses to Stress
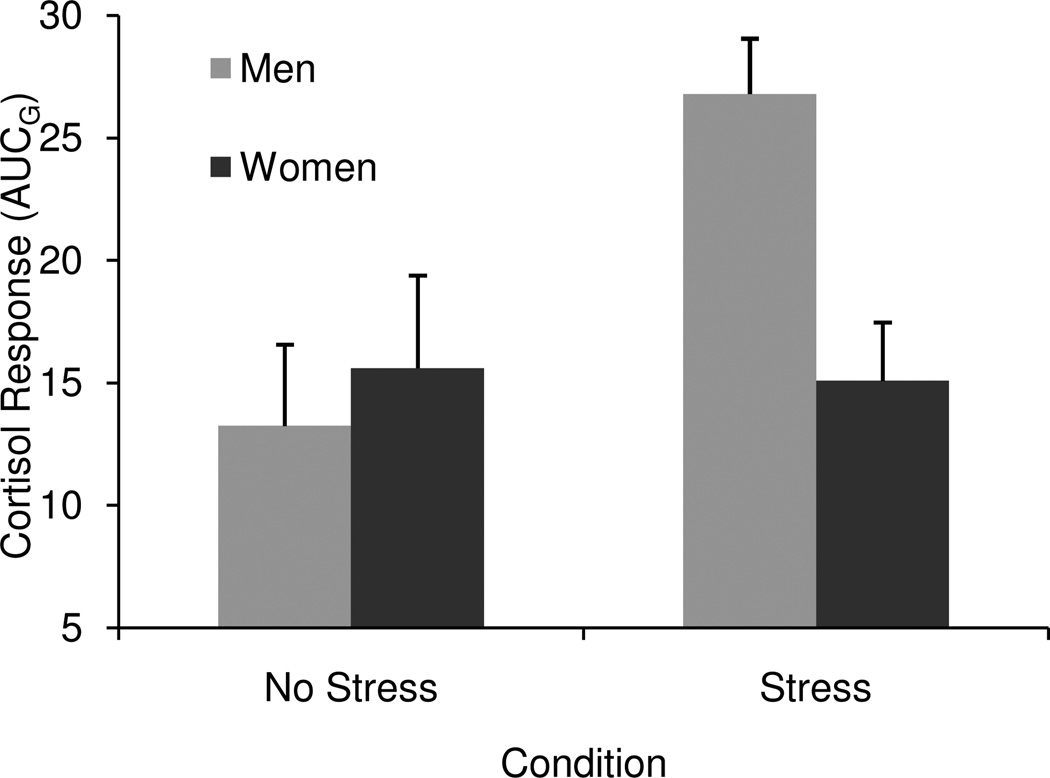
The main effect of condition (β = 0.22, t (71) = 2.06, p < .05) and the condition X gender interaction (β = 0.47, t (71) = 2.35, p < .05) were significant. Independent samples t-tests revealed that men in the stress condition had larger cortisol responses than men in the No Stress condition (t (33) = −2.63, p < .05), but there were no differences in cortisol between conditions for women (t (39) = 0.15, p = .88). Mean cortisol responses for men and women in each condition are presented in Figure 1. These findings suggest that the stress manipulation did not succeed in eliciting a cortisol response among women.
Figure 1.
Estimated marginal means and standard errors for cortisol production, by gender and condition.
Do recent depressive and anxious symptoms predict smaller cortisol responses to acute stress?
We predicted that higher levels of each of depressive and anxious symptoms would predict smaller cortisol responses to the stressor. To test this prediction, we added symptoms, the group X symptoms interaction, the gender X symptoms interaction, and the group X gender X symptoms interaction. In light of the gender X group interaction, if there was an effect of symptoms, this should be evident in the three-way interaction term. As noted previously, we ran separate regressions for each symptom score (distress, somatic symptoms, and anhedonic symptoms). There were no main effects of symptoms in any of the models, and the two and three way interaction terms were not significant.
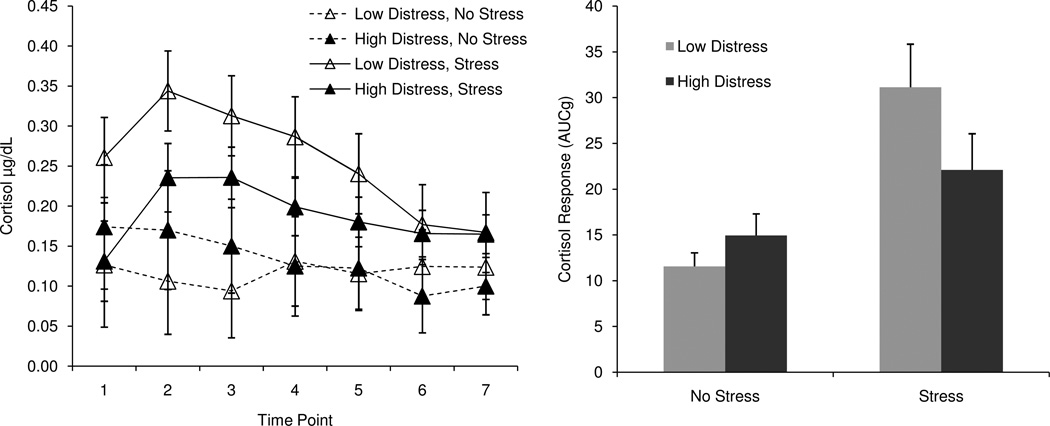
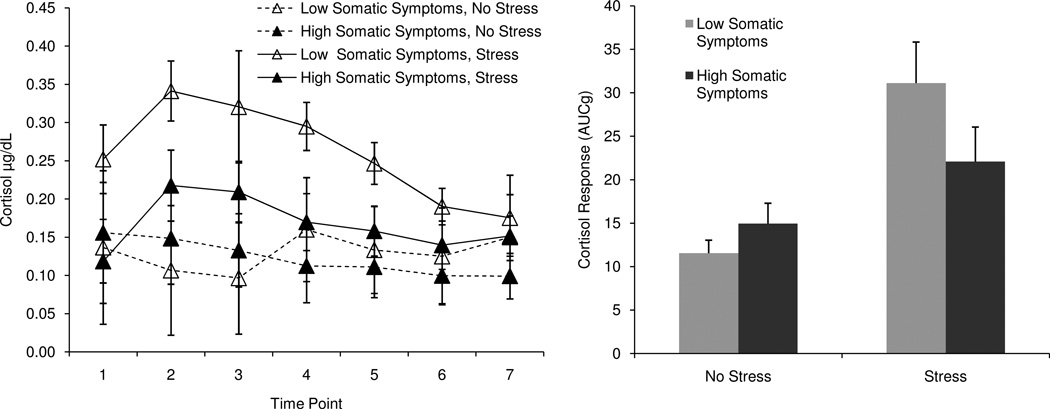
However, given the number of predictors relative to the sample size, this test was somewhat underpowered, and we suspected that this level of power might not be sufficient to detect a three-way interaction (Cohen & Cohen, 2003). To fully test whether symptoms were related to cortisol responses to stress, we split the sample by gender and condition and regressed symptoms on cortisol response in each of these four groups. Consistent with our predictions, two types of symptoms – general distress and somatic symptoms – were related to cortisol responses, but only among men in the Stress group. Higher levels of distress (F(2, 32) = 7.03, β = −0.36, p < .05, f2 = 0.19) and somatic symptoms (F(2, 32) = 7.51, β = −0.38, p < .05, f2 = 0.22) were associated with smaller cortisol responses among men in the Stress group. There were no significant relationships between symptoms and cortisol among women in the stress group, or among men or women in the non-stress group (all p’s > .05). Anhedonic symptoms were not related to cortisol responses in any of our tests (all p’s > .05). Figure 2 illustrates this pattern of results, depicting mean cortisol response curves and total production for men high and low in distress (as determined by a median split). Figure 3 depicts similar information for men high and low in somatic symptoms. When entered simultaneously, distress and somatic symptoms were not significant predictors, most likely because the variables were highly correlated (r = 0.83, p < .001)1.
Figure 2.
Cortisol response curves and total production for men high and low in distress, by condition.
Figure 3.
Cortisol response curves and total production for men high and low in somatic symptoms, by condition.
Potential Mediators
As discussed previously, a number of factors could explain the relationship between negative affective symptoms and cortisol responses, including baseline cortisol level, subjective responses to the task, and health behaviors. We evaluated these potential mediators in male participants in the stress condition (n = 25).
Baseline Cortisol
Among men in the stress condition, higher levels of distress were associated with marginally lower baseline cortisol levels (β = −0.38, p = .06), and lower baseline cortisol was associated with smaller cortisol responses (β = 0.73, p < .001). To test whether baseline cortisol mediated the association between distress and cortisol response, we entered distress and baseline cortisol as predictors in a linear regression with cortisol response as the dependent variable. Adding baseline cortisol as a predictor rendered the association between distress and cortisol response non-significant, although the Sobel test failed to reach significance (z = −1.86, p = .06).
Subjective Responses
Next, we examined whether subjective experience during the task mediated the relationship between negative affect and cortisol. Men reporting higher levels of distress appraised the task as more threatening (β = .40, p < .05), and afterwards rated the experience as more stressful (β = 0.40, p < .05), felt less satisfied with their performance (β = −0.42, p < .05), felt more helpless (β = 0.63, p = .001), and reported larger increases in anxiety (β = .46, p < .05). Men with more somatic symptoms felt more helpless (β = 0.49, p < .05) and reported larger increases in negative emotion (β = .47, p < .05) and anxiety (β = 0.52, p = .05). However, subjective responses were not related to cortisol, so we did not conduct further tests of mediation.
Health Behaviors
Finally, we examined whether health behaviors mediated the relationship between symptoms and cortisol. Using data for men in the stress condition, we regressed distress or somatic symptoms onto each of the following potential mediators: alcohol use (number of drinks consumed in previous week), caffeine use (amount consumed in previous 24 hours), and sleep quality. None of the men in the stress condition reported taking any aspirin or ibuprofen in the previous 72 hours, so medication use was not tested as a mediator. Men with more distress or somatic symptoms reported poorer sleep over the previous month (βdistress = 0.44, βsomatic = 0.34, both p’s < .05), but sleep quality was not associated with cortisol and therefore we did not conduct further tests of mediation.
Discussion
This study examined whether recent depressive and anxious symptoms were related to cortisol responses to acute stress in a non-clinical population. As predicted, in a sample of individuals with a normal range of symptoms, men reporting higher levels of distress and somatic symptoms had smaller cortisol responses to a laboratory stressor. These findings suggest that the experience of sub-clinical depressive and anxious symptoms is associated with alterations in HPA function similar to those observed in major depression. The pattern of diminished responding observed in our sample is consistent with a dysregulation hypothesis of depression (Siever & Davis, 1985), which maintains that depression and other affective disorders are characterized by a dysregulation of neural systems involved in affect regulation, rather than an absolute increase or decrease in activity in these systems. Our findings are also consistent with reports that depressed patients show flatter diurnal cortisol rhythms than do patients with PTSD and other affective disorders (Yehuda et al., 1996). Anhedonic symptoms were not related to cortisol responses, suggesting that different types of negative affective symptoms have may different implications for stress physiology. Consistent with this premise, patterns of diurnal cortisol secretion vary between clinical depression subtypes (i.e. atypical vs. melancholic depression; Gold et al., 1995), and some depressed patients do not show cortisol non-suppression in response to the dexamethasone challenge (see Burke et al., 2005a, for discussion). Our data suggest there may be similar heterogeneity among individuals experiencing sub-clinical negative affective symptoms.
Depressive and anxious symptoms were not related to cortisol responses among women, presumably because the stressor did not elicit a cortisol response among women. This finding is consistent with other evidence that men show larger cortisol responses to laboratory stressors, particularly achievement-oriented tasks like the public speaking task used in this study. Men may be more physiologically responsive to achievement-oriented stressors and women may be more physiologically responsive to relational stressors (Stroud et al., 2002). As the stressor task used in our study was a prototypical achievement stressor, our finding that men showed larger cortisol responses is consistent with previous work.
We tested three potential mediators of the association between depressive and anxious symptoms and cortisol responding: baseline cortisol, subjective responses to the task, and health behaviors. We found evidence that alterations in baseline cortisol may partially account for the association between depressive and anxious symptoms and cortisol responses to acute stress. Men reporting higher levels of distress had lower baseline cortisol, which statistically mediated the association between distress and cortisol response. Although this may seem at odds with previous reports of elevated baseline cortisol in distressed and clinically depressed individuals (see Burke et al., 2005a, and Miller et al., 2007, for reviews), it is worth noting that ours not have been a true baseline measure. We deliberately collected our baseline measure one hour after participants arrived at the laboratory, in order to allow them to acclimate to the laboratory setting. However, participating in a laboratory experiment is a novel experience and the mere process of arriving at a laboratory may elicit cortisol responding in some individuals. In addition, the events that occurred during the first hour of the session (i.e., filling out questionnaires, undergoing a skin irritation procedure) may have elicited additional cortisol responding. Thus, the differences in baseline cortisol we observed may reflect differences in cortisol responses to the laboratory context. In other words, individuals reporting more distress in the previous week may have shown smaller cortisol responses both to the novelty of the laboratory context and to the stressor task.
The association between depressive and anxious symptoms and cortisol responses could not be explained by subjective responses to the task. Although men reporting higher levels of distress and somatic symptoms found the task more aversive and experienced more negative emotion in response to the task, these subjective responses were not related to cortisol responses. This finding is consistent with other reports that subjective experience and physiology are not closely correlated (Mauss et al., 2005). The discrepancy between subjective experience and cortisol responding may be especially pronounced for individuals with depression or sub-clinical levels of depressive symptoms. Consistent with this premise, Peeters and colleagues (2003) found that negative affect was less closely related to cortisol levels in daily life for depressed patients compared to healthy controls.
The association between symptoms and cortisol response could also not be explained by health behaviors. Men reporting more distress and somatic symptoms reported poorer sleep, but sleep was not related to cortisol responses. Although clinical depression is associated with alterations in sleep quality, our data suggest that the decrements in sleep quality associated with normal levels of depressive and anxious symptoms may not be sufficient to alter cortisol responses to acute stress.
There are several limitations to this study. First, we assessed negative affect at only one time point by asking participants to self-report on their feelings over the previous week. Though retrospective reports of emotion are not weighted averages and tend to be biased by the most intense portions of emotional episodes and the end of these episodes (Kahneman, 2003), the fact that our measures of distress and somatic symptoms were related to cortisol responding in men is noteworthy. Future research ought to use daily diary or experience-sampling methods to investigate how real-time reports of sub-clinical depressive and anxious symptoms are related to cortisol responding to acute stress, and whether changes in symptoms over time are related to changes in cortisol responding within individuals. Second, we had limited information about basal cortisol function, as our baseline measure may have been altered by the novel laboratory setting. Future work is needed to understand how diurnal patterns of cortisol release and other aspects of daily functioning are related to responses to acute stressors. Finally, a relatively small sample size limited our ability to conduct mediation analyses. Subsequent studies utilizing larger sample sizes will be useful in testing other potential mediators. Given the modest sample size, the fact that baseline cortisol statistically mediated the association between distress and men’s cortisol responses suggests that this may be one important pathway by which affective symptoms influence cortisol responding to stressors.
This study suggests that the pattern of blunted cortisol responding observed in clinical depression may represent a more general association between depressive and anxious symptoms and HPA function. Our data suggest that normal between-person variation in recent depressive and anxious symptoms may predict cortisol responding to acute stress. These findings highlight the importance of attending to the physiological consequences of negative affective symptoms along the continuum from normal function to pathology and demonstrate that between-person differences in negative affect within the normative range have meaningful consequences for physiology.
Acknowledgements
We wish to thank Chris Dunkel Schetter for thoughtful comments on the manuscript and Janice Kiecolt-Glaser and the OSU Stress and Health Study for their support.
Role of Funding Sources
This project was supported by a University Fellowship from the University of California, Los Angeles; a National Science Foundation Fellowship from the UCLA Interdisciplinary Relationship Science Training Program; a Summer Research Mentorship Award from the University of California, Los Angeles; a Graduate Research Award from the American Psychological Association (APA), Division 38; an Alumni Grant for Graduate Research and Scholarship from the Graduate School, The Ohio State University; an APA Dissertation Research Award; the Department of Psychology, The Ohio State University; and MO1-RR-0034 (Ohio State University General Clinical Research Center). These sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To address any concerns that the support manipulation, included as part of another study, did not alter the pattern of results, we regressed support condition (support vs. no support), gender, and the support X gender interaction on cortisol response. This test revealed no effect for support or the support X gender interaction. In addition, we added symptoms and all interactions to this model, running separate tests for each symptom score. Again, there were no main or interaction effects for support on cortisol, leading us to conclude that the presence (or absence) of a confidant did not influence cortisol responding and did not moderate the relationship between symptoms and cortisol, for either men or women. As an additional test of this conclusion, we split the sample by gender and regressed symptoms, support, and the symptoms X support interaction on cortisol. These tests revealed no effects of support on cortisol.
Conflict of Interest
All authors declare that they have no conflicts of interest that could inappropriately influence, or be perceived to influence, their work.
Contributors
Theodore Robles wrote the protocol and collected the data. Kathryn Brooks conducted the literature search, performed the analyses, and wrote the first draft of the manuscript. Both authors contributed to and have approved the final manuscript.
Contributor Information
Kathryn P. Brooks, 1285 Franz Hall, Department of Psychology, University of California, Los Angeles, Los Angeles, CA 90095, kathryn.p.brooks@gmail.com, Fax: 310-206-5895
Theodore F. Robles, Box 951563, 1285 Franz Hall, Department of Psychology, University of California, Los Angeles, Los Angeles, CA 90005, robles@psych.ucla.edu, Fax: 310-206-5895
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ahrens T, Deuschle M, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosomatic Med. 2008;70:461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005a;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Burke HM, Fernald LC, Gertler PJ, Adler NE. Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosomatic Med. 2005b;67(2):211–216. doi: 10.1097/01.psy.0000156939.89050.28. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Breier A, Albus M, Pickar D, Zahn T, Owen M, Paul S. Controllable and uncontrollable stress in humans: Alterations in mood and neuroendocrine and psychophysiological function. Am J Psychiatry. 1987;144:1419–1425. doi: 10.1176/ajp.144.11.1419. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Hillsdale, NJ: Erlbaum; 2003. [Google Scholar]
- Croes S, Merz P, Netter P. Cortisol reaction in success and failure condition in endogenous depressed patients and controls. Psychoneuroendocrinology. 1993;18:23–35. doi: 10.1016/0306-4530(93)90052-m. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Archives of General Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- Gold PW, Licinio J, Wong ML, Chrousos GP. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci. 1995;771:716–729. doi: 10.1111/j.1749-6632.1995.tb44723.x. [DOI] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: Mapping bounded rationality. American Psychologist. 2003;58:696–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic-pituitary-adrenal axis. Psychosomatic Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The 'Trier Social Stress Test' - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Cauter EV. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Willhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosom Med. 2003;65(5):836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Griffiths J, Merali Z, Anisman H. Variations of lymphocyte subsets associated with stress in depressive populations. Psychoneuroendocrinology. 1996;21:659–671. doi: 10.1016/s0306-4530(96)00030-3. [DOI] [PubMed] [Google Scholar]
- Robles TF. Stress, social support, and delayed skin barrier recovery. Psychosomatic Med. 2007;69:807–815. doi: 10.1097/PSY.0b013e318157b12e. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273(5276):749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. Overview: toward a dysregulation hypothesis of depression. Am J Psychiatry. 1985;142:1017–1031. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23(4):353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Steptoe A, Wardle J. Positive affect and biological function in everyday life. Neurobiology of Aging. Special Issue: Aging, Diabetes, Obesity, Mood and Cognition. 2005;26(Suppl1):S108–S112. doi: 10.1016/j.neurobiolaging.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Stroud L, Salovey P, Epel E. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, Leitten CL. Subjective, physiological, and behavioral effects of threat and challenge appraisal. J Pers Soc Psychol. 1993;65:248–260. [Google Scholar]
- Trestman RL, Coccaro EF, Bernstein D, Lawrence T, Gabriel SM, Horvath TB, Siever LJ. Cortisol responses to mental arithmetic in acute and remitted depression. Biological Psychiatry. 1991;29:1051–1054. doi: 10.1016/0006-3223(91)90361-o. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher M, Trestman R, Levengood R, Siever L. Cortisol regulation in posttraumatic stress disorder and major depression: A hronobiological analysis. Biological Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]