Abstract
Rationale:
Prior studies comparing abnormalities in pulmonary function between HIV-infected and HIV-uninfected persons in the current era are limited.
Objectives:
To determine the pattern and severity of impairment in pulmonary function in HIV-infected compared to HIV-uninfected individuals.
Methods:
Cross-sectional analysis of 300 HIV-infected and 289 HIV-uninfected men enrolled from 2009-2011 in two clinical centers of the Lung HIV Study. Participants completed pre- and post-bronchodilator spirometry, diffusing capacity (DLCO) measurement, and standardized questionnaires.
Results:
Most participants had normal airflow; 18% of HIV-infected and 16% of HIV-uninfected men had airflow obstruction. The mean percent predicted DLCO was 69% in HIV-infected vs. 76% in HIV-uninfected men (p<0.001). A moderately to severely reduced DLCO of ≤60% was observed in 30% of HIV-infected compared to 18% of HIV-uninfected men (p<0.001), despite the fact that 89% of those with HIV were on antiretroviral therapy. A reduced DLCO was significantly associated with HIV and CD4 cell count in linear regression adjusting for smoking and other confounders. The DLCO was lowest in HIV-infected men with CD4 cell counts <200 compared to those with CD4 cell counts ≥200 and to HIV-uninfected men. Respiratory symptoms of cough, phlegm and dyspnea were more prevalent in HIV-infected patients particularly those with abnormal pulmonary function compared to HIV-uninfected patients.
Conclusions:
HIV infection is an independent risk factor for reduced DLCO, particularly in individuals with a CD4 cell count below 200. Abnormalities in pulmonary function among HIV-infected patients manifest clinically with increased respiratory symptoms. Mechanisms accounting for the reduced DLCO require further evaluation.
Keywords: Pulmonary function, FEV1, DLCO, gas exchange, COPD, HIV/AIDS
INTRODUCTION
The aging of the HIV-infected population has resulted in a changing spectrum of disease. Mortality related to AIDS-defining conditions has decreased markedly for those on effective antiretroviral therapy (ART),1, 2 whereas co-morbid non-AIDS-defining conditions now account for the majority of deaths in recent studies.2-6 Since the early days of the AIDS epidemic, pulmonary diseases have been among the most frequent complications of HIV.7-9 Consistent with other co-morbid complications, the spectrum of pulmonary diseases now reflects a substantial burden of non-AIDS defining chronic diseases, especially chronic obstructive pulmonary disease (COPD).10-15
While some of the burden of pulmonary disease is explained by the high prevalence of smoking, HIV infection appears to confer an independent increase in the risk for chronic lung diseases, particularly COPD.10, 11, 16-18 Pulmonary function abnormalities and respiratory symptoms are common in HIV-infected patients, with approximately 20% having irreversible airflow obstruction on spirometry, and >30% reporting cough, phlegm, wheeze or dyspnea.19-21 However, prior studies directly comparing pulmonary function between HIV-infected and HIV-uninfected persons in the current ART era are limited, and importantly have not assessed post-bronchodilator spirometry or diffusing capacity of the lung for carbon monoxide (DLCO).12, 19, 20 A low DLCO was first noted in HIV-infected patients prior to the combination ART era,22, 23 and while recent studies of HIV-infected persons have found that a substantial proportion have an abnormally reduced DLCO,14, 20 these have not included an HIV-uninfected comparator group.
Therefore, we sought to determine the pattern and severity of impairment in pulmonary function in a large cohort HIV-infected and HIV-uninfected individuals. We also asked whether pulmonary function was associated with markers of HIV severity. These analyses utilized data from HIV-infected and HIV-uninfected men participating in two clinical centers of the Lung HIV Study,24 a multicenter study of pulmonary disease in HIV infection sponsored by the Lung Division of the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI). Some of these results have been reported previously in the form of an abstract.25
METHODS
Overview of population
We performed a cross-sectional analysis of 300 HIV-infected and 289 HIV-uninfected men enrolled from 2009-2011 in the Lung HIV Study, a longitudinal cross-cohort collaboration examining pulmonary complications in the ART era.24 Participants from two Lung HIV Clinical Centers that obtained DLCO measurements in HIV-infected and uninfected patients were included, namely the pulmonary substudy of the Multicenter AIDS Cohort (MACS)26 and the Examinations of HIV Associated Lung Emphysema (EXHALE) Study, a substudy of the Veterans Aging Cohort Study (VACS).27, 28 The MACS pulmonary study enrolled patients from the Pittsburgh and Los Angeles sites, and the EXHALE study enrolled patients from the Atlanta, Bronx, Houston and Los Angeles VA Medical Centers. Only men were included in these analyses because MACS is an all-male cohort and very few women were enrolled in EXHALE. All participants signed written informed consent, and the study was approved by the Institutional Review Boards at each participating center.
HIV-infected and uninfected outpatients in the MACS and VACS were approached for participation. Those with acute respiratory infections or illnesses in the past 4 weeks were excluded. A history of chronic lung diseases was not required for enrollment. Within the EXHALE study, HIV-infected and uninfected participants were matched by current smoking status; those with a history of lung diseases other than COPD or asthma, including lung cancer, were excluded. In the MACS substudy, consecutive HIV-infected and uninfected participants were selected to reflect the distributions within the parent study of smoking and the presence of respiratory symptoms in order to avoid bias in recruiting primarily smokers or symptomatic individuals who might be more willing to participate in a study of lung function.
Pulmonary function testing
All participants completed pulmonary function tests (PFTs) at study enrollment per American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines,29, 30 including pre- and post-bronchodilator spirometry and single breath DLCO. Within both studies, PFTs were obtained by certified, trained respiratory technicians or research personnel. PFTS were obtained in the clinical pulmonary function laboratories at the associated medical center in the EXHALE study, and within research pulmonary function laboratories in the MACS substudy. PFT results were reviewed by site pulmonologists or study investigators for quality control; those not meeting ATS criteria for acceptability and reproducibility were not included in these analyses. Airflow obstruction was defined as a ratio of the post-bronchodilator forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) below 0.70.31 In secondary analyses, airflow obstruction was defined as an FEV1/FVC ratio below the lower limit of normal (LLN); as results were similar, we present the results using a ratio below 0.70. Predicted normal values (% predicted) for spirometry were determined using Hankinson formulas32, and using Neas formulas for DLCO.33 Both include adjustments for age, race/ethnicity, gender, and height; % predicted DLCO values were also corrected for hemoglobin. A positive bronchodilator response was present if the FEV1 and/or FVC increased by at least 12% and 200 mL following bronchodilator administration.34 Severity of airflow obstruction was graded based on the FEV1.31 Impairment in DLCO corrected for hemoglobin was based upon ATS/ERS standards, with a threshold of ≤60% of predicted indicating moderate or greater impairment, and ≤40% of predicted reflecting severe impairment.34
Other data collection
Participants completed a standardized questionnaire assessing history of lung diseases, smoking and other exposures using previously published instruments.24 Similar questions were used in the EXHALE and the MACS substudy. Smoking status and pack-years of smoking were defined consistently. Participants who reported <100 lifetime cigarettes were defined as never smokers; as current smokers if they had smoked within the past 12 months; and as former smokers if they had quit >12 months ago. Pack-years were calculated based upon number of cigarettes smoked per day on average and years smoked; never smokers were considered to have zero pack-years of exposure. Within the MACS substudy, recent drug use was defined as within 6 months, and within EXHALE, recent use was defined as within 12 months. Questions regarding respiratory symptoms of usual cough, usual phlegm, and prior attacks of wheezing that have been associated with shortness of breath were taken from previously published instruments;35 shortness of breath with activity was quantified using the modified Medical Research Council (MRC) dyspnea score.36, 37 The MRC dyspnea score was dichotomized at 2 and above as suggested in published guidelines,31 corresponding to the need to stop for breath when walking at one’s own pace on level ground or greater limitation. HIV viral load (copies/ml) and CD4 cell counts (cells/μl) were obtained within 12 months of pulmonary function testing. Recorded nadir CD4 cell count prior to PFTs was dichotomized as > or <200. ART use was captured from the MACS and VACS datasets.26, 27
Statistical analysis
To ensure that results were similar within each cohort, analyses were first stratified by cohort. As results were consistent with similar associations and magnitude of difference in PFT results by HIV status, we proceeded with the combined EXHALE and MACS cohort analyses. Baseline characteristics in HIV-infected and HIV-uninfected men were compared using t-tests and Wilcoxon rank-sum tests for parametric or non-parametric continuous variables respectively, and chi-squared tests for categorical variables. To determine the association of HIV with impairment in pulmonary function, potential risk factors were first examined in bivariate models. Then, multivariable linear regression models were used with % predicted DLCO as the outcome, and included HIV, an indicator variable for clinical center, smoking and other potential confounders. Predictors that were significant at p<0.1 in bivariate analyses were included in the multivariable models and were retained if statistically significant at p<0.05 or if they had a substantive effect on other coefficients.
Because smoking is a major potential confounder, we modeled smoking in several ways, including by deciles of pack-years. The risk of impaired pulmonary function did not increase linearly, but displayed a threshold effect at 35 pack-years; this level was more strongly associated with PFT results than smoking status. Therefore, we modeled smoking exposure as 0 pack-years, 0-34, and >35 pack-years in multivariable analysis.
To determine the association of pulmonary function impairment with markers of HIV, we stratified participants by HIV and CD4 cell count, assuming those without HIV to have a CD4 cell count ≥350. HIV-infected participants were divided into groups by CD4 cell count ≥350, 200-349, and <200 cells/μl. Because associations were similar in multivariable regression for those with CD4 cell counts of ≥350 cells/μl and 200-349 cells/μl, these groups were combined in final analysis. We also compared associations with HIV RNA >500 or <500 copies/ml, using HIV-uninfected participants as the referent group. This study was adequately powered at >80% to detect a 7.5% relative difference in means for FEV1, FVC, FEV1/FVC and % predicted DLCO between HIV-infected and HIV-uninfected groups. There was also sufficient power to detect an absolute difference of 10% for FEV1/FVC<0.70 and DLCO ≤60% predicted (power of 82% and 79%, respectively).
RESULTS
Demographic and clinical characteristics
Our cohort included 300 HIV-infected and 289 HIV-uninfected men with a mean age of 54 (Table 1); 51% were from VA sites. The VA and MACS patients were comparable, although the VA patients were slightly older and more likely to be current smokers (data not otherwise shown). The combined cohort was racially and ethnically diverse. Overall, more HIV-infected men were current smokers compared to HIV-uninfected men (47% vs. 35%, p=0.007) and had more pack-years of smoking. HIV-infected men were also more likely to have smoked marijuana and used injection drugs (Table 1). As very few participants had used injection drugs recently, current and former use were combined into a composite variable reflecting ever use in subsequent analyses. HIV-infected men were also more likely to report a history of prior bacterial or Pneumocystis pneumonia.
Table 1.
Characteristics of HIV-infected compared to HIV-uninfected participants
| Characteristics | HIV+, N=300 | HIV−, N=289 | p-value |
|---|---|---|---|
| Age, mean years. (SD) | 54 (8) | 54 (9) | 0.8 |
| Race/ethnicity | 0.3 | ||
| White | 43% | 50% | |
| Black | 47% | 40% | |
| Hispanic | 9% | 9% | |
| Other | 1% | 1% | |
| Smoking status | 0.007 | ||
| Current | 47% | 35% | |
| Former | 27% | 30% | |
| Never | 26% | 35% | |
| Smoking pack-years among ever smokers, median (SD) |
26 (13-40) | 20 (8-37) | 0.07 |
| Injection drug use | 0.001 | ||
| Current | 3% | 0% | |
| Former | 23% | 15% | |
| Never | 74% | 85% | |
| Marijuana use | 0.05 | ||
| Current | 32% | 24% | |
| Former | 50% | 50% | |
| Never | 26% | 18% | |
| Prior bacterial pneumonia | 28% | 16% | <0.001 |
| Prior Pneumocystis pneumonia | 10% | 2% | <0.001 |
| Prior tuberculosis | 6% | 5% | 0.6 |
| CD4 cell count, median (IQR) | 493 (354-698) | -- | |
| CD4 cell count <200 cells/μl | 8% | -- | |
| HIV viral load ≤500 copies/ml | 84% | -- | |
| On Antiretroviral therapy | 89% | -- | |
| Hemoglobin, mean (SD) | 14.3 (1.5) | 14.7 (1.2) | 0.002 |
Prevalence of PFT abnormalities in HIV-infected compared to HIV-uninfected men
The majority of both HIV-infected and HIV-uninfected men had normal airflow on spirometry (Table 2), with an average FEV1 post-bronchodilator of >90% of predicted. There was no significant difference in the proportion of HIV-infected compared to HIV-uninfected participants with an FEV1/FVC<0.70 post-bronchodilator or with a positive bronchodilator response. Overall, 18% of HIV-infected and 16% of HIV-uninfected men met criteria for airflow obstruction (p=0.5).
Table 2.
PFT results by HIV status
| PFT results | HIV+ | HIV− | p-value |
|---|---|---|---|
| FEV1 in L, pre-BD, mean (SD) | 3.16 (0.8) | 3.23 (0.8) | 0.4 |
| FEV1, %predicted pre-BD, mean (SD) | 90 (19) | 91 (19) | 0.5 |
| FEV1 in L, post-BD, mean (SD) | 3.31 (0.8) | 3.39 (0.8) | 0.3 |
| FEV1, %predicted post-BD, mean | 95 (19) | 96 (19) | 0.4 |
| FVC in L, pre-BD, mean (SD) | 4.28 (0.9) | 4.35 (0.9) | 0.6 |
| FVC, %predicted pre-BD, mean (SD) | 95 (16) | 96 (18) | 0.9 |
| FVC in L, post-BD, mean (SD) | 4.34 (0.9) | 4.39 (0.9) | 0.7 |
| FVC, %predicted post-BD, mean (SD) | 97 (15) | 97 (16) | 0.9 |
| FEV1/FVC post-BD, mean (SD) | 0.76 (0.1) | 0.78 (0.1) | 0.4 |
| FEV1/FVC<0.70 | 18% | 16% | 0.5 |
| Bronchodilator responsiveness* | 16% | 13% | 0.2 |
| Raw DLCO, mean (SD) | 21.9 (6.2) | 23.9 (6.3) | 0.001 |
| DLCO**, %predicted, mean (SD) | 69 (19) | 76 (18) | <0.001 |
| DLCO ≤60% predicted, n% | 30% | 18% | <0.001 |
BD = Bronchodilator; DLCO = diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; SD = standard deviation
Bronchodilator responsiveness was present if the FEV1 and/or FVC increased by at least 12% and 200 mL following bronchodilator administration.
Percent predicted DLCO values are corrected for hemoglobin.
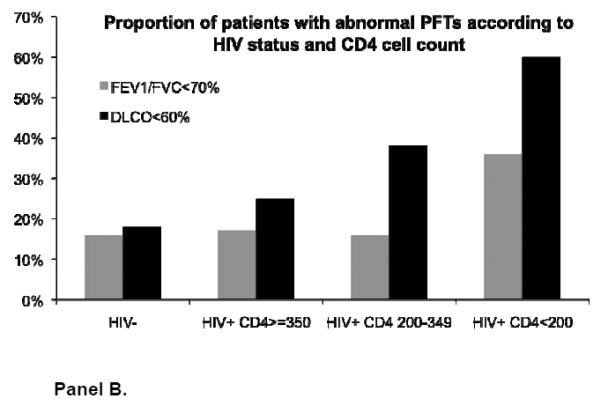
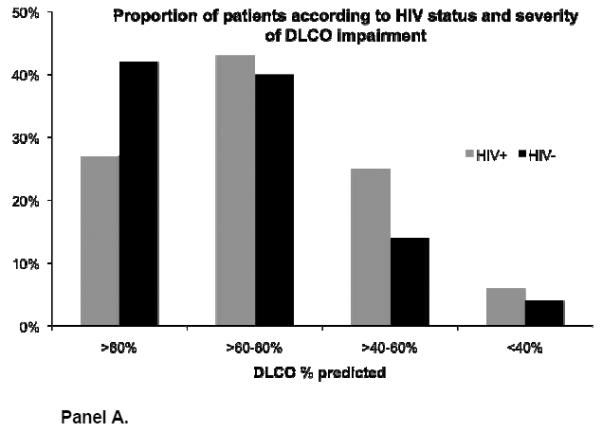
Mean DLCO was significantly lower in HIV-infected compared to HIV-uninfected men (Table 2). Overall, 30% of HIV-infected participants had a DLCO that was ≤60% of predicted compared to 18% of HIV-uninfected participants (p=0.001). HIV-infected participants were also more likely to have greater DLCO impairment across all ATS/ERS severity categories of DLCO (Figure 2). Patients with a DLCO ≤60% predicted were significantly more likely to have ever smoked compared to those with a higher DLCO. Among HIV-infected participants, 92% of those with a DLCO ≤60% vs. 66% of those with a DLCO >60% had ever smoked (p<0.005).
The predominant pulmonary function abnormality observed was an isolated reduction in DLCO. An equal proportion (61%) of HIV-infected and HIV-uninfected participants with a DLCO ≤60% of predicted did not have concomitant airflow obstruction. Among participants with airflow obstruction, the mean % predicted DLCO was substantially lower among those with HIV compared to those without, with a mean (standard deviation (SD)) of 56 (21) vs. 67 (22) respectively, p=0.008. Among participants without airflow obstruction, the mean % predicted DLCO (SD) was also significantly lower among HIV-infected compared to HIV-uninfected men: 73 (17) vs. 77 (17), p=0.005.
PFT results stratified by markers of HIV disease severity
In bivariate analyses stratified by HIV and CD4 cell count, airflow obstruction was present in 36% of HIV-infected men with a CD4 cell count <200 cells/μl compared to 16% with a CD4 cell count 200 to 349, 17% with a CD4 cell count ≥350, and 16% of HIV-uninfected men (p=0.09) (Figure 1). There was no statistically significant relationship in the prevalence of airflow obstruction according to HIV viral load: 24% with HIV viral load >500 had airflow obstruction compared to 17% with viral load ≤500 copies/ml and 16% of HIV-uninfected participants (p=0.4).
However, DLCO was associated with both recent as well as nadir CD4 cell count; the relationship appeared more strongly related to recent CD4 cell count (Supplemental Table 1). HIV-infected men with a recent CD4 cell count <200 as well as those with a CD4 between 200 to 349 had a significantly lower DLCO when compared to HIV-infected men with a CD4 cell count ≥350 and to HIV-uninfected men (Figure 1). The % predicted DLCO (mean (SD)) was 58 (12) in HIV-infected men with CD4 <200 cells/μl; 67 (17) in those with CD4 200 to <350; 72 (19) in those with CD4 ≥350; and 76 (18) in HIV-uninfected men, p=0.001.
DLCO was also significantly associated with HIV viral load (Supplemental Table 1). The mean % predicted DLCO (SD) in HIV-infected participants with a viral load of >500 copies/ml was 66 (18) compared to 71 (19) in the HIV-infected participants who were virally suppressed, and 76 (18) in the HIV-uninfected (p=0.001). Overall, 34% of HIV-infected men with a viral load >500 copies/ml had a DLCO ≤60% of predicted normal, compared to 30% of HIV-infected men with HIV RNA below this level and 18% of HIV-uninfected men, p=0.002.
HIV as an independent risk factor for decreased DLCO
After evaluating factors associated with DLCO in bivariate analyses, we determined independent risk factors for % predicted DLCO, corrected for hemoglobin, in multivariable linear regression (Table 4). Although prior Pneumocystis pneumonia, tuberculosis, and injection drug use were significantly associated with DLCO in bivariate analyses, they were no longer significant in multivariable analyses. HIV viral load and CD4 cell count were both associated with DLCO but were collinear; CD4 was chosen because the association with DLCO was stronger. Thus, after adjusting for race-ethnicity, pack-years of smoking and clinical center, we found that HIV status remained significantly associated with a decreased DLCO % predicted. Stratifying participants by HIV and recent CD4 cell count, DLCO was significantly lower in the HIV-infected groups with a CD4 cell count <200 (beta-coefficient −11.6, 95% confidence interval [CI] −18.4 to −4.8) and with a CD4 cell count ≥200 cells/μl (beta-coefficient −3.7, 95% CI −6.4 to −0.88) when compared to those without HIV infection.
Table 4.
Prevalence of respiratory symptoms according to HIV status and PFT results
| HIV-infected | HIV-uninfected | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Respiratory symptom | DLCO≤60% | DLCO>60% | p-value | DLCO≤60% | DLCO>60% | p-value |
|
| ||||||
| Usual cough | 43% | 21% | <0.001 | 26% | 18% | 0.2 |
| Usual phlegm | 42% | 28% | 0.02 | 29% | 22% | 0.3 |
| Wheeze | 26% | 25% | 0.8 | 29% | 23% | 0.3 |
| MRC dyspnea≥2 | 29% | 9% | <0.001 | 16% | 9% | 0.17 |
|
| ||||||
| FEV1/FVC<0.70 | FEV1/FVC≥0.70 | p-value | FEV1/FVC<0.70 | FEV1/FVC≥0.70 | p-value | |
|
| ||||||
| Usual cough | 53% | 22% | <0.001 | 21% | 19% | 0.8 |
| Usual phlegm | 59% | 27% | <0.001 | 20% | 25% | 0.5 |
| Wheeze | 43% | 22% | 0.001 | 33% | 22% | 0.1 |
| MRC dyspnea≥2 | 31% | 11% | <0.001 | 11% | 10% | 0.7 |
p-values represent comparisons by PFT strata within HIV-infected or within HIV-uninfected participants.
DLCO results are presented as above or below 60% of predicted normal.
Association of PFT results with respiratory symptoms
To assess the clinical significance of abnormal PFT results with patient-reported outcomes, we compared the association between respiratory symptoms and abnormalities in PFTs. Compared to HIV-uninfected participants, HIV-infected participants were significantly more likely to report usual cough (28 vs. 20%, p=0.03) and usual phlegm (33% vs. 24%, p=0.03), whereas the prevalence of wheezing was similar (26% vs 24%, p=0.7). HIV-infected participants tended to have greater dyspnea on exertion (15 vs 10% with MRC dyspnea score of 2 or greater, p=0.058). HIV-infected participants with abnormal lung function defined by an FEV1/FVC ratio <0.70 or a DLCO ≤60% of predicted were significantly more likely to have usual cough, phlegm and dyspnea compared to HIV-infected participants who did not meet criteria for fixed airflow obstruction or who had a higher DLCO (Table 4). In contrast, the difference between the proportions of HIV-uninfected participants with respiratory symptoms according to PFT results was not as marked. Restricted to those with a DLCO ≤60% predicted, HIV-infected compared to HIV-uninfected participants were more likely to have cough (42% vs. 26%, p=0.04), although the difference in those with phlegm (42% vs. 29%, p=0.1) and dyspnea (29% vs. 16%, p=0.1) was not statistically significant. Restricted to those with an FEV1/FVC<0.70, HIV-infected compared to HIV-uninfected participants were significantly more likely to have cough (53% vs. 21%, p=0.001), phlegm (58% vs. 20%, p<0.001) and dyspnea (31% vs. 11%, p=0.02), whereas the proportions with wheezing were similar. Among persons without pulmonary function abnormalities, the prevalence of respiratory symptoms was equivalent by HIV status.
DISCUSSION
In the first large-scale and multicenter cohort to examine pulmonary function including DLCO in HIV-infected and HIV-uninfected men in the current ART era, we found that HIV infection was an independent predictor of decreased DLCO after accounting for smoking and other potential confounders. Additionally, markers of greater HIV severity, including higher HIV viral load, recent CD4 cell count, and a nadir CD4 cell count <200 were all associated with lower DLCO. In multivariable models, DLCO was significantly lower in the HIV-infected groups with a CD4 cell count <200 and with a CD4 cell count ≥200 cells/μl when compared to those without HIV infection. Measures of airflow and the likelihood of airflow obstruction were similar in HIV-infected and HIV-uninfected participants, although the proportion with airflow obstruction tended to be greater in those with a CD4 cell count <200. Notably, our cohort had a high prevalence of smoking, even in the HIV-uninfected participants that was substantially higher than the general population.
The prevalence of a decreased DLCO, despite the fact that 89% of the HIV-infected men were on ART, was striking. A DLCO less than 60% of predicted, which is consistent with moderate to severe impairment, was significantly more likely in HIV-infected compared to uninfected men, and was present in 30% of those with HIV. The prevalence of a reduced DLCO is important to consider in light of published guidelines and common clinical practice that often involve only screening spirometry to evaluate for respiratory disease.31 In our cohort, spirometry alone would have missed a substantial gas exchange abnormality in many of the HIV-infected and uninfected participants; considering HIV-infected individuals, 61% of those with moderate to severely reduced DLCO had an isolated reduction without accompanying airflow obstruction.
These data are significant because both FEV1 and DLCO are important clinical indicators of patient outcomes. The FEV1, in particular, is an established measure associated with increased mortality.31, 38 However, a DLCO <85% predicted, in the absence of other pulmonary impairment, is also associated with increased mortality in the general population.39 Of PFT measures, DLCO was most closely associated with mortality in a recent study.40 DLCO has been predictive of morbidity and mortality in a number of specific pulmonary diseases as well.41-43
However, the clinical implications of reduced pulmonary function are not fully understood in HIV-infected populations. We found that HIV-infected patients with a low DLCO or airflow obstruction were significantly more likely to have increased respiratory symptoms, including usual cough and phlegm, as well as dyspnea compared to HIV-infected patients with higher DLCO and with no airflow obstruction. Further, respiratory symptoms were notably more prominently expressed among HIV-infected patients with abnormal pulmonary function than among HIV-uninfected patients. A DLCO ≤80% predicted has been associated with a greater prevalence of respiratory complaints in HIV-infected persons in another study.14 In addition, a reduced DLCO could play a role in the decreased functional capacity44 and aerobic capacity in HIV-infected persons.45 Additional longitudinal studies of patient outcomes are required to fully evaluate the significance of our findings.
There are multiple diseases that can contribute to impaired DLCO. The differential diagnosis includes emphysema, interstitial lung diseases (ILD), pulmonary vascular diseases (such as pulmonary arterial hypertension (PAH), chronic thromboembolic disease, or from injection drug use), as well as non-pulmonary diseases, such as congestive heart failure (CHF) with pulmonary edema. Our data suggest that the impairment in DLCO may not be attributable solely to emphysema, given that many participants had an isolated reduction in DLCO with normal airflow. Nonetheless, emphysema may still be a significant contributor, as a greater prevalence of radiographic emphysema in the absence of airflow obstruction among HIV-infected compared to uninfected persons has been reported early in the combination ART era.16, 46, 47 An increased risk of ILD,10 PAH,13, 48 and CHF49 have also been reported in HIV-infected persons. Combined emphysematous and interstitial changes, as a consequence of smoking may also occur, 50 although the extent to which this manifests among HIV-infected persons has not been explored. Of note, patients in the EXHALE study were more likely to have a lower DLCO compared to MACS participants; this finding may reflect unmeasured confounding related to socioeconomic status, occupational or environmental exposures, and extent of drug use that Veterans may share in common, but that differ from MACS participants. Additional work understanding which disease processes are causing the decrease in DLCO will have significant implications for appropriate management of HIV-infected persons.
The pathologic mechanisms by which HIV may contribute independently to a decrease in DLCO are uncertain. The increased inflammation observed in HIV-infected individuals, particularly when more severely immunosuppressed,51, 52 may play a significant role. Higher circulating peripheral neutrophil counts, reflective of greater inflammation, have been associated with a reduced DLCO in the general population.33 Lymphocytic alveolitis in HIV-infected persons may play a role in reduced DLCO, particularly among those with lower CD4 cell counts.53 Upregulation of matrix metalloproteinase expression in alveolar macrophages may be particularly important in HIV-associated emphysema.54 In addition, the presence of subacute infection or greater likelihood of colonization with microorganisms may contribute to the pathogenesis of increased lung inflammation and impaired DLCO. While prior studies have found that a declining DLCO in HIV-infected patients with acute respiratory symptoms is suggestive of PCP,55 it is important to note that our population consisted of stable HIV-infected outpatients without a recent change in respiratory symptoms.
The association of HIV infection with airflow obstruction remains controversial. Although we have previously shown that COPD prevalence is increased in HIV-infected persons based on ICD-9 or self-reported diagnoses,56 there was no difference in the prevalence of airflow obstruction defined by spirometry when comparing HIV-infected to HIV-uninfected men in our current cohort. These results are similar to work by Drummond and colleagues,12 although these authors did find an association between airflow obstruction and substantially higher HIV viral load, defined as >200,000 copies/ml, suggesting a potential pathogenetic role of HIV. There were too few participants with an HIV viral load >200,000 copies/ml (n=2) in our cohort to use this stratification, which may account for the differences between these two studies. Other studies have found an association between airflow obstruction and ART use.14, 20 Because 89% of the HIV-infected patients were on ART, we had limited power to examine the relationship between ART and pulmonary function.
In contrast to our results and those of Drummond, Madeddu and colleagues from Italy found a lower FEV1, FEV1/FVC ratio, and increased prevalence of airflow obstruction in HIV-infected individuals compared to age and smoking-matched HIV-uninfected controls (23% vs. 8%, p=0.008) that remained significant in multivariable analysis.15 The majority of these patients had undetectable viral load. While residual confounding or other unmeasured factors between our cohorts may explain these differences, the Italian cohort was significantly younger, with a mean age of 42. One possible explanation is that HIV infection may contribute to a decline in lung health through accelerated cellular senescence, resulting in an earlier presentation of COPD that was more pronounced in their younger cohort. It is also possible that in our older cohort, men with more severely compromised lung function have already died, minimizing differences between those with and without HIV, as the mortality rate among HIV-infected individuals still exceeds that of the uninfected.
Our study has a number of strengths. This is the first study to compare results of pre- and post-bronchodilator spirometry and DLCO in HIV-infected and uninfected men in the current era. The participants were enrolled from the MACS and VACS, two large and multicenter ongoing HIV cohort studies with carefully characterized clinical data. PFTs were measured systematically in a standardized fashion. Our cohort was also racially and geographically diverse, with centers located throughout the US.
Our study has certain limitations. Due to technical issues, we were unable to systematically obtain measures of carboxyhemoglobin at all sites and therefore were unable to adjust the DLCO for carboxyhemoglobin. However, all participants were asked to refrain from smoking for at least 6 hours prior to testing, DLCO was corrected for hemoglobin, and adjusted for other potential confounders in multivariable models. Pulmonary function testing was of necessity performed at multiple labs, but all laboratories operated per ATS standards, trained personnel obtained PFTs at each site, and we included an adjustment for clinical center.29, 30, 57 Finally, our results may not be generalizable to other studies or populations, as our cohort was a research cohort restricted to men.
In summary, HIV-infected men had a significantly decreased DLCO compared to HIV-uninfected men even after adjusting for smoking and other potential confounders, and despite widespread use of ART. Overall, 30% of HIV-infected men had a DLCO ≤60% of predicted normal, with the majority having an isolated reduction in diffusing capacity. Furthermore, a lower CD4 cell count was associated with a greater likelihood of a decreased DLCO. An impaired DLCO and fixed airflow obstruction were also more likely to be associated with chronic cough, phlegm and dyspnea in HIV-infected compared to uninfected participants. Whether the decreased DLCO is due to emphysema or other processes such as pulmonary vascular disease or interstitial lung disease requires further evaluation, and will have significant implications for patient care and for our understanding of the pathogenesis of HIV-related lung disease.
Supplementary Material
Table 3.
Multivariable linear regression model for risk factors associated with percent predicted DLCO
| Risk factor | Beta coefficient | 95% CI | p-value |
|---|---|---|---|
| Race/ethnicity | |||
| Black | 1.0 | −4.4 to 6.6 | 0.7 |
| Hispanic/other | −5.8 | −9.4 to −2.1 | 0.002 |
| HIV status and CD4 cell count | |||
| HIV uninfected (referent) | -- | -- | -- |
| HIV infected, CD4 ≤200 | −3.7 | −6.5 to −0.88 | 0.01 |
| HIV infected, CD4 <200 | −11.6 | −18.4 to −4.8 | 0.001 |
| Smoking exposure | |||
| Never (referent) | -- | ||
| >0 to <35 pack-years | −4.8 | −8.1 to −1.5 | 0.004 |
| ≥35 pack-years | −15.3 | −19.3 to −11.3 | <0.001 |
| EXHALE (VACS) site | −7.3 | −10.9 to −3.7 | <0.001 |
Percent predicted DLCO values were also corrected for hemoglobin.
CI = confidence interval
Acknowledgments
Funding Sources:
NIH/NHLBI R01-HL090342 (KC); K24-HL087713 (LH); HL090335 (LH); R01-HL090313 (PTD), R01-HL090483 (GK), R01-HL090316 (WNR), R01-HL090331 (BWT), R01-HL090339 (AM).
Footnotes
This work was presented, in part, at the Conference on Retrovirus and Opportunistic Infections (CROI), Seattle, WA, 2012.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Brettle R, Kirk O, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002 Aug 16;16(12):1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 3.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite RS, Justice AC, Chang CC, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005 Aug;118(8):890–898. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 6.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009 Aug 24;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis. 1990 Jun;141(6):1582–1598. doi: 10.1164/ajrccm/141.6.1582. [DOI] [PubMed] [Google Scholar]
- 8.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990 May;141:1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JM, Hansen NI, Lavange L, et al. Respiratory disease trends in the Pulmonary Complications of HIV Infection Study cohort. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1997 Jan;155(1):72–80. doi: 10.1164/ajrccm.155.1.9001292. [DOI] [PubMed] [Google Scholar]
- 10.Crothers K, Huang L, Goulet JL, et al. HIV Infection and Risk for Incident Pulmonary Diseases in the Combination Antiretroviral Therapy Era. Am J Respir Crit Care Med. 2011 Feb 1;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirk GD, Merlo C, P OD, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007 Jul 1;45(1):103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012 Apr;67(4):309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc. 2011 Jun;8(3):308–312. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary Function Abnormalities in HIV-infected Patients During the Current Antiretroviral Therapy Era. Am J Respir Crit Care Med. 2010 Jun;3 doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madeddu G, Fois AG, Calia GM, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2012 Sep 13; doi: 10.1007/s15010-012-0330-x. [DOI] [PubMed] [Google Scholar]
- 16.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000 Mar 7;132(5):369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 17.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000 Oct;118(4):1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 18.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012 May 15;26(8):1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003 Jun;123(6):1977–1982. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
- 20.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4(7):e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010 Sep 15;182(6):790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell DM, Fleming J, Pinching AJ, et al. Pulmonary function in human immunodeficiency virus infection. A prospective 18-month study of serial lung function in 474 patients. Am Rev Respir Dis. 1992 Sep;146(3):745–751. doi: 10.1164/ajrccm/146.3.745. [DOI] [PubMed] [Google Scholar]
- 23.Rosen MJ, Lou Y, Kvale PA, et al. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. American journal of respiratory and critical care medicine. 1995 Aug;152(2):738–745. doi: 10.1164/ajrccm.152.2.7633736. [DOI] [PubMed] [Google Scholar]
- 24.Crothers K, Thompson BW, Burkhardt K, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011 Jun;8(3):275–281. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crothers K, Kleerup E, Wongtrakool C, et al. HIV Infection Is Associated with Impaired Pulmonary Diffusing Capacity; Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections2012; Seattle, WA. [Google Scholar]
- 26.Detels R, Jacobson L, Margolick J, et al. The multicenter AIDS Cohort Study, 1983 to. Public Health. 2012 Mar;126(3):196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006 Aug;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 29.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005 Oct;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2013 [Updated 2013] http://www.goldcopd.org/
- 32.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 33.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med. 1996 Feb;153(2):656–664. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005 Nov;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 35.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978 Dec;118:1–120. 6 Pt 2. [PubMed] [Google Scholar]
- 36.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999 Jul;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959 Aug 29;(5147):257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003 May;58(5):388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neas LM, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol. 1998 Jun 1;147(11):1011–1018. doi: 10.1093/oxfordjournals.aje.a009394. [DOI] [PubMed] [Google Scholar]
- 40.Ward H, Cooper B, Miller MR. Validation of lung function prediction equations from patient survival data. Eur Respir J. 2012 May;39(5):1181–1187. doi: 10.1183/09031936.00104911. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson MK, Dignam JJ, Siddique J, Vigneswaran WT, Celauro AD. Diffusing capacity predicts long-term survival after lung resection for cancer. Eur J Cardiothorac Surg. 2012 May;41(5):e81–86. doi: 10.1093/ejcts/ezs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra S, Shah SJ, Thenappan T, Archer SL, Rich S, Gomberg-Maitland M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J Heart Lung Transplant. 2010 Feb;29(2):181–187. doi: 10.1016/j.healun.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson MK, Gaissert HA, Grab JD, Sheng S. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg. 2009 Dec;138(6):1297–1302. doi: 10.1016/j.jtcvs.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 44.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the veterans aging cohort study. AIDS Patient Care STDS. 2011 Jan;25(1):13–20. doi: 10.1089/apc.2010.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006 Nov;22(11):1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 46.Diaz PT, King MA, Pacht ER, et al. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999 Jul;160(1):272–277. doi: 10.1164/ajrccm.160.1.9812089. [DOI] [PubMed] [Google Scholar]
- 47.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992 Jan 15;116(2):124–128. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- 48.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA. 2008 Jan 23;299(3):324–331. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 49.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011 Apr 25;171(8):737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011 Mar 10;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012 Jul;55(1):126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twigg HL, Soliman DM, Day RB, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999 May;159:1439–1444. doi: 10.1164/ajrccm.159.5.9808031. 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- 54.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol. 2009 Oct;86(4):913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stansell JD, Osmond DH, Charlebois E, et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1997;155(1):60–66. doi: 10.1164/ajrccm.155.1.9001290. [DOI] [PubMed] [Google Scholar]
- 56.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006 Nov;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 57.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005 Jul;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


