Abstract
Study Objective
To determine whether the methacholine challenge method used for albuterol can be applied to assess long-acting β2-adrenergic agonist (LABA) bioequivalence, which would require a sufficiently steep dose-response curve.
Design
Prospective, unblinded, randomized, 2-way crossover study.
Setting
University medical center clinical research laboratory.
Patients
Ten adults, aged 21–58 years, with mild asthma (forced expiratory volume in 1 sec [FEV1] ≥ 70% predicted) and a baseline provocational concentration of methacholine required to decrease FEV1 by 20% (PC20) of 4 mg/ml or less completed the study.
Intervention
Patients were randomized to receive a single dose of either 12 or 24 μg of formoterol delivered by a dry powder inhaler; 3–7 days later, at the same time of day, they received the other dose.
Measurements and Main Results
The FEV1 was measured before and 1 hour after dosing, followed by performance of a methacholine challenge. Statistical analysis was performed by the 2-sample regression method for crossover studies. The dose-response curve for bronchodilatation was flat; the mean ± SD increase in FEV1 after formoterol 12 and 24 μg was 14 ± 5% and 14 ± 8%, respectively (p>0.05). In contrast, the geometric mean PC20 (95% confidence interval) was 7 mg/ml (2–22 mg/ml) after the 12-μg dose and 16 mg/ml (5–45 mg/ml) after the 24-μg dose (p<0.001).
Conclusion
Bioassay by methacholine challenge will be useful for bioequivalence studies of LABAs. A sample of at least 28 patients will be required for formoterol when methacholine challenge is performed in an optimal manner. The sample size may differ for other LABAs.
Keywords: methacholine, bioassay, formoterol, long-acting β2-adrenergic agonist, LABA
Inhaled long-acting β2-adrenergic agonists (LABAs) are recommended in the treatment of asthma as maintenance therapy when combined with an inhaled corticosteroid or when used alone to prevent exercise-induced bronchospasm.1 In addition, they are recommended alone or in combination with a long-acting inhaled anti-cholinergic or an inhaled corticosteroid as maintenance therapy for chronic obstructive pulmonary disease.2 Formoterol, salmeterol, and arformoterol are the only LABAs currently available in the United States, and they are marketed in various formulations alone or in combination with an inhaled corticosteroid.
Although patents are still in force for several drug products containing a LABA (i.e., drug and device), the patents on the drug substance (i.e., the drug alone) have expired. Therefore, pharmaceutical firms have developed or are interested in developing alternative delivery devices for two of these drugs, and in some cases attempts are being made to develop a generic version.
Obtaining U.S. Food and Drug Administration (FDA) approval of a generic LABA includes a requirement for an in vivo dose-response study to establish bioequivalence,3 and under varying circumstances, such a study is required by the European Medicines Agency4 and Canada’s Human Protection Branch.5 The purpose of such a study is to determine whether a test and reference product meet equivalence criteria based on a bioassay sensitive to differences in dose. If the study design is not sufficiently sensitive to detect a difference in two doses of the reference product, then it would not be possible to detect a difference between the test and reference products in the amount of drug delivered to the airways.6
In the United States, pharmacokinetic studies are not sufficient, by themselves, to establish a dose-response relationship,3 since plasma concentrations have not been proven to indicate where in the airways the drug is delivered and do not distinguish drug absorbed from the lungs and drug absorbed from the swallowed portion of the dose in the gastrointestinal tract.7 Thus, a pharmacodynamic end point is required to demonstrate the amount of drug delivered to the relevant receptors in the airways.8
Conventional bronchodilator studies, where the forced expiratory volume in 1 second (FEV1) is measured after a dose over time, have been used, but often the dose-response curve with this pharmacodynamic end point is relatively flat.9, 10 That is, during typical daytime studies in patients with stable asthma, one actuation may produce the same improvement in FEV1 as two actuations, since the response lies on the upper plateau portion of the dose-response curve.10
Dose-response studies of albuterol have demonstrated that bronchoprovocation with histamine or methacholine are the most sensitive methods of determining differences in doses when delivered by generic and brand chlorofluorocarbon (CFC) metered-dose inhalers (MDIs),11 between a CFC MDI and dry powder inhaler (DPI),12 and between CFC and hydrofluoroalkane (HFA) MDIs.13
Bronchoprovocation with methacholine has been used to compare the intensity and/or duration of formoterol delivered by a DPI or MDI.14–16 However, none of these studies has provided sufficient information to determine whether methacholine challenge can detect a 2-fold or smaller difference in dose and, thus, be useful as a bioassay to determine bioequivalence. Also, they do not provide information on the sample size required for a bioequivalence study. Since the Aerolizer (Foradil Aerolizer; Schering Corp., Kenilworth, NJ) is the only DPI formulation of formoterol available in the United States, it will serve as the reference product for subsequent studies of new delivery devices or generic formulations of this drug.
Thus, the purpose of this study was to determine whether a methacholine-based clinical bioassay can be used to evaluate bioequivalence of formoterol (Aerolizer) and other formoterol-containing formulations. This would require that a highly significant dose-response relationship be present, which, in turn, requires a sufficiently steep dose-response slope relative to the response variability observed in the study. In addition, we propose possible improvements in study design that may further increase the statistical power of these bioequivalence methods.
Methods
Patients
The study protocol was approved by the University of Florida Institutional Review Board (IRB), and all patients read and signed an informed consent form before any procedures were begun. Since the formoterol higher dose of 24 μg was above the FDA-approved dose of 12 μg, and methacholine concentrations were above labeled concentrations, the protocol was submitted to the FDA and approved as an investigator-sponsored investigational new drug application (IND no. 101,246).
Patients aged 18–60 years with mild stable asthma were eligible for study inclusion. The protocol called for patients to have a baseline FEV1 of 70% or greater and a provocational concentration of methacholine required to decrease FEV1 by 20% (PC20) of 4 mg/ml or less. Patients were required to be nonsmokers for at least 1 year and to have had a smoking history of not more than 10 pack-years. Women of child-bearing age were required to have had a negative pregnancy test, were not breastfeeding, and were using an acceptable method of contraception. All patients were required to be able to perform acceptable and reproducible spirometry according to the American Thoracic Society (ATS)–European Respiratory Society (ERS) guidelines.17 The inhalation technique of the DPI (Aerolizer) was reviewed on each study day and performed in accordance with the product’s package insert. Patients were allowed to use an oral inhaled or intranasal corticosteroid as long as the dosage was stable for at least 4 weeks and entrance criteria were met.
Patients were excluded if they had a severe asthma exacerbation requiring hospitalization in the previous 12 months, if they required a short course of systemic corticosteroids for asthma in the previous 4 weeks, if they had a respiratory infection in the previous 4 weeks or during the study, or if they were unable to withhold drugs that might interfere with the methacholine challenge for the recommended times.18 Patients were also excluded if they had a history of any pulmonary disease other than asthma, or history of clinically significant cardiovascular, renal, neurologic, liver, or endocrine dysfunction. Patients with well-controlled hypertension, hypercholesterolemia, or diabetes mellitus were not excluded as long as their maintenance drug therapy would not interfere with the methacholine challenge (e.g., β-blockers).
Study Design
This prospective, unblinded, randomized, 2-way crossover study consisted of three visits. All study visits were either in the morning or afternoon, at the same time of day for each patient. The first visit was a screening visit to determine study eligibility and baseline PC20, followed by two subsequent visits for study drug randomization and administration. The second and third visits were each scheduled 3–7 days after the previous visit. At the second visit, patients were randomized to receive a single dose of either 12 or 24 μg of formoterol delivered by a dry powder inhaler; they received the other dose 3–7 days later at the third visit. During the second and third visits, vital signs and spirometry were measured before administration of study drug. After the initial screening visit, FEV1 had to be 60% or greater and within 10% of the previous visit on each study day and within 20% of the screening visit. Albuterol was not used for at least 6 hours, and LABAs and leukotriene modifiers were not used for at least 36 hours before each challenge. If a patient was taking an inhaled corticosteroid, the morning dose was withheld. Patients then inhaled the single dose of formoterol 12 or 24 μg. The FEV1 and methacholine challenge were performed 1 hour after dosing. At the conclusion of the challenge, inhaled albuterol was administered until FEV1 returned to 90% or greater of that study day’s baseline value.
Study Drug Administration
Formoterol fumarate was delivered by a DPI, 12 μg/capsule (Foradil Aerolizer). Patients inhaled rapidly and deeply through the inhaler mouthpiece and held their breath for 10 seconds. The capsule was inspected; if there was any powder remaining, the inhalation process was repeated immediately. Patients inhaled one capsule for the 12-μg nominal dose and two capsules for the 24-μg nominal dose.
Spirometry
Spirometry was performed with a pneumotach spirometer (KoKo; Quantum Research, Louisville, CO). Spirometry was performed, at least in triplicate, according to standards set by the ATS-ERS Task Force.17
Methacholine Challenge
Methacholine challenges were performed in accordance with the ATS guideline for the five-breath dosimeter method (KoKo Digidoser; Quantum Research).18 The same nebulizer was used on all three visits for a given patient. A total of three nebulizers were used in the study. Methacholine dilutions of 0.0625, 0.25, 1, 4, 16, and 64 mg/ml were prepared by the Investigational Drug Service of Shands Hospital Pharmacy at the University of Florida by using an aseptic technique, from 100-mg vials of FDA-approved methacholine chloride powder (Provocholine; Methapharm, Brantford, Canada). Each concentration contained a final volume of 2 ml dispensed in unit-dose syringes and was stored frozen for up to 5 months.19 The solutions were allowed to warm to room temperature before use, and any unused syringes were discarded after the challenge.
Data Analysis
Bronchodilator response to each dose of formoterol was calculated as the percent change in FEV1 from the study day baseline value to 1 hour after administration, before the methacholine challenge began. The 1-hour postdose PC20 was interpolated by the KoKo software program from the log-linear slope of the last two concentrations and percentage decrease in FEV1.
Statistical Analysis
No formal sample size calculation was performed. However, based on previous experience,20 it was judged that completion by 10 patients would be sufficient to meet the study objectives.
The regression method of Shuster was used to compare the natural logarithm of postdose PC20 values.21 This method takes the period 2 less period 1 difference (irrespective of treatment assignment) and compares the two treatment orderings. This effect size estimate is superior to the 1-sample t test in that it is unbiased when the actual sizes assigned to the orderings differ, is more efficient, and adjusts for carryover effects. The dependent primary variable was the difference in the change in natural log PC20 (period 2 less period 1). The FEV1 was compared in the same way, except logs were not used. A p value less than 0.05 (2-sided) was considered to indicate a statistically significant difference.
Results
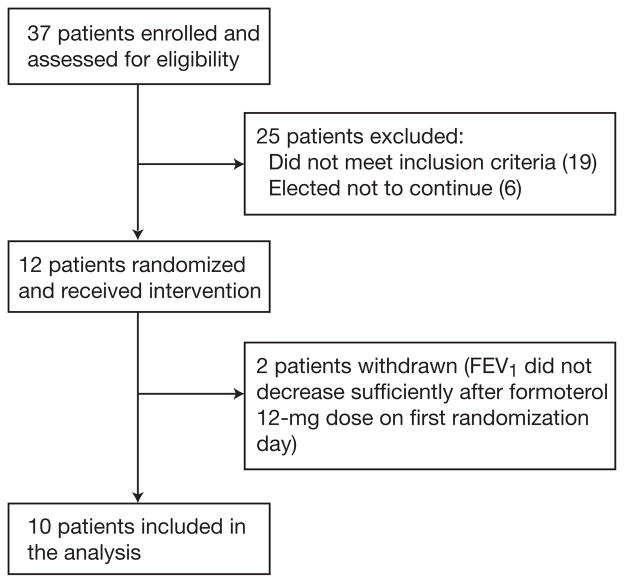
Between April and November 2008, 37 patients were enrolled in the study (i.e., they signed the informed consent form), but 25 were excluded during the screening visit. In two patients (patient nos. 13 and 31), the FEV1 decreased only 17% during the screening visit. Therefore, the PC20 was extrapolated.22 Since the screening PC20 was greater than 4 mg/ml in those two patients (4.7 mg/ml each) and did not strictly meet the inclusion criteria of 4 mg/ml or less, the University of Florida IRB granted an exception to include them in the study. Thus, 12 patients were randomized. Of these patients, in two who received the 12-μg dose of formoterol on the first randomization study day, the FEV1 did not decrease sufficiently to allow determination of PC20, so both of these patients were withdrawn since it was unlikely that a PC20 could be measured after the higher dose. In four patients, PC20 could not be measured after the 24-μg dose, so a censored PC20 of 64 mg/ml was assigned. Thus, 10 patients (aged 21–58 yrs) completed the study and were included in the analysis (Figure 1).
Figure 1.
Schematic of the patient selection process. FEV1 = forced expiratory volume in 1 second.
At the time of study enrollment, all patients required asthma drugs: four used a combination product containing fluticasone and salmeterol, two used fluticasone alone, and one used budesonide DPI; all patients used an albuterol MDI as needed (Table 1). The mean ± SD baseline FEV1 was 2.6 ± 0.8 L (79.5 ± 6.8% predicted), and the baseline geometric mean PC20 was 1.1 mg/ml (95% confidence interval [CI] 0.56–2.2 mg/ml; Table 1).
Table 1.
Demographics, FEV1, and Methacholine PC20 During the Screening Visit for the 10 Study Patients
| Patient No. | Sex/Age (yrs) | FEV1, L (% predicted) | PC20 (mg/ml) | Previous Therapy |
|---|---|---|---|---|
| 3 | M/29 | 4.07 (89) | 0.61 | Fluticasone-salmeterol, albuterol p.r.n. |
| 7 | F/26 | 2.40 (82) | 0.62 | Albuterol p.r.n. |
| 12 | F/52 | 1.85 (75) | 0.42 | Fluticasone, albuterol p.r.n. |
| 13 | M/25 | 3.17 (78) | 4.70 | Fluticasone-salmeterol, albuterol p.r.n. |
| 20 | F/29 | 2.79 (90) | 0.61 | Albuterol p.r.n. |
| 21 | F/54 | 1.91 (78) | 1.38 | Fluticasone-salmeterol, albuterol p.r.n. |
| 29 | F/49 | 1.89 (69) | 0.34 | Fluticasone-salmeterol, albuterol p.r.n. |
| 30 | M/25 | 3.35 (84) | 2.27 | Budesonide, albuterol p.r.n. |
| 31 | F/58 | 1.71 (78) | 4.70 | Fluticasone, albuterol p.r.n. |
| 36 | M/21 | 3.08 (72) | 1.24 | Albuterol p.r.n. |
| Mean ± SD | 36.8 ± 14.5 | 2.6 ± 0.8 (79.5 ± 6.8) | ||
| Geometric mean | 1.1 (95% CI 0.56–2.2) |
FEV1 = forced expiratory volume in 1 second; PC20 = provocational concentration of methacholine required to decrease FEV1 by 20%; CI = confidence interval.
Bronchodilator Response
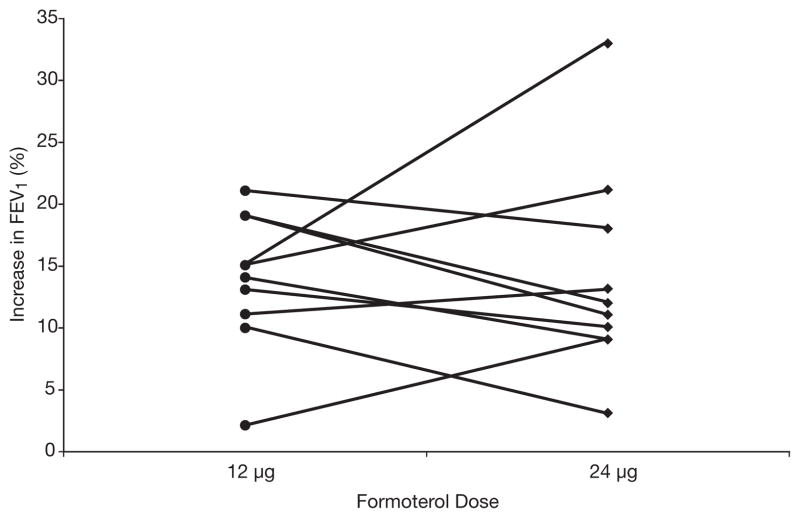
Seven patients demonstrated an increase in FEV1 of 12% or greater after 12 μg of formoterol, and five patients demonstrated this level of response after 24 μg (Table 2). There was no significant dose-response relationship; the mean ± SD increase in FEV1 was 14 ± 5% after 12 μg and 14 ± 8% after 24 μg (Figure 2).
Table 2.
Bronchodilator Response After the 12- and 24-μg Doses in the 10 Study Patients
| Patient No. | 12-μg Dose
|
24-μg Dose
|
||||
|---|---|---|---|---|---|---|
| Baseline FEV1, L (% predicted) | Postdose FEV1, L (% predicted) | Postdose % Increase in FEV1 | Baseline FEV1, L (% predicted) | Postdose FEV1, L (% predicted) | Postdose % Increase in FEV1 | |
| 3 | 3.97 (87) | 4.74 (104) | 19 | 4.22 (92) | 4.71 (103) | 12 |
| 7 | 2.73 (93) | 3.13 (106) | 15 | 2.69 (90) | 3.25 (109) | 21 |
| 12 | 1.72 (70) | 1.94 (78) | 13 | 1.91 (77) | 2.10 (85) | 10 |
| 13 | 3.23 (79) | 3.59 (88) | 11 | 3.17 (78) | 3.58 (88) | 13 |
| 20 | 2.51 (80) | 2.98 (95) | 19 | 2.74 (88) | 3.05 (98) | 11 |
| 21 | 1.90 (78) | 2.09 (86) | 10 | 1.89 (76) | 1.94 (80) | 3 |
| 29 | 2.08 (76) | 2.13 (78) | 2 | 2.06 (75) | 2.25 (82) | 9 |
| 30 | 3.28 (82) | 3.74 (93) | 14 | 3.40 (85) | 3.71 (93) | 9 |
| 31 | 1.55 (71) | 1.88 (86) | 21 | 1.55 (71) | 1.83 (84) | 18 |
| 36 | 2.58 (60) | 2.97 (69) | 15 | 2.75 (64) | 3.66 (85) | 33 |
|
| ||||||
| Mean ± SD | ||||||
|
| ||||||
| 2.55 ± 0.77 (78 ± 9) | 2.92 ± 0.93 (88 ± 12) | 14 ± 5 | 2.64 ± 0.82 (80 ± 9) | 3.01 ± 0.95 (91 ± 10) | 14 ± 8a | |
FEV1 = forced expiratory volume in 1 second.
p>0.05 compared with the 12-μg dose.
Figure 2.
Bronchodilator response measured as percent increase in forced expiratory volume in 1 second (FEV1) 1 hour after administration of single doses of formoterol 12 μg and 24 μg, on separate days, at the same time of day, in the 10 patients who completed the study. No significant dose-response relationship was noted; the mean ± SD increase in FEV1 was 14 ± 5% after the 12-μg dose and 14 ± 8% after the 24-μg dose (p>0.05).
Methacholine Response
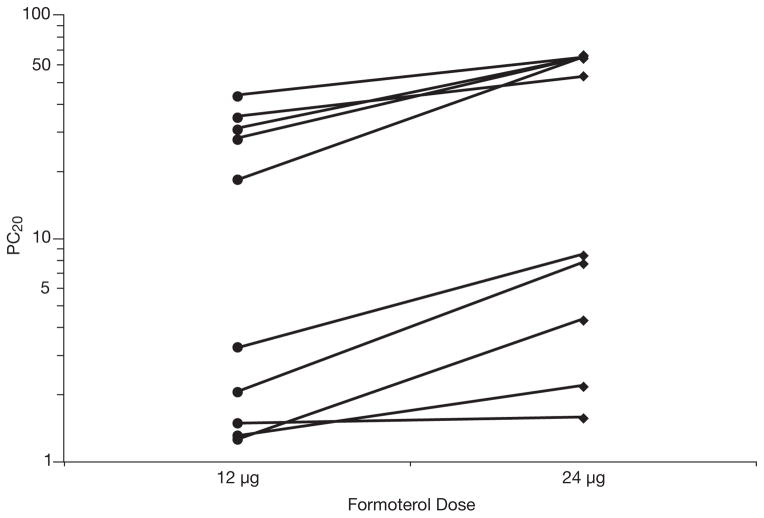
The PC20 was increased from the screening value in nine patients after 12 μg and in all patients after 24 μg (Table 3). There was a significant dose-response relationship; the geometric mean was 7 mg/ml (95% CI 2–22 mg/ml) after 12 μg and 16 mg/ml (95% CI 5–45 mg/ml) after 24 μg (p<0.001; Figure 3). The PC20 ratio after 24 μg compared with after 12 μg was increased in all but one patient (patient no. 29), with a geometric mean of 2.2-fold (95% CI 1.6–3.0-fold; Table 3). When compared with the screening values, the PC20 increased 6-fold (95% CI 2–16-fold) after 12 μg and 14-fold (95% CI 6–33-fold) after 24 μg (Table 3). Only one patient (patient no. 36) did not demonstrate an increase in PC20 from the screening visit after 12 μg, but this patient did demonstrate a 3.5-fold increase from screening after 24 μg.
Table 3.
Response to Methacholine Challenge 1 Hour After Dosing in the 10 Study Patients
| Patient No. | PC20 (mg/ml)
|
PC20 Ratio, 24 μg:12 μg | Fold Change in PC20 from Screening
|
||
|---|---|---|---|---|---|
| 12 μg | 24 μg | 12 μg | 24 μg | ||
| 3 | 1.29 | 2.17 | 1.68 | 2.10 | 3.60 |
| 7 | 27.75 | 64.00 | 2.30 | 44.80 | 103.00 |
| 12 | 2.03 | 7.71 | 3.80 | 4.80 | 18.30 |
| 13 | 43.30 | 64.00 | 1.48 | 9.21 | 13.62 |
| 20 | 34.80 | 52.50 | 1.50 | 57.10 | 86.10 |
| 21 | 3.19 | 8.40 | 2.60 | 2.30 | 6.10 |
| 29 | 1.47 | 1.57 | 1.07 | 4.30 | 4.60 |
| 30 | 30.75 | 64.00 | 2.08 | 13.50 | 28.20 |
| 31 | 18.00 | 64.00 | 3.60 | 3.80 | 13.62 |
| 36 | 1.24 | 4.30 | 3.46 | 1.00 | 3.50 |
|
| |||||
| Geometric Mean (95% confidence interval) | |||||
|
| |||||
| 7 (2–22) | 16 (5–45)a | 2.2 (1.6–3.0) | 6 (2–16) | 14 (6–33) | |
PC20 = provocational concentration of methacholine required to decrease forced expiratory volume in 1 second by 20%.
p≤0.001.
Figure 3.
Provocational concentration of methacholine required to decrease the forced expiratory volume in 1 second by 20% (PC20) measured 1 hour after administration of single doses of formoterol 12 μg and 24 μg, on separate days, at the same time of day, in 10 patients who completed the study. A significant dose-response relationship was noted; the geometric mean (95% confidence interval) was 7 mg/ml (2–22 mg/ml) after the 12-μg dose and 16 mg/ml (5–45 mg/ml) after the 24-μg dose (p<0.001).
None of the patients required prednisone, an emergency department visit, or hospitalization for asthma during the study, and no adverse events were reported.
Discussion
The results of this study demonstrated a highly significant dose-response relationship for formoterol-induced inhibition of methacholine challenge. The geometric mean PC20 increased an average of 2.2-fold between 12 and 24 μg (p<0.001), with only 10 patients completing the study. This indicates that methacholine challenge methods that have been used successfully to assess equivalence of short-acting β2-adrenergic receptor agonist (SABA) formulations12 can be applied to evaluate bioequivalence of LABA formulations.
The statistical power of a bioassay is a function of the ratio of within-patient variability (s), estimated from the root mean squared error of the analysis of variance of log10 PC20, and slope of the dose-response curve (b), estimated by the difference in log10 PC20 divided by the difference in log10 formoterol dose.23 The lower the s:b ratio, the greater the statistical power and the steeper the dose-response curve relative to the response variability. In our study, the s:b ratio was 0.116. This value is considerably lower than in previous studies of albuterol where s:b ratios as high as 1.0 or more have been observed (see Figure 2A in reference 12).
With use of the slope and within-patient variability in our study, generation of simulated data sets suggests that a sample size of 28 patients would be required to determine a relative potency of 0.95 of test and reference products with a 90% CI of 0.80–1.25 (i.e., FDA requirement for bioequivalence3). Several measures were taken to decrease the within-patient variability in PC20 in our study. These included selecting patients with high reactivity to methacholine who could perform reliable and reproducible spirometry, stabilizing the baseline FEV1 before administration of saline, excluding patients with greater than 10% decrease in FEV1 after administration of saline, having the methacholine dilutions prepared by the investigational pharmacy service and freezing the samples until use, and selecting the highest FEV1 after each methacholine concentration rather than the lowest. In addition, the respiratory therapist who performed the challenges was certified in performing spirometry and had extensive training and experience in performing challenges. Without attention to such details, within-patient variability will be much larger and, thus, a larger sample will be required. For example, if the within-patient variability is increased 3-fold, simulations suggest a sample of 75 patients would be required for the same slope, effect size, and CI as in this study. However, the above calculations are based on the Finney straight line analysis with potency on the dose axis, not the response axis.23 This assumes a linear dose-response curve. Hence, lack of linearity would limit the reliability of this power computation. Nevertheless, the data from this study are linear when the screening visit PC20 is taken as a zero dose.
In contrast, the dose-response relationship for bronchodilatation was not significant. After both doses, the mean increase in FEV1 was 14%. This is consistent with previous studies indicating that bronchodilatation often does not distinguish between doses in daytime studies of patients with stable asthma.9, 10–13, 24 Patients were included in this study only if their FEV1 was at least 70% predicted at the screening visit. Thus, on average, the response was on the upper portion of the dose-response curve. That is, the dose producing 50% of the maximum effect (ED50) for bronchodilatation would be expected to be much higher than the ED50 for methacholine.25
No significant correlation was found between FEV1 or PC20 measured during the screening visit and postdose PC20 ratio for 24 μg:12 μg, suggesting that these screening tests may not adequately predict response. Of interest, visual inspection of Figure 3 suggests that there may have been two distinct groups of responders: those who had a large increase in PC20 with both doses (upper group) and those who had a much smaller response (lower group). One possible explanation for the different groups is chance alone, whereas another is downregulation of β-receptors (e.g., tachyphylaxis) from regular use of LABAs or frequent use of as-needed albuterol.26 Unfortunately, we did not capture the frequency of use of as-needed albuterol. We did, however, identify four patients at screening who used a combination inhaled corticosteroid-LABA product before the study. In spite of observing the ATS recommended washout period of 36 hours for the LABA,18 three of these four patients had a postdose PC20 ratio for 24 μg:12 μg of less than 2.0, indicating a blunted dose-response relationship. Tachyphylaxis has been reported after discontinuing a LABA,27 but it is unclear how long it lasts and, therefore, how long the washout period should be.
Although our results indicate that the methods in this study can be used, without modification, to assess equivalence of drug delivery to the lung by different inhaled formoterol formulations, we did learn several lessons that could prove useful in the design of subsequent SABA and LABA bioequivalence studies. First, we propose that the maximum methacholine concentration should be increased to 128 mg/ml to be able to determine PC20 in patients with a greater bronchoprotective effect than 64 mg/ml. This would minimize “top-censoring” of the data. Concentrations as high as 256 mg/ml have been used safely.28 Second, to reduce possible tachyphylaxis of bronchoprotective effects of β-agonists, LABAs should be withheld for a longer period than recommended by ATS (e.g., 3 wks) and patients who require SABAs more frequently than 3 times/week should be excluded. Third, an additional screening day should be added to perform methacholine challenge after the highest dose of reference product that will be used in the study to exclude patients who do not demonstrate a bronchoprotective effect (i.e., a PC20 4-fold higher than after the screening PC20 measured in absence of β2-agonist effect). Such additional screening also will identify the occasional patient in whom a PC20 cannot be determined after the higher reference dose at 128 mg/ml of methacholine.
Increasing the screening PC20 from 4 mg/ml or less to 8 mg/ml or less is likely to decrease screening failures without adversely affecting the outcome. In a subsequent study where this was done, 3 of 21 randomized patients with a PC20 greater than 4 mg/ml but less than or equal to 8 mg/ml would have been unnecessarily excluded (unpublished data, L. Hendeles, 2011). Using the tidal breathing method is another method of decreasing the number of screening failures. One group of investigators found that 25% of patients would have a PC20 greater than 8.0 mg/ml by dosimeter when their PC20 was less than 8.0 mg/ml by the tidal breathing method.29 In addition, tachyphylaxis from frequent use of an SABA may be circumvented by substituting ipratropium HFA MDI for as-needed bronchodilatation, but the onset of action is slower30 and it does not block exercise-induced bronchospasm.31 Tiotropium could be substituted for LABAs, but this drug has an effect on methacholine PC20 for 72 hours,32 which makes its use less practical than simply excluding patients who require combination therapy with an inhaled corticosteroid and LABA.
These recommendations modestly increase the complexity of the study and will increase the number of screening failures, but since this is a clinical bioassay, the extra efforts are likely to enrich the study sample with patients most likely to yield a steep dose-response curve and to decrease within-patient variability.
An additional modification may be needed for future LABA bioequivalence studies. It is likely that FDA and other regulatory agencies will require a second methacholine challenge, 8–12 hours after dosing, to confirm that the duration of action of the test product is similar to the reference product. This second challenge should be added to subsequent studies. In fact, in one recent study of an experimental once-daily LABA, the authors performed repeat challenges at 4, 8, 24, and 32 hours after each dose.28
Conclusion
The results of this study indicate that methacholine bronchoprovocation can successfully be used to assess bioequivalence of pulmonary delivery of formoterol by different DPIs or MDI formulations. Incorporating recommendations from lessons learned from this study may further increase the statistical power of this method. It is likely that this methodology will be useful for other LABAs, such as salmeterol, but sample sizes will be different if either within-patient variability or the slope of the dose-response curve differs substantially from those observed in this study.
Acknowledgments
Supported by an investigator-initiated grant from Teva/IVAX Pharmaceuticals, and from the U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau, Pediatric Pulmonary Center fellowship training grant (T72MC000002), and National Institutes of Health (NIH) Research Facilities Construction Program C06 (grant RR17568). Dr. Shuster is supported by NIH General Clinical Research Center grants (M01RR00082 and U54RR025208).
The authors thank Sue McGorray, Ph.D., Division of Biostatistics, University of Florida, for performing the s:b ratio calculations, and Harold Iverson, Ph.D., Teva Pharmaceuticals, for performing the sample size simulations based on the Finney method. We also thank our study coordinators, Carmen Lowell, R.R.T., and Alice Boyette, L.P.N., for their meticulous conduct of the study.
References
- 1.National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: guidelines for the diagnosis and management of asthma. [Accessed October 4, 2010];Full report. 2007 Available from www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 2.National Heart, Lung, and Blood Institute. [Accessed October 4, 2010];Global initiative for chronic obstructive lung disease. Updated 2009. Available from http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2003.
- 3.Adams WP, Ahrens RC, Chen M-L, et al. Demonstrating bioequivalence of locally acting orally inhaled drug products (OIPs): workshop summary report. J Aerosol Med. 2010;23:1–29. doi: 10.1089/jamp.2009.0803. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency, Committee for Medicinal Products for Human Use. [Accessed October 5, 2010];Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003551.pdf.
- 5.Health Products and Food Branch, Health Canada. [Accessed December 2, 2010];Scientific advisory committee on respiratory and allergy therapies (SAC-RAT) 2009 Nov 13; Available from http://www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/sci-com/resp_allerg/sacrat_rop_ccstrta_crd_2009-11-13-eng.php.
- 6.Ahrens RC. On comparing inhaled β-adrenergic agonists. Ann Allergy. 1991;67:296–8. [PubMed] [Google Scholar]
- 7.Borgström L, Nilsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–70. doi: 10.1023/a:1015951402799. [DOI] [PubMed] [Google Scholar]
- 8.Rogers DF, Ganderton D. Determining equivalence of inhaled medications. Respir Med. 1995;89:253–61. doi: 10.1016/0954-6111(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 9.Blake KV, Hoppe M, Harman E, et al. Relative amount of albuterol delivered to lung receptors from a metered-dose inhaler and nebulizer solution: bioassay by histamine bronchoprovocation. Chest. 1992;101:309–15. doi: 10.1378/chest.101.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Hendeles L, Beaty R, Ahrens R, et al. Response to inhaled albuterol during nocturnal asthma. J Allergy Clin Immunol. 2004;113:1058–62. doi: 10.1016/j.jaci.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 11.Stewart BA, Ahrens RC, Carrier S, et al. Demonstration of in vivo bioequivalence of a generic albuterol metered-dose inhaler to Ventolin. Chest. 2000;117:714–21. doi: 10.1378/chest.117.3.714. [DOI] [PubMed] [Google Scholar]
- 12.Ahrens RC, Hendeles L, Clarke WR, et al. Therapeutic equivalence of Spiros dry powder inhaler and Ventolin metered dose inhaler. Am J Respir Crit Care Med. 1999;160:1238–43. doi: 10.1164/ajrccm.160.4.9806101. [DOI] [PubMed] [Google Scholar]
- 13.Parameswaran KN, Inman MD, Ekholm BP, et al. Protection against methacholine bronchoconstriction to assess relative potency of inhaled β2-agonist. Am J Respir Crit Care Med. 1999;160:354–7. doi: 10.1164/ajrccm.160.1.9812035. [DOI] [PubMed] [Google Scholar]
- 14.Becker AB, Simons FER, McMillan JL, et al. Formoterol, a new long-acting selective β2-adrenergic receptor agonist: double-blind comparison with salbutamol and placebo in children with asthma. J Allergy Clin Immunol. 1989;84:891–5. doi: 10.1016/0091-6749(89)90385-0. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth BJ, Sims EJ, Das SK, et al. Bronchoprotection with formoterol via dry powder and metered-dose inhalers in patients with asthma. Ann Allergy Asthma Immunol. 2005;95:283–90. doi: 10.1016/S1081-1206(10)61226-4. [DOI] [PubMed] [Google Scholar]
- 16.Ramsdale EH, Otis J, Kline PA, Gontovnick LS, Hargreave FE, O’Byrne PM. Prolonged protection against methacholine-induced bronchoconstriction by the inhaled β2-agonist formoterol. Am Rev Respir Dis. 1991;143:998–1001. doi: 10.1164/ajrccm/143.5_Pt_1.998. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force: standardisation of lung function testing. Eur Respir J. 2005;26:319–38. [Google Scholar]
- 18.American Thoracic Society. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 19.Asmus MJ, Vaughan LM, Hill MR, et al. Stability of frozen methacholine solutions in unit-dose syringes for bronchoprovocation. Chest. 2002;121:1634–7. doi: 10.1378/chest.121.5.1634. [DOI] [PubMed] [Google Scholar]
- 20.Hendeles L, Khan YR, Shuster JJ, et al. Comparison of 2 dosimeters by methacholine challenge. J Allergy Clin Immunol. 2007;120:1218–19. doi: 10.1016/j.jaci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Shuster JJ. Design and analysis of experiments. In: Ambrosius WT, editor. Topics in biostatistics. Totawa, NJ: Humana Press; 2007. pp. 235–60. [Google Scholar]
- 22.Jokic R, Davis EE, Cockcroft DW. Methacholine PC20 extrapolation [letter] Chest. 1998;114:1796–7. doi: 10.1378/chest.114.6.1796-a. [DOI] [PubMed] [Google Scholar]
- 23.Finney DJ. Statistical methods in biological assay. 3. London, United Kingdom: Charles Griffin & Co., Ltd; 1978. pp. 211–12. [Google Scholar]
- 24.Ahrens RC, Harris JB, Milavetz G, et al. Use of bronchial provocation with histamine to compare the pharmacodynamics of inhaled albuterol and metaproterenol in patients with asthma. J Allergy Clin Immunology. 1987;79:876–82. doi: 10.1016/0091-6749(87)90235-1. [DOI] [PubMed] [Google Scholar]
- 25.Creticos PS, Adams WP, Petty BG, et al. A methacholine challenge dose-response study for development of a pharmacodynamic bioequivalence methodology for albuterol metered-dose inhalers. J Allergy Clin Immunol. 2002;110:713–20. doi: 10.1067/mai.2002.129036. [DOI] [PubMed] [Google Scholar]
- 26.Lipworth B, Tan S, Devlin M, et al. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104:431–8. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 27.Aziz I, Hall IP, McFarlane LC, et al. β2-Adrenoceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. J Allergy Clin Immunol. 1998;101:337–41. doi: 10.1016/S0091-6749(98)70245-3. [DOI] [PubMed] [Google Scholar]
- 28.O’Byrne PM, van der Linde J, Cockcroft DW, et al. Prolonged bronchoprotection against inhaled methacholine by inhaled BI 1744, a long-acting β2-agonist, in patients with mild asthma. J Allergy Clin Immunol. 2009;124:1217–21. doi: 10.1016/j.jaci.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft DW, Davis BE. The bronchoprotective effect of inhaled methacholine by using total lung capacity inspirations has a marked influence on the interpretation of the test result. J Allergy Clin Immunol. 2006;117:1244–8. doi: 10.1016/j.jaci.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Ullah MI, Newman GB, Saunders KB. Influence of age on response to ipratropium and salbutamol in asthma. Thorax. 1981;36:523–9. doi: 10.1136/thx.36.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bundgaard A, Rasmussen FV, Madsen L. Pretreatment of exercise-induced asthma in adults with aerosols and pulverized tablets. Allergy. 1980;35:639–45. doi: 10.1111/j.1398-9995.1980.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;154:876–80. doi: 10.1164/ajrccm.154.4.8887578. [DOI] [PubMed] [Google Scholar]