Abstract
Background
Prior studies have documented decreased pregnancy rates and early menopause in female cancer survivors; however, infertility rates and reproductive interventions have not been studied. This study investigates infertility and time to pregnancy among female childhood cancer survivors, and analyzes treatment characteristics associated with infertility and subsequent pregnancy.
Methods
The Childhood Cancer Survivor Study (CCSS) is a cohort study including five-year cancer survivors from 26 institutions who were <21 years old at the time of diagnosis between January 1, 1970, and December 31, 1986, and a sibling control group. CCSS females ages 18–39 years reporting they had ever been sexually active (3,531 survivors and 1,366 female controls) were studied. Self-reported infertility, medical treatment for infertility, the time to first pregnancy in survivors and siblings, and the risk of infertility in survivors by demographic, disease, and treatment variables were analyzed.
Findings
Survivors had an increased risk of clinical infertility (>1 year of attempts at conception without success) compared to siblings which was most pronounced at early reproductive ages (≤24 years Relative Risk (RR)=2·92, 95% Confidence Interval (CI) 1·18–7·20; 25–29 years RR=1·61, 95% CI 1·05–2·48; 30–39 years RR=1·37, 95% CI 1·11–1·69). Despite being equally likely to seek treatment for infertility, survivors were less likely to be prescribed medication for treatment of infertility (RR=0·57, 95% CI 0·46–0·70). Increasing doses of uterine radiation and alkylating agent chemotherapy were most strongly associated with infertility. Although survivors had an increased time to pregnancy interval (p=0·032), 64·2% (292/455) with infertility achieved a pregnancy.
Interpretation
A more comprehensive understanding of infertility after cancer is critical for counseling and decision-making regarding future attempts at conception as well as fertility preservation.
Significant improvements in cancer therapy have dramatically increased the five-year survival rate for childhood cancers, which now exceeds 80%.1 When considering the long term effects of cancer therapy, infertility is reported as a primary concern, particularly among female survivors.2, 3 Menstrual cyclicity is not sensitive in identifying gonadotoxic effects of therapy and many childhood cancer survivors are at risk for unrecognized infertility.4
The risk of non-surgical premature menopause is increased for cancer survivors with a cumulative incidence of 8% by age 40.5 In addition, cancer survivors are less likely to become pregnant when compared to their siblings.6, 7 Likelihood of pregnancy as a measure of fertility does not take into account individual desires for childbearing or attempts at pregnancy and thus does not assess the prevalence of infertility. Furthermore, self-reported parenthood does not reflect time to pregnancy or the use of infertility treatments in those who conceive. Therefore, prior studies may underestimate the risk of infertility in childhood cancer survivors.
In this study, we aim to quantify the risk of infertility in childhood cancer survivors using clinical definitions of infertility, and to identify disease and treatment characteristics in childhood cancer that increase the risk of infertility. Additionally, we evaluate if the length of time to pregnancy is longer in survivors than siblings who conceive.
Materials and Methods
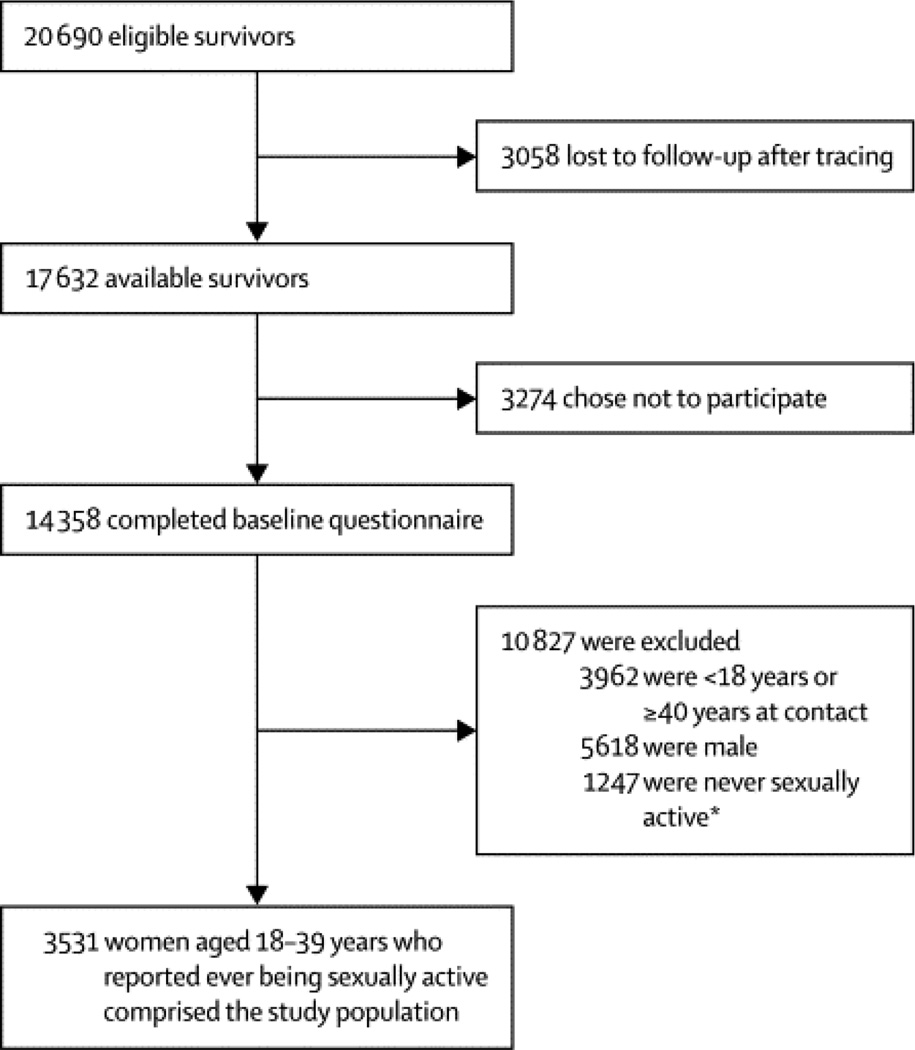
Details of the Childhood Cancer Survivor Study (CCSS) study design, cohort characteristics, and baseline data collection have been published previously.8, 9 In brief, CCSS is a collaborative study of 26 clinical centers in the United States and Canada that assembled a cohort of five-year cancer survivors who were diagnosed with an eligible malignancy before age 21 between January 1, 1970 and December 31, 1986. Eligible malignancies included leukemia, CNS cancer, Hodgkin lymphoma, non-Hodgkin lymphoma (NHL), Wilms (kidney) tumor, neuroblastoma, soft tissue sarcoma, or bone tumor. In subjects who had survived at least five years but subsequently died, the next-of-kin, typically parents or a spouse, was contacted. In addition, survivors were asked to identify all their living siblings, from which a random sample of the closest aged sibling was asked to participate. 4,775 eligible siblings were contacted and 4,023 participated (84·3%).9 For this study, female subjects between 18 and 40 years of age at completion of the baseline questionnaire between 1994 and 2005 who reported they had ever been sexually active were included. 14.8% of eligible survivors were unable to be located after intensive tracing efforts (3,058/20,690). Among survivors contacted, 81.4% (14,358/17,632) provided a baseline questionnaire (Figure 1).
Figure 1. Flowchart of the cancer survivor cohort included in the infertility analyses.
Previously published CCSS data indicates no differences in clinical or demographic characteristics amongst participants and non-participants other than a higher rate of nonparticipation among the next-of-kin of those subjects who had died and a somewhat lower portion of males among participants.8 The baseline questionnaire collected information on demographics, medical care, and medical conditions, including reproductive history. The questions asked of siblings were identical to those acquired from survivors, with the exception of cancer-specific questions. Individuals who reported a pregnancy were sent a supplemental pregnancy/offspring questionnaire.
The institutional review board at each participating institution approved this study. Informed consent was obtained from all participants.
Exposure variables
Data regarding disease and treatment characteristics (type and dose of chemotherapeutic agents; radiation field size, site, and dose) were collected by medical record abstraction at each collaborating institution.5
The total exposure to alkylating agents (AA) was quantified by reporting an alkylating agent score (AA score), which accounted for the number of AA and the doses of each individual agent (in total dose per square meter of body surface area). A distribution of doses of each AA was determined and each subject was assigned a score of 0 to 3, with 0 being no exposure and 1, 2, and 3 representing the lower, middle, and upper tertile of doses of that drug, respectively. Doses of individual AA used to derive the AA score have been previously published.6 Individual drug scores for each subject were summed, and an overall AA score of 0 to 3 was assigned to each subject.10 Individual chemotherapeutic agents, bone marrow transplant, history of relapse, and history of second malignancy prior to completion of the baseline questionnaire were considered as additional exposures.
Radiation therapy (RT) doses to the ovaries, uterus, and hypothalamus/pituitary were estimated as previously described by Stovall et al.11, 12 RT doses are reported in gray (Gy). Maximum ovarian RT dose was highly correlated with uterine RT dose, i.e. the majority of subject’s maximum ovarian dose and uterine dose were within the same RT category. Given the high correlation between these variables, it was not possible to fit a multivariable model that included both uterine RT dose and maximum ovarian RT dose; therefore, uterine dose is reported in this study to represent both uterine and gonadal exposure. Any shielding used during RT was accounted for in dose estimations.
Infertility questions
Two definitions of infertility were used. The first, ‘clinical infertility’ used the commonly accepted definition for infertility, and included anyone who responded yes to the question, “Was there ever a period in your life when you and a partner tried for one year or more to become pregnant, without success?”.13 The second definition of infertility, ‘total infertility’, included women with ‘clinical infertility’ as well as those who reported ovarian failure. Ovarian failure was defined as never having had a menstrual period or menstrual periods stopping five years or more from time of baseline questionnaire. Total infertility is inclusive of both those women who attempted to become pregnant for one year or more, without success (the ‘clinical infertility’ group) as well as those with ovarian failure who may not have attempted pregnancy. Only subjects with ‘clinical infertility’ answered nested questions regarding the use of infertility services.
Pregnancy questions
Participants who reported at least one pregnancy at any point after their cancer diagnosis on the baseline questionnaire were asked to complete a pregnancy questionnaire. The pregnancy questionnaire asked if the pregnancy was planned, and if so, how many months the participant tried to get pregnant. This study used data only from the first reported pregnancy. Time to first pregnancy among survivors and siblings as well as between cancer treatment groups was compared.
Statistical analysis
Subjects were asked to recall any instance of infertility that occurred up to the time of the baseline questionnaire. Subjects were not asked when they began trying to become pregnant or when they experienced infertility, therefore we were not able to account for individual differences in follow-up time. Infertility was treated as a binary outcome. The covariates examined in statistical analyses were chosen on the basis of their clinical relevance and previously established associations with infertility. Inclusion of covariates in the multivariable models was based on clinical judgment and examination of the univariate estimates.
Risk of total infertility and clinical infertility were first evaluated in a model comparing survivors and siblings. All subsequent analyses including sociodemographic, behavioral, and treatment variables were performed on the group reporting clinical infertility. Univariate analyses were performed using a Chi-squared or Fisher’s exact test. Potential factors for inclusion in multivariable models included age at baseline questionnaire, race, education level, smoking status, and body mass index (BMI). It was hypothesized that the risk of infertility would increase with age and that the relative risk comparing survivors to siblings would decline with age at baseline questionnaire. Thus, interaction terms between age at baseline questionnaire and survivor-sibling group were also considered. In a second model of infertility risk in the survivor group, the effects of disease and treatment factors were evaluated. In addition to factors considered for inclusion in the first model, potential risk factors included history of bone marrow transplant, history of relapse, history of a second malignancy prior to the baseline questionnaire, AA score, RT exposures (any RT, RT to the abdomen, RT to the brain, RT to the head, RT to the pelvis, total body irradiation (TBI)), uterine RT dose, and use of chemotherapy agents. Variables strongly correlated with chemotherapy and RT treatment variables were excluded from the multivariable model. All relative risk (RR) estimates and 95% confidence intervals (CI) were determined using a modified Poisson regression with robust variance estimates.14
Using data from the supplemental pregnancy questionnaire, time to first conception was compared between survivors and siblings. Survivors and siblings were included if they reported at least one planned pregnancy. In these analyses, histograms showed a highly skewed distribution even after transforming the data. To describe differences between groups defined by survivor status (survivor versus sibling), or among survivors, between treatment exposures, empirical cumulative distribution plots were examined and Wilcoxon rank-sum or Kruskal-Wallis tests were used. All tests were two-sided with a 0·05 significance level without adjustment for multiple tests. All analyses were performed using Statistical Analysis Software, version 9·2 (SAS Institute).
Role of the funding source
The study sponsor had no role in study design; in collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. JN and WL had full access to raw data. The corresponding author (SB) had full access to all of the data and the final responsibility to submit for publication.
Results
Median age at the time of the baseline questionnaire was 27·6 years (interquartile range (IQR) 23·5–32·3 years) in the survivor group, and 33·6 years (IQR 24·4–33·6 years) in the control group. Demographics of the two groups are listed in Table 1. The majority of participants were still menstruating at the time of the baseline questionnaire (82·0% [2894/3531] of survivors and 86·8% [1186/1366] of siblings).
Table 1.
Demographic characteristics of female survivors of childhood cancer and of siblings at completion of the baseline questionnaire.
| Characteristic | Survivors (N=3531) |
Siblings (n=1366) |
|---|---|---|
| Age | N (%) | N (%) |
| <20 | 227 (6.4%) | 80 (5.9%) |
| 20–24 | 749 (21.2%) | 238 (17.4%) |
| >24–29 | 1040 (29.5%) | 371 (27.2%) |
| >29–34 | 916 (25.9%) | 364 (26.7%) |
| >34–39 | 542 (15.4%) | 275 (20.1%) |
| >39 | 57 (1.6%) | 38 (2.8%) |
| Race | ||
| White non-Hispanic | 2952 (83.6%) | 1197 (87.6%) |
| Hispanic | 80 (2.3%) | 14 (1.0%) |
| Black | 172 (4.9%) | 44 (3.2%) |
| Other | 315 (8.9%) | 66 (4.8%) |
| Unknown | 12 (<1%) | 45 (3.3%) |
| Education | ||
| Not HS graduate | 278 (7.9%) | 70 (5.1%) |
| Completed HS | 645 (18.3%) | 229 (16.8%) |
| Post HS, some college | 1287 (36.5%) | 470 (34.4%) |
| College graduate | 1107 (31.4%) | 551 (40.3%) |
| Unknown | 214 (6.1%) | 46 (3.4%) |
| Marital status | ||
| Married/Living as married | 1908 (54.0%) | 879 (64.4%) |
| Widowed/Divorced/Separated | 354 (10.0%) | 108 (7.9%) |
| Never married | 1103 (31.2%) | 356 (26.1%) |
| Unknown | 166 (4.7%) | 23 (1.7%) |
| Smoking status | ||
| Current smoker | 688 (19.5%) | 343 (25.1%) |
| Former smoker | 465 (13.2%) | 239 (17.5%) |
| Never smoked | 2321 (65.7%) | 779 (57.0%) |
| Unknown | 57 (1.6%) | 5 (0.4%) |
| BMI | ||
| <18.5 | 332 (9.4%) | 68 (5.0%) |
| 18.5 – <25 | 2054 (58.2%) | 820 (60.0%) |
| 25.0 – <30 | 660 (18.7%) | 252 (18.5%) |
| >=30 | 410 (11.6%) | 193 (14.1%) |
| Unknown | 75 (2.1%) | 33 (2.4%) |
Survivors had an increased risk compared to siblings of both clinical and total infertility. The relative risk was more pronounced when the 107 survivors with ovarian failure were included in the definition of infertility (‘total infertility’, Table 2). After adjusting for known sociodemographic and behavioral risk factors, the relative risk of clinical infertility in survivors was 1·48 compared to siblings (95% CI 1·23–1·78). This adjusted risk of clinical infertility was more pronounced at younger ages at study participation (≤24 years RR=2·92, [95% CI 1·18–7·20], p=0·020; 25–29 years RR=1·61, [95% CI 1·05–2·48], p=0·029; 30–40 years RR=1·37, [95% CI 1·11–1·69], p=0·0035).
Table 2.
Comparison of infertility and the use of medical services for infertility between survivors and siblings.
| Outcome variable | Survivors (N=3531) |
Siblingsa (N=1366) |
RRb (95% CI) | Pb |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Total infertilityc | ||||
| Unknown | 206 (5.8%) | 29 (2.1%) | ||
| No | 2763 (78.3%) | 1190 (87.1%) | ||
| Yes | 562 (15.9%) | 147(10.8%) | 1.54 (1.30–1.82) | <0.0001 |
| Clinical infertilityd | ||||
| Unknown | 178 (5.0%) | 14 (1.0%) | ||
| No | 2898 (82.1%) | 1215 (89.0%) | ||
| Yes | 455 (12.9%) | 137 (10.0%) | 1.34 (1.12–1.60) | 0.0015 |
| Visit to a doctor for infertility | N=455 | N=137 | ||
| Unknown | 3 (0.7%) | 3 (2.2%) | ||
| No | 137 (30.1%) | 34 (24.8%) | ||
| Yes | 315 (69.2%) | 100 (73.0%) | 0.93 (0.83–1.05) | 0.25 |
| Doctor found a reason for infertility | N=315 | N=100 | ||
| Unknown | 11 (3.5%) | 0 (0.0%) | ||
| No | 96 (30.5%) | 25 (25.0%) | ||
| Yes | 208 (66.0%) | 75 (75.0%) | 0.91 (0.80–1.05) | 0.19 |
| Medication to help you get pregnant | N=208 | N=75 | ||
| Unknown | 3 (1.4%) | 0 (0.0%) | ||
| No | 118 (56.7%) | 19 (25.3%) | ||
| Yes | 87 (41.8%) | 56 (74.7%) | 0.57 (0.46–0.70) | <0.0001 |
Sibling group is the reference group when calculating relative risks.
Unadjusted relative risks (RR) are displayed and are based on available data. P-values are two sided and p<0.05 was considered statistically significant.
Total infertility refers to the overall prevalence of infertility including those reporting trying for > 1 year without success and those with ovarian failure (defined as never getting a menstrual period or last period > 5 years before the baseline questionnaire).
Clinical infertility is defined as trying for > 1 year without success. Only those subjects reporting yes to this definition of infertility went on to answer the subsequent nested questions about the use of infertility services.
Comparison of survivors and siblings that reported clinical infertility revealed no differences in the likelihood of visiting a doctor for infertility. Survivors were slightly less likely to have the doctor find a reason for their infertility, although this difference did not reach statistical significance. However, survivors were significantly less likely to receive medication to help them become pregnant (RR=0·57, 95% CI 0·46–0·70) (Table 2).
Subsequent analyses analyzed demographic and behavioral factors in survivors and their associations with clinical infertility. As seen in Table 3, unadjusted increases in risk of infertility were observed in women who were older at childhood cancer diagnosis, older at study entry, that were currently or previously married, in those with high BMI, lower educational attainment, and in smokers. In adjusted models (data not shown), only associations with older age (>29 vs. 24–29) at the time of the baseline questionnaire (RR=1·68, 95% CI 1·26–2·24), marital status (RR=7·91, 95% CI 4·58–13·68 if currently married), and BMI >30 kg/m2 (RR=1·71, 95% CI 1·30–2·26) remained significant. Notably, a significant association between age at primary diagnosis and infertility was not observed after adjustment.
Table 3.
The unadjusted relative risk of clinical infertility by demographic, disease and treatment characteristics in survivors of childhood cancer.
| Variable | Na | Unknown n (%)b |
No n (%)b |
Yes n (%)b |
RR (95% CI)c | P |
|---|---|---|---|---|---|---|
| Age at diagnosis | ||||||
| 0–4 | 633 | 32 (5.1%) | 542 (85.6%) | 59 (9.3%) | 0.55 (0.42–0.73) | <0.0001 |
| 5–9 | 771 | 34 (4.4%) | 658 (85.3%) | 79 (10.3%) | 0.60 (0.47–0.78) | 0.0001 |
| 10–14 | 1123 | 46 (4.1%) | 918 (81.8%) | 159 (14.2%) | 0.83 (0.68–1.02) | 0.078 |
| ≥15– <21 | 890 | 62 (7.0%) | 681 (76.5%) | 147 (16.5%) | Referent | |
| Unknown | 114 | 4 (3.5%) | 99 (86.8%) | 11 (9.7%) | ||
| Age at baseline questionnaire | ||||||
| <20 | 227 | 39 (17.2%) | 176 (77.5%) | 12 (5.3%) | 0.54 (0.30–0.95) | 0.033 |
| 20–24 | 749 | 16 (2.1%) | 695 (92.8%) | 38 (5.1%) | 0.44 (0.31–0.62) | <0.0001 |
| >24–29 | 1040 | 30 (2.9%) | 890 (85.6%) | 120 (11.5%) | Referent | |
| >29–34 | 916 | 41 (4.5%) | 716 (78.2%) | 159 (17.4%) | 1.53 (1.23–1.90) | 0.0001 |
| >34–39 | 542 | 47 (8.7%) | 383 (70.7%) | 112 (20.7%) | 1.90 (1.51–2.41) | <0.0001 |
| >39– <40 | 57 | 5 (8.8%) | 38 (66.7%) | 14 (24.6%) | 2.27 (1.40–3.66) | 0.0008 |
| Unknown | 0 | |||||
| Education | ||||||
| Not HS graduate | 278 | 33 (11.9%) | 205 (73.7%) | 40 (14.4%) | Referent | |
| Completed HS | 645 | 44 (6.8%) | 509 (78.9%) | 92 (14.3%) | 0.94 (0.67–1.32) | 0.71 |
| Post HS, some college | 1287 | 89 (6.9%) | 1036 (80.5%) | 162 (12.6%) | 0.83 (0.60–1.14) | 0.24 |
| College graduate | 1107 | 5 (0.5%) | 980 (88.5%) | 122 (11.0%) | 0.68 (0.49–0.94) | 0.021 |
| Unknown | 214 | 7 (3.3%) | 168 (78.5%) | 39 (18.2%) | ||
| Race | ||||||
| White non-Hispanic | 2952 | 155 (5.3%) | 2419 (81.9%) | 378 (12.8%) | Referent | |
| Hispanic | 80 | 2 (2.5%) | 69 (86.3%) | 9 (11.3%) | 0.85 (0.46–1.59) | 0.62 |
| Black | 172 | 11 (6.4%) | 137 (79.7%) | 24 (14.0%) | 1.10 (0.75–1.61) | 0.61 |
| Other | 315 | 10 (3.2%) | 264 (83.8%) | 41 (13.0%) | 0.99 (0.74–1.34) | 0.97 |
| Unknown | 12 | 0 (0.0%) | 9 (75.0%) | 3 (25.0%) | ||
| Marital status | ||||||
| Married/Living as married | 1908 | 28 (1.5%) | 1514 (79.4%) | 366 (19.2%) | 8.70 (5.75–13.17) | <0.0001 |
| Widowed, Divorced, Separated | 354 | 9 (2.5%) | 295 (83.3%) | 50 (14.1%) | 6.48 (4.01–10.45) | <0.0001 |
| Never married | 1103 | 75 (6.8%) | 1005 (91.1%) | 23 (2.1%) | Referent | |
| Unknown | 166 | 66 (39.8%) | 84 (50.6%) | 16 (9.6%) | ||
| Smoking status | ||||||
| Current smoker | 688 | 13 (1.9%) | 566 (82.3%) | 109 (15.8%) | 1.27 (1.04–1.56) | 0.020 |
| Former smoker | 465 | 6 (1.3%) | 393 (84.5%) | 66 (14.2%) | 1.13 (0.88–1.45) | 0.32 |
| Never smoked | 2321 | 122 (5.3%) | 1920 (82.7%) | 279 (12.0%) | Referent | |
| Unknown | 57 | 37 (64.9%) | 19 (33.3%) | 1 (1.8%) | ||
| BMI | ||||||
| <18.5 | 332 | 46 (13.9%) | 262 (78.9%) | 24 (7.2%) | 0.72 (0.48–1.08) | 0.11 |
| 18.5 – <25 | 2054 | 97 (4.7%) | 1730 (84.2%) | 227 (11.1%) | Referent | |
| 25.0 – <30 | 660 | 17 (2.6%) | 546 (82.7%) | 97 (14.7%) | 1.30 (1.04–1.62) | 0.020 |
| ≥30 | 410 | 11 (2.7%) | 301 (73.4%) | 98 (23.9%) | 2.12 (1.71–2.62) | <0.0001 |
| Unknown | 75 | 7 (9.3%) | 59 (78.7%) | 9 (12.0%) | ||
| Primary diagnosis | ||||||
| Leukemia | 1006 | 46 (4.6%) | 850 (84.5%) | 110 (10.9%) | Referent | |
| Hodgkin lymphoma or NHL | 927 | 44 (4.8%) | 709 (76.5%) | 174 (18.8%) | 1.72 (1.38–2.14) | <0.0001 |
| CNS | 323 | 17 (5.3%) | 270 (83.6%) | 36 (11.2%) | 1.03 (0.72–1.46) | 0.88 |
| Solid tumor | 1275 | 71 (5.6%) | 1069 (83.8%) | 135 (10.6%) | 0.98 (0.77–1.24) | 0.86 |
| Radiation | ||||||
| Any RT | ||||||
| No | 962 | 43 (4.5%) | 828 (86.1%) | 91 (9.5%) | Referent | |
| Yes | 2194 | 110 (5.0%) | 1774 (80.9%) | 310 (14.1%) | 1.50 (1.21–1.87) | 0.0003 |
| Unknown | 375 | 25 (6.7%) | 296 (78.9%) | 54 (14.4%) | ||
| Abdomen RT | ||||||
| No | 2225 | 97 (4.4%) | 1871 (84.1%) | 257 (11.6%) | Referent | |
| Yes | 853 | 50 (5.9%) | 670 (78.6%) | 133 (15.6%) | 1.37 (1.13–1.66) | 0.0013 |
| Unknown | 453 | 31 (6.8%) | 357 (78.8%) | 65 (14.4%) | ||
| Brain or head RT | ||||||
| No | 2156 | 104 (4.8%) | 1771 (82.1%) | 281 (13.0%) | Referent | |
| Yes | 924 | 43 (4.7%) | 772 (83.6%) | 109 (11.8%) | 0.90 (0.73–1.11) | 0.34 |
| Unknown | 451 | 31 (6.9%) | 355 (78.7%) | 65 (14.1%) | ||
| Pelvis RT | ||||||
| No | 2494 | 108 (4.3%) | 2104 (84.4%) | 282 (11.3%) | Referent | |
| Yes | 583 | 39 (6.7%) | 437 (75.0%) | 107 (18.4%) | 1.66 (1.36–2.04) | <0.0001 |
| Unknown | 454 | 31 (6.8%) | 357 (78.6%) | 66 (14.5%) | ||
| TBI | ||||||
| No | 3041 | 145 (4.8%) | 2517 (82.8%) | 379 (12.5%) | Referent | |
| Yes | 36 | 2 (5.6%) | 24 (66.7%) | 10 (27.8%) | 2.25 (1.32–3.81) | 0.0027 |
| Unknown | 454 | 31 (6.8%) | 357 (78.6%) | 66 (14.5%) | ||
| Radiation dose (Gy) | ||||||
| Uterine | ||||||
| No RT | 962 | 43 (4.5%) | 828 (86.1%) | 91 (9.5%) | Referent | |
| 1–5 | 1799 | 79 (4.4%) | 1494 (83.1%) | 226 (12.6%) | 1.33 (1.05–1.67) | 0.016 |
| 5.1–10 | 81 | 5 (6.2%) | 55 (67.9%) | 21 (25.9%) | 2.79 (1.85–4.22) | <0.0001 |
| 10.1–20 | 86 | 5(5.8%) | 63 (73.3%) | 18 (20.9%) | 2.24 (1.43–3.53) | 0.0005 |
| >20 | 120 | 11 (9.2%) | 82 (68.3%) | 27 (22.5%) | 2.50 (1.71–3.66) | <0.0001 |
| Unknown | 483 | 35 (7.3%) | 376 (77.9%) | 72 (14.9%) | ||
| Pituitary | ||||||
| No RT | 964 | 43 (4.5%) | 830 (86.1%) | 91 (9.4%) | Referent | |
| 1–20 | 1482 | 75 (5.1%) | 1194 (80.6%) | 213 (14.4%) | 1.53 (1.22–1.93) | 0.0003 |
| 20.1–30 | 430 | 8 (1.9%) | 357 (83.0%) | 65 (15.1%) | 1.56 (1.16–2.10) | 0.0034 |
| >30 | 171 | 15 (8.8%) | 140 (81.9%) | 16 (9.4%) | 1.04 (0.63–1.72) | 0.88 |
| Unknown | 484 | 37 (7.6%) | 377 (77.9%) | 70 (14.5%) | ||
| Alkylating agent score | ||||||
| 0 | 1510 | 55 (3.6%) | 1276 (84.5%) | 179 (11.9%) | Referent | |
| 1 | 543 | 25 (4.6%) | 460 (84.7%) | 58 (10.7%) | 0.91 (0.69–1.20) | 0.51 |
| 2 | 429 | 17 (4.0%) | 363 (84.6%) | 49 (11.4%) | 0.97 (0.72–1.30) | 0.82 |
| 3 | 318 | 13 (4.1%) | 242 (76.1%) | 63 (19.8%) | 1.68 (1.30–2.18) | <0.0001 |
| Unknown | 731 | 68 (9.3%) | 557 (76.2%) | 106 (14.5%) | ||
| History of BMT | ||||||
| No | 3302 | 163 (4.9%) | 2714 (82.2%) | 425 (12.9%) | Referent | |
| Yes | 83 | 15 (18.1%) | 56 (67.5%) | 12 (14.5%) | 1.30 (0.77–2.19) | 0.32 |
| Unknown | 146 | 0 (0.0%) | 128 (87.7%) | 18 (12.3%) | ||
| History of relapse | ||||||
| No | 3183 | 124 (3.9%) | 2646 (83.1%) | 413 (13.0%) | Referent | |
| Yes | 348 | 54 (15.5%) | 252 (72.4%) | 42 (12.1%) | 1.06 (0.79–1.42) | 0.71 |
| Unknown | 0 | |||||
| History of second malignancy before baseline | ||||||
| No | 3388 | 156 (4.6%) | 2798 (82.6%) | 434 (12.8%) | Referent | |
| Yes | 143 | 22 (15.4%) | 100 (69.9%) | 21 (14.7%) | 1.29 (0.87–1.93) | 0.21 |
| Unknown | 0 |
N includes survivors that reported have ever been sexually active.
n (%) are out of total N.
RR is based on available data.
Specific diagnosis and treatment variables that were associated with clinical infertility were analyzed. In unadjusted models, as shown in Table 3, those with a history of lymphoma, those who received any RT, TBI, and RT to the abdomen or pelvis, had an increased risk of infertility. Additionally, increasing dose of RT to the uterus, exposure to higher cumulative doses of AA (AA score of 3; see Methods), and pituitary radiation doses between 1 to 30 Gy significantly increased the risk of infertility. In adjusted models, uterine RT doses greater than 5 Gy (RR=2·48, [95% CI 1·54–4·01] for 5·1–10 Gy; RR=2·02, [95% CI 1·27–3·23] for 10·1–20 Gy; RR=1·95, [95% CI 1·19–3·19] for >20 Gy) and the AA score of 3 (RR=1·48, 95% CI 1·10–1·99) remained significant.
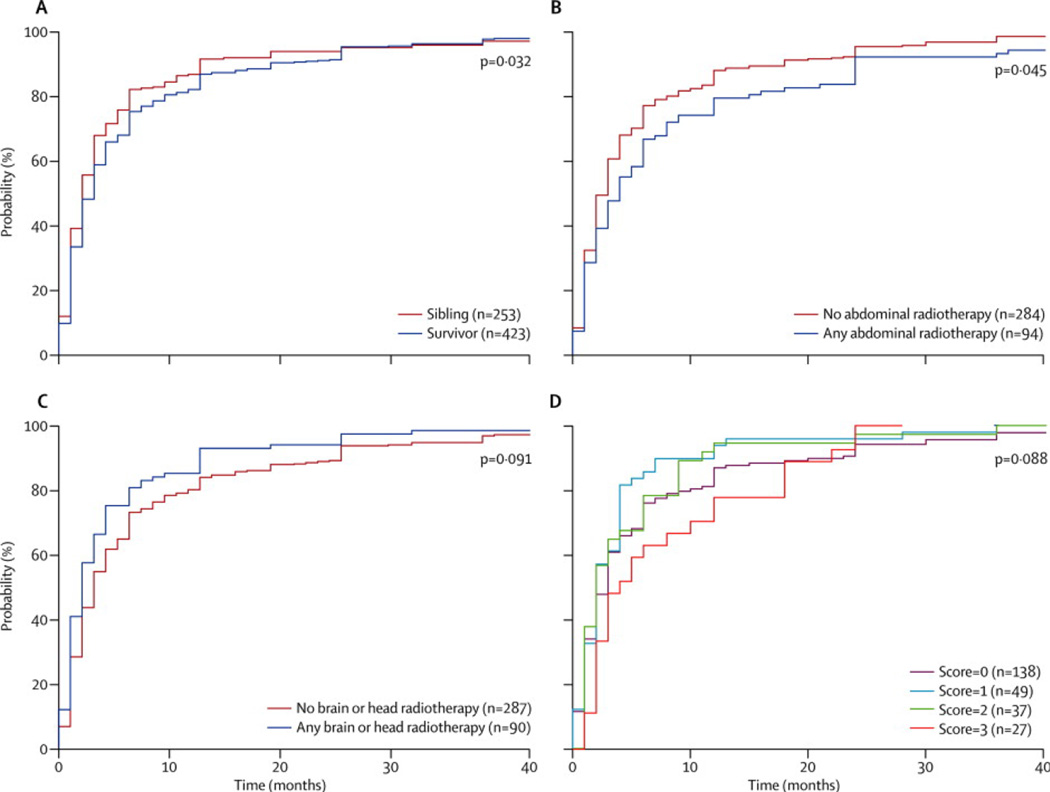
There were 704 participants who reported a planned first pregnancy. Data regarding time to first conception were available for 676 pregnancies (423 survivors and 253 siblings). As shown in Figure 2, survivors took significantly longer to become pregnant than siblings (p=0·032). For example, 13.0% (55/423) of survivors required more than twelve months of attempts to achieve pregnancy, compared to 8·3% (21/253) of siblings. When survivors were grouped according to cancer treatment, survivors who had received abdominal RT took significantly longer to become pregnant than those who did not, and there was a trend toward increasing time to pregnancy in those who had received high doses of AA. Interestingly, survivors that received RT to the brain or head had a significantly decreased time to pregnancy compared to other survivors.
Figure 2. Distribution of time to first pregnancy among survivors and siblings.
The distribution of time to first pregnancy in months was summarized using the empirically estimated cumulative distribution function in CCSS participants who reported having a first planned pregnancy and who completed the pregnancy questionnaire. In each panel, the horizontal axis indicates number of months to first pregnancy and the vertical axis indicates estimated probabilities. The height of the distribution curve at x months indicates the estimated probability of pregnancy in subjects achieving pregnancy in x months or less. For presentation purposes, plots were truncated at 40 months, however all available data were used in estimating distributions and group comparisons. Upper left panel, p=0.032; upper right panel p=0.045; lower left panel p=0.0091; lower right panel p=0.088.
Finally, survivors with clinical infertility were analyzed to characterize the likelihood of achieving a pregnancy. Nearly two-thirds of survivors with self-reported clinical infertility achieved a pregnancy (292/455, 64·2%). In adjusted analyses, infertile survivors who received more than 10 Gy of uterine RT were less likely to become pregnant (RR=0·33, 95% CI 0·14–0·75 for 10·1–20 Gy and RR=0·21, 95% CI 0·08–0·60 for >20 Gy) than those who were not exposed to uterine RT. Additionally, those with AA scores of 2 or 3 were less likely to become pregnant (RR=0·59, 95% CI 0·43–0·82 and RR=0·77, 95% CI 0·62–0·97 respectively).
Comment
These data demonstrate an increased risk for infertility in childhood cancer survivors starting at a very young reproductive age. RT to pelvic organs and treatment regimens containing AA increased the risk for infertility in a dose dependent fashion. Overall, survivors had a slightly increased time to pregnancy interval, and were less likely to be given medical treatment for infertility; however nearly two-thirds of survivors with clinical infertility reported a pregnancy. Treatment for childhood malignancy has variable effects on reproductive function, and counseling regarding likelihood of fertility in survivors remains challenging. Prior studies have characterized risk factors for childlessness or pregnancy, but were not evaluating a group of women who desired pregnancy and reported an inability to conceive within a year. Clinicians caring for survivors who request information on likelihood of pregnancy or success of treatment for infertility have a paucity of data on which to base their recommendations. To our knowledge, this is the first large study of female childhood cancer survivors which quantifies the risk of infertility using a clinical definition and characterizes the use and success of infertility treatments in this setting.
Interestingly, survivors who had received RT to the brain had a shorter time to pregnancy than other survivors. Survivors who receive high doses of brain RT may experience significant neurocognitive impairments and may be less likely to partner or attempt pregnancy.15 The shortened time to pregnancy in this survivor group in our study likely reflects selection bias for a good prognosis group that received low dose RT that did not impact ovarian reserve.
It is concerning that survivors were half as likely to be medically treated for infertility as their siblings. Data are lacking as to why providers did not prescribe infertility medications, but do raise concern regarding a provider bias against treating cancer survivors for infertility. It is possible that providers assessed the chance of success as poor and therefore the decision was made not to attempt therapy, or that survivors were less motivated to take medications after having been treated extensively in the past for another condition. Alternatively, reproductive medicine providers may have been uncomfortable with perceived medical co-morbidities.
One major challenge for providers is quantifying the risk of reproductive dysfunction after cancer treatment. Menstruation is not sensitive in identifying women with diminished ovarian reserve. Indeed, in the present study, despite an increased risk of infertility, the majority of cancer survivors were still menstruating. Uterine RT has been demonstrated to negatively impact reproductive function independently of the ovarian effect of RT. Small uterine volumes, impaired blood flow, and endometrial damage may play a role in infertility or adverse pregnancy outcomes (increased low birth weight and miscarriage rates, and lower live birth rates) in survivors that received uterine RT.16–19
This is the most comprehensive study of infertility in childhood cancer survivors that we are aware of to-date. The strengths of this study include the large sample size, a well-characterized cohort, availability of detailed cancer treatment information, and the inclusion of an appropriate control group. We elected to report achievement of pregnancy, as opposed to live birth, in estimations of fertility. Importantly, previous CCSS work did not demonstrate an overall increased risk in miscarriage or still birth in survivors compared to siblings, despite a lower live birth rate in survivors. Additionally, previous reports have shown an increased rate of elective termination of pregnancy in young survivors, lowering the live birth rate but not reflecting fertility potential. Therefore, we believe that in this cohort pregnancy gives a more comprehensive view of fertility.19
Our study has a number of limitations that should be considered when evaluating the observed risk of infertility in survivors. It is important to acknowledge that women with ovarian failure were not included in the analysis of likelihood of pregnancy; the percentage of infertile survivors achieving pregnancy would be lower if that group was included. Because of the young age of our cohort (median 27·6 years), as well as the lower marriage rate in survivors, we may also be underestimating the overall burden of infertility in their lifespan of survivors as they marry, age, and attempt pregnancy. Previous reports in childhood cancer survivors have shown diminished ovarian reserve even with regular menstruation and timely puberty 20–22 and studies of adult cancer survivors have concluded that amenorrhea underestimates infertility.23, 24 Similarly, survivors may also be at risk for secondary infertility if they desire more than one child.
Another significant limitation of these data is that participants answered infertility questions starting in 1994, when use of assisted reproduction such as in vitro fertilization was less common and less successful. Additionally, details regarding fertility diagnoses and specifics of infertility treatment were limited by subject recall, and records for infertility treatment were not available. Survivors may have been more aware of the risks of infertility due to treatment, and therefore over reported infertility. Finally, antineoplatic therapies have evolved toward gonadal sparing regimens in some cases, and the late reproductive effects of these more contemporary regimens may be less pronounced. It is important to note that alkylating agents and pelvic radiation remain anticancer therapies today. This underlines the importance of the current analysis, particularly in light of the advances in reproductive technology.
Modern reproductive medicine offers increased options for fertility preservation prior to therapy. The American Society of Clinical Oncology recommends that oncologists should refer “interested and appropriate” patients to reproductive specialists as soon as possible.25 As assisted reproductive outcomes after cancer therapy appear to be poor, fertility preservation interventions are preferred at diagnosis when possible.26 Fertility preservation techniques prior to or early in cancer therapy may include oophoropexy or cryopreservation of oocytes, ovarian tissue, or embryos depending upon demographic and clinical factors as well as resource availability. Importantly, multiple live births have now been reported from ovarian tissue freezing.27–29
In conclusion, some female childhood cancer survivors are at risk for infertility at a young reproductive age, regardless of age at diagnosis. Pelvic RT and high dose AA chemotherapy are the most significant treatment related risk factors. Although time to pregnancy is slightly increased in cancer survivors, it is encouraging that nearly two-thirds of survivors that reported trying to conceive for at least a year without success can eventually achieve a pregnancy. Collaboration between oncologists and reproductive medicine providers may increase timely access to fertility preservation for those in need, and prevent provider bias when treating cancer survivors for infertility.
Research in Context
Systematic Review: Published works regarding fertility in cancer survivors typically report amenorrhea, pregnancy, and/or live birth as an outcome to estimate fertility. We were familiar with earlier CCSS work by Dr. Green et al (JCO, 2009) which reported on relative risk of pregnancy in childhood cancer survivors compared to siblings. Questioning survivors directly regarding attempts at conception may reveal differences in attempts and desires for children, as well as differences in the need to use medical treatment for infertility to achieve a pregnancy. A search was performed using the PubMed database with combinations of the following terms: fertility, infertility, parenthood, live birth, cancer, and survivorship. Our search was limited to publications including human subjects, and publications in English. Inclusion criteria for studies that reported fertility/infertility in childhood or young adult cancer survivors were examined. In addition, review articles addressing ovarian function and/or fertility after cancer treatment were reviewed. All previously published work used surrogate measures of fertility (most often pregnancy or parenthood). Additionally, the majority of publications have included relatively small numbers of childhood cancer survivors. Finally, no publications were encountered describing the use of infertility treatment or time to pregnancy in female childhood cancer survivors. Since beginning work on this study, Letourneau et al (Cancer, 2012) published of a survey using direct questions about infertility in adult female cancer survivors (aged 18–40). To our knowledge, no previous studies to-date including childhood cancer survivors have used direct measures of infertility.
Interpretation: These data support earlier work demonstrating that cancer therapies have negative gonadal effects on young female cancer survivors leading to an increased risk of infertility and childlessness. Consistent with previous reports, pelvic radiation and alkylating agent chemotherapy are significant treatment related risk factors for infertility. Our data differ from other studies which suggest that earlier age at cancer diagnosis is protective against the development of infertility. This is likely due to the fact that our multivariable models adjusted for treatment related factors (radiation and chemotherapy doses) and sociodemographic variables known to be associated with infertility. We hypothesize that the age distribution of disease and associated treatments accounts for the lower risk of infertility by young age at diagnoses, rather than a different biologic effect of cancer therapies on reproductive organs at a younger age.
Additionally, for those survivors who have infertility but are still menstruating, these data provide useful information for survivors regarding the likelihood of pregnancy, taking into account treatments they may have received. Furthermore, we provide data regarding the length of time to conception in survivors able to conceive. Importantly, the increased risk of infertility is seen in cancer survivors at very young ages, even though the majority of young female cancer survivors resume menstruation (over 50% of our cohort was <30 years of age when they answered questions about infertility). This highlights that ongoing menstrual function does not equate to normal fecundity. Clinicians should alert cancer survivors with ongoing ovarian function in survivorship that they are at risk for infertility, and refer to reproductive specialists for consideration of fertility preservation if they are not ready to attempt conception at young reproductive ages.
Acknowledgements
JN and WL had full access to raw data. The corresponding author (SB) had full access to all of the data and the final responsibility to submit for publication. Supported by grant U24 CA55727 (LLR Principal Investigator) from the National Cancer Institute, support to St. Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC), and support to LD from Swim Across America, Inc (SAA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Barton has no conflicts of interest to report. Dr. Najita has no conflicts of interest to report. Dr. Ginsburg has no conflicts of interest to report. Dr. Leisenring has no conflicts of interest to report. Dr. Stovall has no conflicts of interest to report. Ms. Weathers has no conflicts of interest to report. Dr. Sklar has no conflicts of interest to report. Dr. Robison has no conflicts of interest to report. Dr. Diller has no conflicts of interest to report.
Contributions:
Design and conduct of the study: Sara Barton, Julie Najita, Elizabeth Ginsburg, Wendy Leisenring, Marilyn Stovall, Charles Sklar, Leslie Robison, Lisa Diller.
Collection, management, analysis, and interpretation of the data: Sara Barton, Julie Najita, Elizabeth Ginsburg, Wendy Leisenring, Marilyn Stovall, Rita Weathers, Charles Sklar, Leslie Robison, Lisa Diller.
Preparation, review or approval of the manuscript: Sara Barton, Julie Najita, Elizabeth Ginsburg, Wendy Leisenring, Marilyn Stovall, Rita Weathers, Charles Sklar, Leslie Robison, Lisa Diller.
References
- 1.Armenian SH, Landier W, Hudson MM, Robison LL, Bhatia S. Children's Oncology Group's 2013 blueprint for research: Survivorship and outcomes. Pediatr Blood Cancer. 2012;60(6):1063–1068. doi: 10.1002/pbc.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connell S, Patterson C, Newman B. Issues and concerns of young Australian women with breast cancer. Support Care Cancer. 2006;14(5):419–426. doi: 10.1007/s00520-005-0003-8. [DOI] [PubMed] [Google Scholar]
- 3.Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 5.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27(16):2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magelssen H, Melve KK, Skjaerven R, Fossa SD. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum Reprod. 2008;23(1):178–186. doi: 10.1093/humrep/dem362. [DOI] [PubMed] [Google Scholar]
- 8.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 9.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 11.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60(2):542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 13.Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5) Suppl:S60. doi: 10.1016/j.fertnstert.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol. 1992;99(5):392–394. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 17.Green DM, Peabody EM, Nan B, Peterson S, Kalapurakal JA, Breslow NE. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20(10):2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 18.Signorello LB, Mulvihill JJ, Green DM, et al. Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet. 2010;376(9741):624–630. doi: 10.1016/S0140-6736(10)60752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187(4):1070–1080. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 20.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–5314. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 21.Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod. 2009;24(4):982–990. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- 22.van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92(10):3869–3874. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 23.Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118(7):1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Kaaij MA, Heutte N, Meijnders P, et al. Premature Ovarian Failure and Fertility in Long-Term Survivors of Hodgkin's Lymphoma: A European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol. 2012;30(3):291–299. doi: 10.1200/JCO.2011.37.1989. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 26.Barton SE, Missmer SA, Berry KF, Ginsburg ES. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil Steril. 2012;97(2):381–386. doi: 10.1016/j.fertnstert.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt KT, Rosendahl M, Ernst E, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95(2):695–701. doi: 10.1016/j.fertnstert.2010.07.1080. [DOI] [PubMed] [Google Scholar]
- 29.Dolmans MM, Jadoul P, Gilliaux S, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet. 2013;30(3):305–314. doi: 10.1007/s10815-013-9952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]