Abstract
Over the past several years, the dominant paradigm in drug development for metastatic renal cell carcinoma (mRCC) has been to more selectively and potently target moieties such as the vascular endothelial growth factor receptor (VEGFR). The effectiveness of this strategy appears to be nearing a plateau, however, underscoring the need for novel approaches. Vaccine-based therapies represent one such approach. Several distinct vaccines are currently being examined in mRCC, each utilizing a distinct mechanism of action. For instance, the autologous dendritic cell vaccine AGS-003 utilizes patient-specific antigens derived from primary tumor tissue. In contrast, the poxvirus vaccine TG4010 produces an antigenic response to MUC1, a cell surface glycoprotein that reduces cell-cell interactions and thereby precludes contact inhibition. Other vaccines elicit a response to a broader spectrum of antigens – for instance, the vaccine IMA901 is based on 9 tumor-associated peptides identified from a novel biotechnology platform combining mass spectroscopy, microarray analysis of RNA expression, and immunogenicity assays. Herein, the current status of vaccine-based therapies for mRCC is described in detail. Furthermore, challenges to clinical implementation (e.g., cost, optimal pairing with targeted agents, appropriate sequencing) are presented.
Keywords: IMA-901, Argos, AGS-003, vaccine, renal cell carcinoma
Introduction
Targeted therapies have drastically impacted the treatment paradigm for metastatic renal cell carcinoma (mRCC). Prior to their advent, the treatment of mRCC was largely confined to immunotherapeutic approaches including interferon-α (IFN-α) and interleukin-2 (IL-2).1 Several phase III studies have demonstrated superior clinical outcomes with targeted agents as compared to IFN-α.2,3 Furthermore, although IL-2 has not been placed head to head against any currently approved targeted drugs in a prospective trial, the agent appears to elicit clinical benefit in only a small subset of patients with the caveat of significant cardiovascular toxicity.4
While targeted therapies have largely supplanted immunotherapeutic approaches in the clinic, there may be a plateau in the clinical effectiveness of these agents. Newer generations of targeted therapeutics aim to more specifically target relevant moieties, such as vascular endothelial growth factor receptor-2 (VEGFR2). While several of these agents (e.g., axitinib and tivozanib) do achieve this aim, this may not translate to a clinical benefit.5,6 For instance, in the phase III TIVO-1 trial comparing sorafenib and tivozanib, 1-year overall survival (OS) appeared to be superior with sorafenib.7 Furthermore, in the recently presented AGILE study comparing sorafenib and axitinib in the front-line setting, no significant difference in progression-free survival (PFS) was observed.8 These datasets have led the investigative community to question the strategy of more aggressively targeting the VEGF-signaling axis.
Interestingly, as focus shifts away from VEGF-directed therapies, there may be a resurgence of immune-based approaches. In particular, programmed death-1 (PD-1) inhibitors, which prevent induction of T-cell anergy, appear to show substantial efficacy even in heavily refractory patients.9,10 Agents inhibiting the ligand of PD-1, PD-L1, may also ultimately prove efficacious in mRCC.11 Vaccine therapies represent a wholly distinct strategy of stimulating the antitumor immune response and have shown promise in other genitourinary malignancies. In the setting of metastatic castration-resistant prostate cancer (mCRPC), the dendritic cell vaccine sipuleucel-T has gained approval on the basis of a placebo-controlled phase III trial demonstrating superior OS.12 These encouraging results have bolstered interest in other vaccine-based therapeutics, both for mCRPC and other therapeutic indications.13 Several vaccine-based therapies have been developed for mRCC. These agents are described in the current review, along with a critical assessment of how they may be incorporated in current treatment paradigms for mRCC.
Multipeptide Vaccines: IMA901
The development of IMA901, a vaccine comprised of tumor associated peptides (TUMAPs), utilized the novel XPRESIDENT platform.14 This platform was used to compare both RNA and HLA ligands derived from normal tissue and tumor tissue derived from 32 patients with RCC. RNA was assessed via microarray, and HLA ligands via mass spectrometry. Once candidate TUMAPs were identified, immunogenicity was assessed using peripheral blood mononuclear cells (PBMCs) derived from healthy donors. A final set of 9 TUMAPs was derived, representing the following antigens:
PLIN2 and APOL1, expressed on the surface of lipid droplets and associated with RCC15
CCND1, a key cell cycle mediator with documented aberrations in RCC16
PRUNE2, overexpressed in genitourinary cancers19
MET, a transmembrane receptor that binds hepatocyte growth factor (HGF), with a well documented role in RCC pathogenesis20-22
RGS5, a cell cycle regulator23
MUC1, a cell surface glycoprotein that can mask surrounding antigenic cell surface proteins24
MMP7, involved in regulation of the extracellular matrix25
IMA901 was first assessed in a phase I study including a total of 28 patients, including 15 treatment-naïve patients and 13 patients with heavily refractory disease.14 The latter cohort had received up to 3 prior lines of therapy, primarily comprised of cytokines with or without cytotoxic agents. Treatment was comprised of a total of 8 injections of IMA901 at days 1, 2, 3, 8, 15, 22, 36 and 64, with GM-CSF prior to delivery. Clinical benefit was assessed at a 3-month benchmark. At this point, 1 partial response (PR) was observed and 11 patients had stable disease (SD).
Immune profiling was performed in 27 of the 28 patients in the phase I experience.14 Importantly, the original formulation also included HBcAg, the antigenic determinant of the Hepatitis B virus, as a means of assessing the immune response. There was no difference in the frequency of clinical benefit amongst patients who did and did not elicit anti-HBV antibodies. However, disease control rates were higher amongst patients who elicited a response to at least one TUMAP (20/27 patients), and highest amongst patients who elicited a response to 2 or more TUMAPs (8/27 patients). Notably, patients with a lower proportion of regulatory T-cells (Tregs) at baseline appeared to have a more profound TUMAP immune response. A more detailed assessment of immune response kinetics was performed in a total of 9 subjects; these studies suggested that the immune response to TUMAPs might be sustained for several months.
A phase II study of IMA901 was subsequently conducted in which 68 patients were randomized to receive either IMA901 alone (n=35), or IMA901 preceded by a single dose of cyclophosphamide at 300 mg/m2.14 The premise for cyclophosphamide use is complex, and involves the theory that priming with the cytotoxic may reduce the burden of Tregs and thereby augment the TUMAP immune response.26 A total of 17 vaccinations were delivered over 9 months on each of the study arms. Baseline characteristics were similar between the two groups. Interestingly, it was found that increasing age was associated with a blunted TUMAP response. Ultimately, amongst those patients that elicited an immune response to IMA901, cyclophosphamide pre-treatment was associated with a significantly prolonged survival (HR=0.38, P=0.04). In contrast, cyclophosphamide pre-treatment had no impact on survival in the subset of patients who lacked an immune response. Supporting the aforementioned hypotheses to explain this observation, Treg quantity was significantly reduced by cyclophosphamide pre-treatment (P=0.013).
Further immune-based assays were performed to identify a more expansive immune profile associated with response.14 Subsets of myeloid derived suppressor cells (MDSCs) were assessed – notably, these cells (akin to Tregs) can stifle the antitumor immune response. Ultimately, elevations of MDSCs characterized by either CD14+HLA−DR−/lo or CD11b+CD14-CD15+ were associated with shorter OS. Expression of the T-cell receptor-ζ (TCR-ζ), which appears to be amplified in the setting of arginine depletion, was positive associated with survival.
IMA901 has recently been assessed in a phase III clinical trial, the results of which are currently pending (Figure 1).27 In this trial, patients were randomized in a 3:2 fashion to receive either sunitinib with IMA901 or sunitinib alone. Interestingly, the dosage and schedule utilized in this study vary from the phase I and II experiences. Specifically, a total of 10 vaccinations with IMA901 were delivered over the course of 4 months. Eligibility for the trial was limited to patients who had received no prior therapy for metastatic disease and those with favorable- or intermediate-risk based on criteria established by Heng et al.28 The primary endpoint for the study was OS, with secondary endpoints including OS based on biomarker-stratified subsets, PFS, safety and immune response. Pre-specified stratification factors in the study included risk group (low v intermediate), geographic region, and nephrectomy status. The results of these studies are eagerly anticipated.
Figure 1.
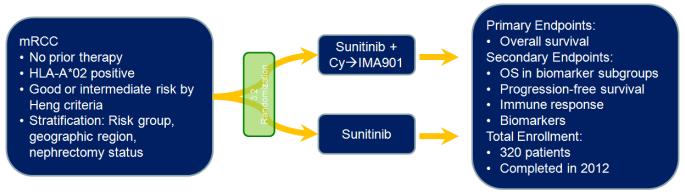
Schema showing the randomization employed in the phase III assessment of IMA901. Note that cyclophosphamide (Cy) is offered as a single pulse dose of 300 mg/m2 prior to initiation of IMA901. IMA901, given with GM-CSF, is delivered over a total of 4 months with a series of 10 consecutive vaccinations. Sunitinib is given on the approved schedule, namely 50 mg daily (4 weeks on, 2 weeks off).
Autologous Vaccines: AGS-003
AGS-003 represents an autologous dendritic cell vaccine, generating through electroporation of tumor-derived RNA into host immune cells.29 These host immune cells presumably become reactive to tumor antigens through IL-12 dependent processes. The approach to clinical evaluation of AGS-003 was distinct from IMA901 – while the preliminary studies of IMA901 focused on monotherapy, AGS-003 was evaluated in combination with sunitinib. The choice of paired therapy with sunitinib was based on mounting preclinical evidence suggesting that sunitinib may decrease Treg function, and thereby augment the antitumor immune response (discussed subsequently).30
A phase II assessment of AGS-003 with sunitinib included a total of 21 patients.31 Because fresh tumor tissue was required, the study was limited to patients who had received cytoreductive nephrectomy. Patients receiving cytoreductive nephrectomy implicitly have de novo metastatic disease, and on this basis are characterized as either intermediate- or poor-risk. Ultimately, by MSKCC criteria, 15 patients (71%) had intermediate-risk disease, while 6 patients (29%) had poor risk disease. Evaluation by the Heng criteria yielded an even larger proportion of patients (10 of 21, or 48%) with poor-risk disease. All but one patient had clear cell histology.
Subsequent to cytoreductive nephrectomy, primary tumor tissue was sent to a central laboratory, along with products from leukapheresis.31 After one 6 week cycle of sunitinib, patients received 5 consecutive doses of AGS-003 every 3 weeks, followed by quarterly administration of sunitinib. Sunitinib and AGS-003 were continued until progression or unacceptable toxicity. An impressive median OS of 30.1 months was achieved in this study. Although no comparator arm was utilized, an evaluation of the International mRCC Database Consortium dataset suggested that that the median OS expectation for a similar population of patients was only 15 months. The primary biomarker assessment paired to the phase II assessment of AGS-003 was an evaluation of memory T-cells.32 Increases in this T-cell subset from baseline to the 5th dose of AGS-003 was associated with prolonged survival.
Based on these encouraging data, a phase III effort (the “ADAPT” study) has been launched (Figure 2).33 This trial will enroll a total of 450 patients randomized in a 2:1 fashion to either sunitinib with AGS-003 or sunitinib alone. Akin to the original phase II design, patients must have de novo metastatic disease, and fresh tumor specimen will be centrally processed along with leukapheresis product. While the original study utilized an induction regimen comprised of 5 doses of AGS-003 every 3 weeks, the ADAPT trial will use a total of 8 doses of AGS-003 over 48 weeks, and treatment with AGS-003 will be offered quarterly thereafter. The study has initiated enrollment, and is designed (with 80% power) to detect a roughly 30% improvement in OS with sunitinib and AGS-003 as compared to sunitinib alone.
Figure 2.
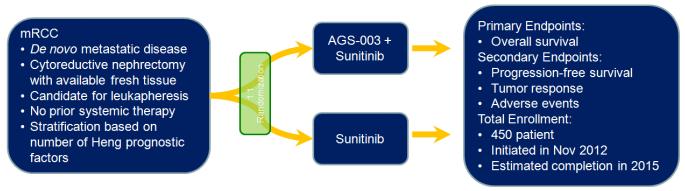
Schema showing the randomization employed in the phase III assessment of AGS-003. AGS-003 is delivered every six weeks for a total of 48 weeks. Patients with stable disease or better at 48 weeks may continue AGS-003 injections quarterly until progressive disease is noted.
Antigen-Directed Vaccines: TG-4010, MVA-5T4
While IMA901 is comprised of multiple antigens, several vaccines have been designed with the intent of generating an immune response to a single antigen. Two examples of this are TG-4010 and MVA-5T4. In the case of TG-4010, the antigen of interest is MUC1, a cell surface glycoprotein. In normal tissue, MUC1 appears to have a protective effect upon epithelial cells by forming a lubricated barrier.34 However, in the setting of cancer, MUC1 overexpression may mask cell surface antigens and thereby stifle the antitumor immune response. Tissue-based studies of MUC1 expression in RCC have suggested that the moiety is an independent prognostic factor.35,36 Furthermore, as noted previously, MUC1 was one of the proteins identified via the XPRESIDENT platform for inclusion in the multi-peptide panel that constitutes IMA901.14
TG-4010 is formulated from a recombinant modified vaccinia virus (MVA) expressing both IL-2 and MUC1 antigen. Two phase I studies of TG-4010 were reported simultaneously, one study conducted in Europe and the other in the US. Notably, these studies included a wide spectrum of malignancies.24 Limited side effects were observed, and 4 of 13 evaluable patients had SD noted for 6-9 months. Consistent with the latent responses that have been observed with several immunotherapeutic agents, one lung cancer patient progressed initially, but later had a response lasting over 1 year. These preliminary results fueled much larger explorations in non-small cell lung cancer (NSCLC).
A phase II study was conducted in mRCC.37 Enrollment in this effort was limited to those patients with documented MUC1 expression by immunohistochemistry, either in primary tumor or metastatic tissue. No prior therapy was permitted in this trial. Ultimately, a total of 37 patients were enrolled. Although no objective responses were observed, 5 of 27 evaluable patients (18%) had SD in excess of 6 months. Notably, the majority of patients that had progressed on TG-4010 alone were treated subsequently with TG-4010 and cytokines. Of 20 evaluable patients, 6 patients (30%) had SD in excess of 6 months. Median OS was 19.3 months for the overall cohort. Notably, an antibody response to MVA or IL-2 was associated with a modest survival advantage. Patients who had a CD8+ T-cell response to MUC1 were also noted to have a slight survival advantage. Given the limited responses and modest survival associated with TG4010, it is unclear whether it will move forward in this malignancy. An ongoing phase III study in non-small cell lung cancer (NSCLC) will randomized patients with MUC1-overexpressing tumors to chemotherapy with or without vaccine.38 This trial may represent the primary development path for this agent.
Important lessons have been gleaned from a phase III study examining MVA-5T4, a distinct antigen-directed vaccine therapy.39 The vaccine is constituted by a MVA that delivers the oncofetal antigen 5T4, a moiety primarily expressed in tumor tissue and placental tissue (but rarely in normal adult tissue). Significant elevations in 5T4 expression have been noted in mRCC. Early encouraging data with MVA-5T4 across a spectrum of malignancies (colorectal cancer, mCRPC, and RCC) led to the establishment of a phase III program in mRCC.40 Patients with treatment-naïve disease were enrolled and randomized in a 1:1 fashion to standard of care therapy with either MVA-5T4 or placebo. A total of 732 patients were randomized; the majority received IFN-α as their standard of care regimen (n=374), while a similar number of patients received IL-2 (n=170) or sunitinib (n=185). The study failed to meet its primary endpoint of improving OS (20.1 months with MVA-5T4 as compared to 19.2 months with placebo; P=0.55). However, there were several subsets that seemed to derive more substantial benefit. For example, a significant improvement in survival with MVA-5T4 (as compared to placebo) was seen amongst those patients that received IL-2 and had a good prognosis by MSKCC criteria (HR 0.54, P=0.046). Unplanned subset analyses based on hematologic parameters suggested that those patients with normal platelets, monocytes and hemoglobin derived greater benefit. Correlative analyses also revealed that an increase in the 5T4 antibody response was associated with improvement in survival, consistent with previous reports.
Rationale for combining vaccines and targeted agents
Early data from several of the vaccine therapies discussed herein are quite encouraging. However, it remains to be seen how these treatments will be incorporated in a crowded landscape of therapies for mRCC. The pivotal trials of AGS-003 and IMA901 assess a combination of vaccine with sunitinib. Recent data from the COMPARZ study, a phase III effort comparing sunitinib and pazopanib in treatment-naïve patients, suggests that pazopanib may have a preferable toxicity profile and yield superior quality of life (QOL), while maintaining similar efficacy.41 These data question the relevance of a sunitinib based regimen. It is problematic to assume that all VEGF-directed therapies will synergize with vaccine-based treatments in a similar fashion. For instance, several preclinical studies have demonstrated that sunitinib may augment the antitumor immune response by decreasing recruitment of myeloid derived suppressor cells (MDSCs) and Tregs to tumor tissue (Figure 3).30,42 In contrast, similar studies assessing sorafenib have demonstrated the opposite effect.43 As such, while agents such as sunitinib form a logical pairing with vaccine-based treatments, agents such as sorafenib may not be ideal. The data from COMPARZ may lead to greater utilization of pazopanib in the first-line setting, but the immune effects of the agent are more poorly characterized.44 Furthermore, only limited data is available for other challengers in the front-line setting, such as axitinib and tivozanib. Exploration of the immune-related effects of pazopanib, axitinib and tivozanib may identify agents amongst these that are best suited for combination with vaccine therapies.
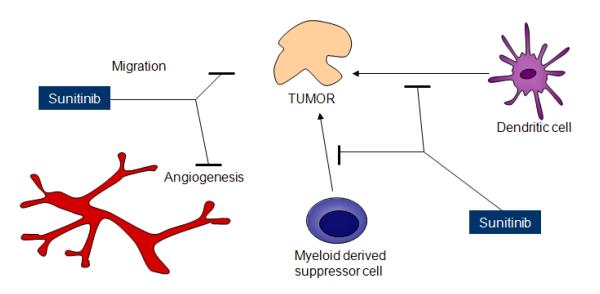
Figure 3.
Schema outlining the proposed mechanisms of action of sunitinib. Although sunitinib is well known to inhibit angiogenesis, abrogation of MDSC recruitment to tumor sites by sunitinib may promote the antitumor immune response, suggesting an alternative mechanism of action.
Beyond combinations with VEGF-directed therapies, it may also be critical to understand the utility of pairing vaccine therapies with mammalian target of rapamycin (mTOR) inhibitors and other emerging therapies. The mTOR inhibitor temsirolimus is appropriate for treatment-naïve patients with poor-risk disease. Although the phase II study of sunitinib with AGS-003 evaluated patients with largely intermediate- and poor-risk disease, in a community based setting, treatment with temsirolimus may be the preferred approach for the latter category.2 As such, studies investigating the combination of temsirolimus with AGS-003 may be warranted. For those patients who progress on VEGF-directed therapies, the mTOR inhibitor everolimus is a preferred second- or third-line option.45 Although synthesis of an autologous vaccine may not be feasible for patients with refractory disease (since fresh tumor specimens are not available for antigen retrieval), combinations of everolimus with multipeptide vaccines may be of utility. Other distinct classes of agents show great promise in mRCC, including fibroblast growth factor (FGFR) inhibitors and MET inhibitors such as dovitinib and cabozantinib, respectively.46,47 It will be critical to assess the efficacy of RCC vaccines in concert with these agents.
Future Directions
While the ideal pairings of vaccine and targeted agent are established, it would be important to consider another key issue in parallel – therapeutic sequencing. The antigenic response elicited by each of the vaccines noted herein is distinct. As such, there may be rationale for patients to be exposed to more than one vaccine-based strategy. The sequence of these vaccines is critical, as it is now well documented that significant attrition occurs across first-, second- and third-line therapy.48 Due to logistical issues (i.e., requirement for fresh tumor), AGS-003 must be offered in the front-line setting, but it remains unclear whether administration of this autologous vaccine would be preferable to a multipeptide vaccine such as IMA901. One can envision subsequent trials comparing these two approaches prospectively – while this would be an immense effort, data pertaining to the “comparative effectiveness” of these strategies is key. Of course, the entry of other “immunotherapeutic” agents, such as PD-1 inhibitors and CTLA4 inhibitors, may further challenge the positioning of vaccine-based therapies.
Comparative effectiveness research is also valuable from the standpoint of evaluating the relative financial burden of vaccine therapies. Important lessons have been learned from the experience with sipuleucel-T in mCRPC – while the agent does yield a modest improvement in OS, it does so at an immense cost to the patient and/or payor.12,49 The cost of vaccine therapies superimposed on the cost of targeted therapies may not be sustainable in the current healthcare climate. One potential measure that could mitigate the cost of vaccine-based therapies is to identify relevant biomarkers that define susceptible populations. For instance, if IMA901 is particularly effective in those patients with low Treg quantities at baseline, it may be better to limit its use to this population. Post-hoc biomarker analyses (i.e., assessing the change in memory T-cells after treatment with AGS-003) may be less useful for this purpose, as the patient would need to embark on therapy in order to establish the biomarker trend. Investing heavily in defining predictive biomarkers now may spare significant costs in the future.
There is no shortage of vaccine-based strategies currently under assessment in mRCC. Table 1 delineates several early efforts not detailed in the current manuscript. While certain vaccine therapies hold incredible promise in mRCC, there are several obstacles to clinical implementation that must be overcome. First, the most relevant synergistic combinations of targeted therapies and vaccine must be identified, as monotherapy with vaccines appear to offer more limited efficacy. With sunitinib possibly being usurped by pazopanib and tivozanib in the front-line setting, the investigative community must work quickly to validate logical pairings. Second, the optimal sequence (or perhaps combination) of vaccine therapies must be established. As noted, this dilemma is not new to mRCC – there is still a lively debate surrounding the optimal sequence of targeted agents for this disease. Finally, the financial burden of these therapies must be addressed. Despite the immense research efforts underway to bring RCC vaccine therapies to the clinic, economic considerations may ultimately hinder clinical implementation.
Table 1.
Summary of recently initiated trials of vaccine based therapies for mRCC. Note that several larger studies are detailed in the manuscript and in the figures enclosed below.
| Study Number | N | Study Design/Agent | Description |
|---|---|---|---|
| NCT0086230350 | 100 | Randomized phase I/II DC-CIK IL-2/IFN-α |
|
| NCT0152282051 | 20 | Phase I Sirolimus DEC-205-NY-ESO-1 fusion protein vaccine |
|
| NCT0091391352 | 24 | Phase 2 DC vaccine Bevacizumab IL-2 IFN-α |
|
Acknowledgements
Dr. Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP) and NIH K12 2K12CA001727-16A1.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 5.Pal SK, Bergerot PG, Figlin RA. Tivozanib: current status and future directions in the treatment of solid tumors. Expert Opin Investig Drugs. 2012;21:1851–9. doi: 10.1517/13543784.2012.733695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael C, Lau C, Josephson DY, Pal SK. Comprehensive overview of axitinib development in solid malignancies: focus on metastatic renal cell carcinoma. Clin Adv Hematol Oncol. 2012;10:307–14. [PubMed] [Google Scholar]
- 7.Motzer RJ, Eisen T, Hutson TE, et al. Overall survival results from a phase III study of tivozanib hydrochloride versus sorafenib in patients with renal cell carcinoma. J Clin Oncol. 2013;31 doi: 10.1200/JCO.2012.47.4940. [Abstr 350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutson TE, Gallardo J, Lesovoy V, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;31 doi: 10.1016/S1470-2045(13)70465-0. [Abstr LBA348] [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Drake CG, Wollner I, et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. Journal of Clinical Oncology. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott DF, Drake CG, Sznol M, et al. A phase I study to evaluate safety and antitumor activity of biweekly BMS-936558 (Anti-PD-1, MDX-1106/ONO-4538) in patients with RCC and other advanced refractory malignancies. Presented at the 2011 Genitourinary Cancers Symposium; 2011. [Abstr 331] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current Opinion in Immunology. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Serum Antibodies to Blood Group A Predict Survival on PROSTVAC-VF. Clinical Cancer Research. 2013;19:1290–9. doi: 10.1158/1078-0432.CCR-12-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt SM, Schag K, Muller MR, et al. Induction of adipophilin-specific cytotoxic T lymphocytes using a novel HLA-A2-binding peptide that mediates tumor cell lysis. Cancer Res. 2004;64:1164–70. doi: 10.1158/0008-5472.can-03-2538. [DOI] [PubMed] [Google Scholar]
- 16.Sukov WR, Ketterling RP, Lager DJ, et al. CCND1 rearrangements and cyclin D1 overexpression in renal oncocytomas: frequency, clinicopathologic features, and utility in differentiation from chromophobe renal cell carcinoma. Hum Pathol. 2009;40:1296–303. doi: 10.1016/j.humpath.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Saino M, Maruyama T, Sekiya T, Kayama T, Murakami Y. Inhibition of angiogenesis in human glioma cell lines by antisense RNA from the soluble guanylate cyclase genes, GUCY1A3 and GUCY1B3. Oncol Rep. 2004;12:47–52. [PubMed] [Google Scholar]
- 18.Behrends S, Vehse K, Scholz H, Bullerdiek J, Kazmierczak B. Assignment of GUCY1A3, a candidate gene for hypertension, to human chromosome bands 4q31.1-->q31.2 by in situ hybridization. Cytogenet Cell Genet. 2000;88:204–5. doi: 10.1159/000015548. [DOI] [PubMed] [Google Scholar]
- 19.Salagierski M, Verhaegh GW, Jannink SA, Smit FP, Hessels D, Schalken JA. Differential expression of PCA3 and its overlapping PRUNE2 transcript in prostate cancer. Prostate. 2010;70:70–8. doi: 10.1002/pros.21040. [DOI] [PubMed] [Google Scholar]
- 20.Burgess TL, Sun J, Meyer S, et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther. 2010;9:400–9. doi: 10.1158/1535-7163.MCT-09-0824. [DOI] [PubMed] [Google Scholar]
- 21.Heymach J, Tran HT, Fritsche HA, et al. Lower baseline levels of plasma hepatocyte growth factor (HGF), IL-6 and IL-8 are correlated with tumor shrinkage in renal cell carcinoma patients treated with pazopanib. Mol Cancer Ther. 2009;8(12 Suppl) Abstr A11. [Google Scholar]
- 22.Schöffski P, Garcia JA, Stadler WM, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU International. 2011 doi: 10.1111/j.1464-410X.2010.09947.x. no-no. [DOI] [PubMed] [Google Scholar]
- 23.Silini A, Ghilardi C, Figini S, et al. Regulator of G-protein signaling 5 (RGS5) protein: a novel marker of cancer vasculature elicited and sustained by the tumor’s proangiogenic microenvironment. Cellular and molecular life sciences : CMLS. 2012;69:1167–78. doi: 10.1007/s00018-011-0862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rochlitz C, Figlin R, Squiban P, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. The Journal of Gene Medicine. 2003;5:690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 25.Fang YJ, Lu ZH, Wang GQ, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. International journal of colorectal disease. 2009;24:875–84. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 26.Walter S, Weinschenk T, Reinhardt C, Singh-Jasuja H. Single-dose cyclophosphamide synergizes with immune responses to the renal cell cancer vaccine IMA901. Oncoimmunology. 2013;2:e22246. doi: 10.4161/onci.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [last accessed October 22, 2012]; NCT01265901: A Randomized, Controlled Phase III Study Investigating IMA901 Multipeptide Cancer Vaccine in Patients Receiving Sunitinib as First-line Therapy for Advanced/Metastatic Renal Cell Carcinoma. Available at http://clinicaltrials.gov/ct2/show/NCT01265901?term=IMA-901&rank=2.
- 28.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 29.Figlin RA, Amin A, Dudek A, et al. Phase II study combining personalized dendritic cell (DC)-based therapy, AGS-003, with sunitinib in metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2012;30:348. [Google Scholar]
- 30.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin A, Dudek A, Logan T, et al. Prolonged survival with personalized immunotherapy (AGS-003) in combination with sunitinib in unfavorable risk metastatic RCC (mRCC) ASCO Meeting Abstracts. 2013;31:357. [Google Scholar]
- 32.Figlin RA, Nicolette CA, Amin A, et al. Monitoring T-cell responses in a phase II study of AGS-003, an autologous dendritic cell-based therapy in patients with newly diagnosed advanced stage renal cell carcinoma in combination with sunitinib. ASCO Meeting Abstracts. 2011;29:2532. [Google Scholar]
- 33. [last accessed April 10, 2013]; NCT01582672: An International Phase 3 Randomized Trial of Autologous Dendritic Cell Immunotherapy (AGS-003) Plus Standard Treatment of Advanced Renal Cell Carcinoma (ADAPT) Available at http://www.clinicaltrials.gov.
- 34.Rochlitz C, Figlin R, Squiban P, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 35.Pajak J, Liszka L, Mrowiec S, Golka D, Lampe P. MUC1 immunoexpression is a virtually constant feature of clear cell renal cell carcinoma metastatic to the pancreas. Advances in anatomic pathology. 2012;19:125–7. doi: 10.1097/PAP.0b013e318248bd97. [DOI] [PubMed] [Google Scholar]
- 36.Aubert S, Fauquette V, Hemon B, et al. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res. 2009;69:5707–15. doi: 10.1158/0008-5472.CAN-08-4905. [DOI] [PubMed] [Google Scholar]
- 37.Oudard S, Rixe O, Beuselinck B, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60:261–71. doi: 10.1007/s00262-010-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. [last accessed April 10, 2013]; NCT01383148: A Phase IIB/III Randomized, Double-blind, Placebo Controlled Study Comparing First Line Therapy With or Without TG4010 Immunotherapy Product in Patients With Stage IV Non-Small Cell Lung Cancer (NSCLC) Available at http://www.clinicaltrials.gov.
- 39.Amato RJ, Hawkins RE, Kaufman HL, et al. Vaccination of Metastatic Renal Cancer Patients with MVA-5T4: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. Clinical Cancer Research. 2010;16:5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 40.Zhang RT, Bines SD, Ruby C, Kaufman HL. TroVax((R)) vaccine therapy for renal cell carcinoma. Immunotherapy. 4:27–42. doi: 10.2217/imt.11.160. [DOI] [PubMed] [Google Scholar]
- 41.Motzer R, Hutson TE, Reeves J, et al. Randomized, open-label, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (mRCC): Results of the COMPARZ trial. Ann Oncol. 2012;23 [Abstr LBA8_PR] [Google Scholar]
- 42.Finke JH, Rini B, Ireland J, et al. Sunitinib Reverses Type-1 Immune Suppression and Decreases T-Regulatory Cells in Renal Cell Carcinoma Patients. Clinical Cancer Research. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 43.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–20. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 44.Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–37. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 45.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 46.Angevin E, Grunwald V, Ravaud A, et al. A phase II study of dovitinib (TKI258), an FGFR- and VEGFR-inhibitor, in patients with advanced or metastatic renal cell cancer (mRCC) ASCO Meeting Abstracts. 2011;29:4551. [Google Scholar]
- 47.Choueiri TK, Pal SK, McDermott DF, et al. Efficacy of Cabozantinib (XL184) in Patients with Metastatic, Refractory Renal Cell Carcinoma. J Clin Oncol. 2012;30 [Abstr. [Google Scholar]
- 48.Vogelzang NJ, Signorovitch JE, Lin PL, et al. Sequential use of targeted therapies for metastatic renal cell carcinoma: A physician survey and chart review of community oncology practices in the United States. ASCO Meeting Abstracts. 2013;31:418. [Google Scholar]
- 49.Vasani D, Josephson DY, Carmichael C, Sartor O, Pal SK. Recent advances in the therapy of castration-resistant prostate cancer: the price of progress. Maturitas. 2011;70:194–6. doi: 10.1016/j.maturitas.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 51.Brichard VG, Lejeune D. GSK’s antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(Suppl 2):B61–71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. New England Journal of Medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]