Abstract
Macrophage migratory inhibitory factor (MIF) is a proinflammatory cytokine shown to promote tumorigenesis. Using the N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) model of bladder cancer, we previously showed that MIF knockout mice display decreased angiogenesis and invasion compared with wild-type. This study examines the role of MIF in bladder cancer via use of oral inhibitors of MIF. In vitro, high-grade bladder cancer cells were treated with recombinant human MIF +/− (rhMIF+/−) inhibitor. Measurements included cell counts, proliferation by 3H-thymidine incorporation (TdR), extracellular signal-regulated kinase (ERK) phosphorylation by western blot analysis, messenger RNA (mRNA) expression by quantitative PCR and protein secretion by enzyme-linked immunosorbent assay. Treatment with rhMIF increased ERK phosphorylation, cell counts, TdR and mRNA expression and protein secretion of vascular endothelial growth factor, which were blocked by specific inhibitors of ERK and MIF. In vivo, 3-month-old male C57Bl/6 mice were given BBN for 22 and 16 weeks in study 1 and study 2, respectively. Mice (n = 8–10 per group) were gavaged with vehicle or doses of MIF inhibitors daily from weeks 16–22 in both studies. Average bladder weights, reflecting tumor mass, tumor stage/burden, mitotic rate and proliferation indices, and microvessel densities were reduced in inhibitor groups versus controls. In summary, MIF promotes bladder cancer via increasing cell proliferation and angiogenesis and oral inhibitors of MIF may prove useful in treatment of this disease.
Introduction
Bladder cancer is the fourth most common solid malignancy in men and the 11th most common in women, representing 7 and 3% of all cancers, respectively. In 2012, there were an estimated 73210 new cases with 14880 deaths (1). Stage at diagnosis is fundamental to outcome. High-grade or muscle-invasive tumors tend to progress and metastasize with up to 50% of muscle-invasive tumors having occult metastatic disease at the time of diagnosis. Invasive and/or metastatic disease carries a relatively poor prognosis with 50% of patients with metastases dying within 2 years of diagnosis. Five year survival rates are as low as 6% (2). There are no feasible tumor markers capable of stratifying bladder cancer patients with regard to progression, prognosis or treatment. Currently used therapies remain disappointing as advanced bladder cancer still proves to be ultimately lethal.
Recent studies have suggested a role for proinflammatory cytokines in promoting tumorigenesis via stimulating cell proliferation, survival and neovascularization and inhibiting apoptosis (3). Macrophage migratory inhibitory factor (MIF) is a widely expressed proinflammatory molecule first described for its ability to inhibit the random migration of macrophages in vitro (4). Its participation in host response to inflammation and defense is well established (5). Additionally, MIF has been shown to contribute to tumorigenesis through many of the same pathways critical to wound healing and inflammation.
MIF has been implicated in lung, breast and prostate cancer, with overexpression shown to correlate with tumor grade/stage and prognosis (6–8). Bladder epithelial cells not only produce MIF but also display upregulation in response to diverse stimuli such as substance P and partial bladder outlet obstruction (9,10). Inhibition of MIF with hyaluronic acid, anti-MIF antibody or MIF antisense was shown to decrease in vitro bladder cancer cell proliferation and cytokine expression (11). In vivo, xenograft studies utilizing cells engineered to exhibit decreased MIF expression via small interfering RNA showed decreased tumor-associated angiogenesis and invasive capacity (12–14).
Using the N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) model of bladder cancer, we showed that MIF null mice developed non-muscle invasive bladder cancers with decreased neovascularity compared with their wild-type counterparts (15). MIF null mice display no developmental or basal phenotype yet show resistance to diverse stimuli, suggesting the possibility of MIF inhibition as a powerful tool for treatment of relevant diseases (16). Oral inhibitors of MIF based on isoxazoline compounds have been developed (Cytokine PharmaSciences, King of Prussia, PA) for human use and are being tested in animal models of non-cancer-related diseases (17,18). In this study, we explored the effects of MIF inhibition on cellular proliferation and angiogenesis in vitro and determined the preclinical efficacy of these novel inhibitors in mice exposed to the well-characterized bladder-specific carcinogen BBN.
Materials and methods
Materials
Recombinant human MIF (rhMIF) and MIF inhibitors (CPSI-2705 and -1306; USA; patent application numbers 20050250826 and PCT/US11/21721) were from Cytokine PharmaSciences. CPSI-1306 is a low molecular weight isoxazoline. CPSI-2705 is an analog of CPSI-1306 in which both the aryl substitution and the amide have been modified (see patent above and ref. 17,18). When evaluated in vitro in a MIF tautomerase assay, CPSI-1306 was found to be 10–50-fold more potent than CPSI-2705 and 100-fold more potent than the literature compound ISO-1. No cytotoxicity was observed for CPSI-1306 when evaluated in vitro in HEPG2 cells and it had an excellent cytochrome P-450 profile (IC50 > 50 µM for CYP1A2, CYP 2C9, CYP 2D6 and CYP 3A4 and IC50 > 8 µM for CYP 2C19). Additionally, in preliminary rat pharmacokinetics studies, CPSI-2705 was shown to have a shorter half-life compared with CPSI-1306 (personal communication). rhMIF was also purchased from R&D Biosystems (Minneapolis, MN) and used as a negative control. This is described by the company as a calibrator protein for MIF immunoassays with no biological activity (19). The extracellular signal-regulated kinase (ERK) inhibitor PD98059 was purchased from Enzo Life Sciences (Farmingdale, NY). BBN was purchased from TCI America (Portland, OR). All other chemicals were purchased from Sigma (St Louis, MO) unless otherwise stated.
Cell culture
Human HTB-5 (high grade, invasive) and HT-1376 (high grade, metastatic) bladder cancer cell lines were obtained from ATCC (Manassas, VA). The UROtsa (benign) urothelial cell line was a gift from Dr Brian Philips, University of Pittsburgh. HTB-5 and HT-1376 cells were cultured in modified Eagle’s medium (103700-021, Invitrogen, Grand Island, NY), and UROtsa cells were cultured in Dulbecco’s modified Eagle’s media, supplemented with 10% heat-inactivated fetal calf serum, 1mM sodium pyruvate, 2mM l-glutamine, 100U/ml penicillin and 50 µg/ml streptomycin at 37°C in a 5% CO2 in air atmosphere. To study the effects of exogenous MIF, HTB-5 cells were treated with rhMIF (0.1–100ng/ml) ± the inhibitor CPSI-1306 (0.5–500nM). To study the effects of endogenous MIF, HT-1376 cells were treated with CPSI-1306 (500nM). All control cultures were treated with the respective vehicles for drugs (<0.1% in concentration).
MIF enzyme-linked immunosorbent assay
Cell culture supernatants from UROtsa, HTB-5 and HT-1376 were assayed for MIF secretion using the Quantikine Human MIF Immunoassay (R&D Systems) as per manufacturer’s instructions.
Real-time (quantitative) PCR
Total RNA was extracted using TRIzol (Invitrogen). RNA (5 μg) was DNase treated (Ambion, Grand Island, NY) and converted to complementary DNA using High Capacity cDNA Archive Kit (Applied Biosystems, Grand Island, NY). Quantitative PCR was performed in 96 well plates using Assays-on-Demand Gene Expression system on a 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters (Applied Biosystems). Glyceraldehyde 3-phosphate dehydrogenase served as the endogenous control. Data analysis was performed using the relative standard curve method.
Cell counts
HTB-5 cells (10000 per well) were plated in 12 well dishes and grown for up to 4 days. Cells were rinsed with phosphate-buffered saline (PBS), suspended with 0.25% trypsin-ethylenediaminetetraacetic acid, centrifuged and resuspended in 1ml of culture medium. An aliquot of 100 µl of cell suspension was counted with a Coulter Counter (Beckman Coulter, Brea, CA).
3H-thymidine incorporation assay
HTB-5 or HT-1376 cells (40000 per well) were plated in 6 well dishes and grown for 2–4 days. Cells were pulsed with 5 µCi/ml 3H-thymidine (NEN Life Science, Waltham, MA) for the last 12h of the culture period and subsequently washed with PBS and extracted with 10% trichloroacetic acid. The trichloroacetic acid insoluble fraction was dissolved in 0.5M NaOH and counted by liquid scintillation. The amount of 3H-thymidine incorporated into each sample was normalized to the cell numbers in parallel cultures.
Western blot analysis
HTB-5 cells (0.3 × 106 per well) were plated in 6 well dishes and cultured to 70–80% confluence. Total cell lysates were obtained following manufacturer’s instructions (Cell Signaling, Danvers, MA). Ten micrograms of total proteins were run on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA). Membranes were washed with 1× Tris-buffered saline containing 0.05% Tween 20 (TBST), blocked in 5% wt/vol non-fat dry milk in 1× TBST and incubated either with rabbit monoclonal phospho-ERK (9101S, Cell Signaling) antibody or with mouse monoclonal total-ERK (9107S) in 5% wt/vol bovine serum albumin in 1× TBST overnight with gentle agitation at 4°C. For AKT, membranes were incubated with either a rabbit polyclonal phospho-pan AKT antibody (ab38449, Abcam, Cambridge, MA) or with a rabbit polyclonal total AKT1/2/3 (ab126811). After washing with TBST, membranes were incubated either with anti-rabbit horseradish peroxidase-conjugated secondary antibody or with anti-mouse secondary antibody (Cell Signaling). Signals were detected with LumiGLO chemiluminescent reagent (Cell Signaling). Bands were scanned for images and densitometry for phosphorylated/total ERK and phosphorylated/total AKT was performed using NIH Image J software (National Institutes of Health, Bethesda, MD).
Vascular endothelial growth factor enzyme-linked immunosorbent assay
Medium was removed from cultured HTB-5 cells, treated ± CPSI rhMIF (100ng/ml) ± CPS1-1306 (50nM), and vascular endothelial growth factor (VEGF) secretion was measured using HUMAN VEGF ELISA KIT (Invitrogen, Life Technologies) following manufacturer’s guidelines.
Animals
C57Bl/6 mice were purchased from Charles River (Wilmington, MA) and maintained at the University of Connecticut Health Center for Laboratory Animal Care under National Institutes of Health guidelines. All procedures were approved by an institutional animal care committee. Animals were housed in a controlled environment with a 12 h light–12 h dark cycle and provided food and water ad libitum.
BBN administration to animals
Three-month-old male C57Bl/6 mice were given BBN (0.05%) in drinking water in brown bottles for 22 weeks in study 1 and for 16 weeks in study 2. In study 1, 30 mice (n = 10 per group) received vehicle (methylcellulose, PEG 300 in a 19:1 ratio) or oral inhibitor CPSI-2705 or CPSI-1306 daily (25mg/kg) from weeks 16 to 22. In the second study, 60 mice (n = ~8 per group) received varying doses of CPSI-1306 (0.001, 0.01, 0.1, 1.0, 10 and 25mg/kg) or vehicle (PEG 400, H2O, 1:1 ratio) daily from weeks 16 to 22. MIF inhibitors were administered by gavage from weeks 16 to 22, corresponding to the time of progression from carcinoma in situ to invasive disease, as determined in our previous studies (15,20). Animals were inspected daily for general health, with weights recorded at least biweekly. Animals were euthanized by CO2 inhalation and death verified by cervical dislocation. Bladders were harvested, weighed, inflated with and placed in 10% buffered formalin in PBS for 24 h and then transferred to PBS.
Histologic analysis
Formalin-fixed bladders were bisected and embedded along the midsagittal plane and serially sectioned for whole-mount pathologic analysis. Evaluation was performed by a single pathologist (P.H.) in a blinded manner. Hematoxylin and eosin (H&E)-stained slides were assessed for tumor stage and grade using the AJCC/UICC and the WHO 2004/ISUP criteria established, respectively, for stage and grade of human bladder urothelial carcinomas (21,22). The mitotic rate was determined by the number of mitotic figures/100 total cells found in five consecutive high-power fields (×40) in the most mitotically active part of the tumor (23).
To assess the degree of inflammation in tumors, both chronic (mononuclear infiltrates) and acute (neutrophilic infiltrate) inflammation status were scored in H&E slides. Since chronic inflammation consisted primarily of lymphocytic aggregates, a scale of 1–3 was utilized for chronic inflammation denoting a single aggregate as 1, two aggregates as 2 and three or more as 3. Similarly, acute inflammation was scored based on intensity and distribution pattern as follows: scale 1 was described as scattered neutrophils, scale 2 was described as denser than scale 1 with adjacent neutrophils touching each other and scale 3 was described as intense crowding and overlapping of adjacent neutrophils.
Immunohistochemistry
As another measure of cell proliferation, we determined a proliferation index by staining bladder sections with proliferating cell nuclear antigen (PCNA) antibody. Sections were deparaffinized and rehydrated in graduated levels of alcohol. Antigen retrieval was performed in 10mM sodium citrate, pH 6.0 at 95°C for 20min. After incubation in 3% hydrogen peroxide, sections were incubated overnight at 4°C with a rabbit polyclonal anti-PCNA antibody (ab15497) at a 1:600 dilution in PBS/bovine serum albumin. Bound anti-PCNA antibody was detected with a secondary biotinylated anti-rabbit antibody (1:200) and visualized using diaminobenzidine. For negative controls, buffer was substituted for the primary antibody. The mean proliferation index for each specimen was determined by counting the number of PCNA-positive-stained cells/100 cells within two selected high-power (×40) fields of tumor, each with evidently higher proportion of PCNA positive tumor nuclei. Means for each group were then calculated.
Microvessel density (MVD) was assessed by immunohistochemical staining for platelet/endothelial cell adhesion molecule 1 (PECAM-1), which highlights the tumor/tissue vascularity. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Non-specific staining was blocked with Power Block™ (BioGenex, San Ramon, CA). Sections were stained with a goat polyclonal anti-PECAM antibody (Santa Cruz Biotechnology, M20 SC-1506) at a 1:500 dilution in PBS/bovine serum albumin. Bound anti-PECAM-1 antibody was detected with a secondary biotinylated horse-anti-goat antibody (1:200) and visualized using diaminobenzidine. MVD was calculated as the mean number of vessels from two determined microvascular ‘hot spots’ at the tumor–stromal interface or intratumoral, as described previously (24). Using an ocular grid, the MVD counts were done in three hotspot areas per specimen at ×200 magnification. Means for each group were then calculated for data analysis.
To assess MIF expression in vivo after administration of inhibitor, immunohistochemistry staining was done for MIF as described previously (15). Briefly, endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Non-specific staining was blocked with Power Block™ (BioGenex). Sections were stained with a rabbit poly clonal anti-MIF antibody (CPM300; Cell Sciences, Canton, MA) at a 1:1000 dilution. Bound antibody was detected with a secondary biotinylated anti-rabbit antibody (1:200) and visualized using diaminobenzidine.
Statistical analysis
Statistical differences were determined using SPSS software (IBM Corp., Somers, NY). Data are means ± SEM. t-Test was used for comparison of two variables, one-way analysis of variance for comparison of more than two independent variables and one dependent variable and two-way analysis of variance for comparison of two independent and two or more dependent variables. Post hoc testing was performed with either Bonferroni or Dunnett’s tests where appropriate.
Results
In vitro: screening of cell lines for endogenous MIF expression/secretion
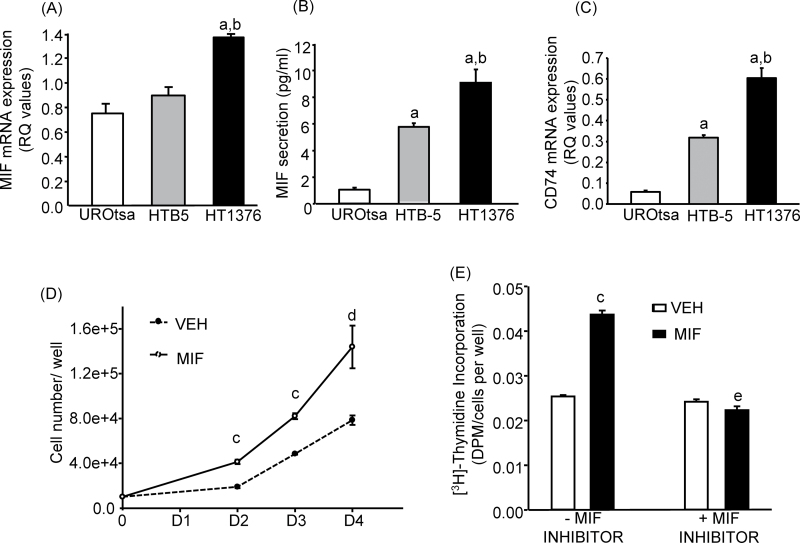
UROtsa, HTB-5 and HT-1376 were evaluated for basal MIF messenger RNA (mRNA) expression (Figure 1A) and its secretion in the medium (Figure 1B). Both MIF mRNA expression and secretion increased from benign (UROtsa) to malignant invasive (HTB-5) to malignant metastatic (HT-1376) cell lines. CD74 is the putative receptor for MIF and has been recently shown to display a positive relationship with MIF upregulation and stage/grade of cancer (25,26). MIF expression/secretion correlated well with the CD74 mRNA expression levels in the three cell lines tested. CD74 mRNA expression was 5- and 10-fold higher, respectively, for HTB-5 and HT-1376 cells compared with UROtsa (Figure 1C). HTB-5 cells were used for further experiments to test the effects of exogenous MIF (rhMIF) as they represented the moderate level of MIF expression and secretion, whereas HT-1376 cells were used to study the effects of endogenous MIF, using the MIF inhibitor, since they represented the highest level of MIF expression and secretion among the cell lines screened.
Fig. 1.
Endogenous MIF and CD74 expression levels and effects of CPSI rhMIF (100ng/ml) and oral MIF inhibitor CPSI-1306 (50nM). Cell lines were screened for endogenous MIF (A) mRNA expression by quantitative PCR, (B) secretion in media by enzyme-linked immunosorbent assay and (C) CD74 mRNA expression by quantitative PCR. HTB-5 cells were treated with the rhMIF or/and CPSI-1306 to evaluate (D) time course for cell numbers and (E) TdR on day 2 of culture, normalized to cell numbers in parallel cultures. Bars/symbols are means ± SEM for n = 3–6 wells of cells. aSignificantly different from UROtsa, P < 0.01. bSignificantly different from HTB-5, P < 0.05. cSignificantly different from vehicle, P < 0.01; d P < 0.05. eSignificant effect of CPSI-1306, P < 0.01.
In vitro: effect of rhMIF on cell growth and proliferation
A time course study was performed using HTB-5 cells to evaluate the effects of rhMIF on cell growth (Figure 1D). rhMIF from CPSI significantly increased cell counts (~2-fold) on culture days 2–4 with similar results noted in another independent experiment. To confirm that the increased cell counts were due to an increase in cell proliferation, HTB-5 cells were treated with rhMIF for 2 days and 3H-thymidine incorporation (TdR) was measured. rhMIF significantly increased TdR, normalized to cell number, up to 70% (Figure 1E) in two independent experiments. Cells were also treated with the MIF inhibitor CPSI-1306. CPSI-1306 did not have any effect on cell proliferation by itself but abrogated the effect of rhMIF (Figure 1E).
In vitro: effect of rhMIF on ERK and AKT phosphorylation
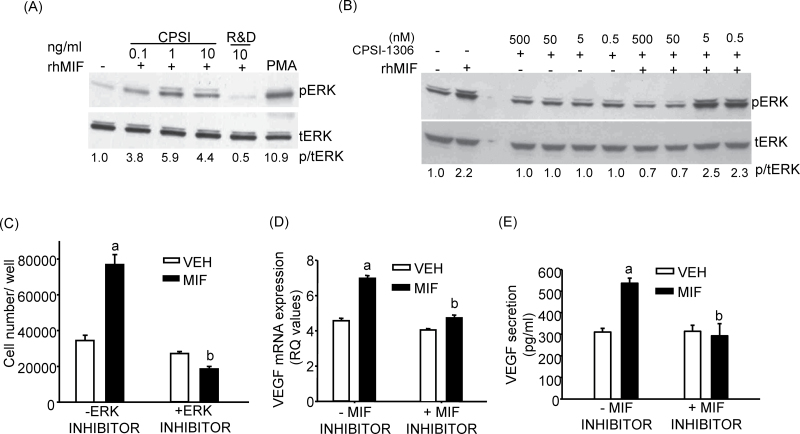
MIF has been shown to stimulate ERK and phosphoinositide-3-kinase (PI3K)/AKT signaling pathways in several cell types and activation of ERK and AKT has been shown to be involved in actions of MIF on tumorigenesis (6,27–29). CPSI rhMIF dose dependently stimulated ERK phosphorylation in HTB-5 cells compared with vehicle (Figure 2A). rhMIF from R&D systems, described as a calibrator for MIF immunoassays only with no bioactivity, was unable to stimulate ERK phosphorylation (Figure 2A). Treatment of HTB-5 cells with CPSI-1306 at doses >5nM antagonized the rhMIF-mediated stimulation of ERK phosphorylation, whereas CPSI-1306 treatment alone had no effect on ERK phosphorylation compared with vehicle (Figure 2B). Phosphorylated/total-ERK densitometry showed that CPSI-1306 blocked the rhMIF activation of ERK for doses 50 and 500nM. In a second similar experiment, CPSI-1306 again blocked the rhMIF activation of ERK for 50nM dose (data not shown). CPSI rhMIF also stimulated AKT phosphorylation in HTB-5 cells and this effect was inhibited by CPSI-1306 (Supplementary Figure 1, available at Carcinogenesis Online). Similar results were obtained in another experiment.
Fig. 2.
Effect of CPSI rhMIF and MIF inhibitor 1306 on ERK phosphorylation, cell counts in the presence of ERK inhibitor and VEGF mRNA and protein secretion. (A) Western blotting for p-ERK and t-ERK in HTB-5 cells treated with various doses of CPSI rhMIF or R&D rhMIF for 20min. HTB-5 cells were also given phorbol myristate acetate (PMA; 1 µM) for 20 min, which served as the positive control for p-ERK. (B) Western blot analysis for p-ERK and t-ERK in HTB-5 cells treated with 10ng/ml rhMIF ± various doses of MIF inhibitor CPSI-1306. Doses of MIF inhibitor were given 45 min prior to addition of rhMIF, which was given for the last 20min. Gels were quantified with Image J software and phosphorylated/total-ERK (p/t-ERK) normalized to vehicle. (C) Effect of ERK inhibitor PD98059 (50 µm) on cell numbers on day 2 in the presence or absence of CPSI rhMIF (100ng/ml). (D) Effect of CPSI rhMIF (100ng/ml) ± CPS1-1306 (50nM) on VEGF mRNA expression in HTB-5 cells at 24 h. (E) Effect of CPSI rhMIF (100ng/ml) ± CPS1-1306 (50nM) on VEGF protein secretion in media in HTB-5 cells at 36 h. Bars are means ± SEM for n = 3–6 wells of cells. aSignificantly different from vehicle, P < 0.01. bSignificant effect of CPSI-1306, P < 0.01.
In vitro: effect of inhibiting ERK on rhMIF-stimulated cell growth
To evaluate the involvement of ERK signaling in mediating the effect of MIF on cell counts, HTB-5 cells were treated with the specific inhibitor of ERK (PD98059, 50 µM). Although, a small, non-significant decrease was observed in cell counts with the ERK inhibitor treatment itself, rhMIF-stimulated increase in cell counts (2.5-fold) was completely inhibited by the addition of ERK inhibitor (Figure 2C). These results indicate that rhMIF may increase cell growth in an ERK-dependent manner.
In vitro: effect of rhMIF+/− inhibitor on VEGF expression
Recent studies have also shown that MIF-induced angiogenesis can lead to tumor progression (12). Treatment of HTB-5 cells with rhMIF for 24 h increased the mRNA expression of VEGF by 2-fold in two independent experiments. Cells were also treated with the MIF inhibitor CPSI-1306. CPSI-1306 had no effect on VEGF mRNA expression but abrogated the effect of rhMIF on VEGF expression (Figure 2D). Similarly, treatment of HTB-5 cells with rhMIF for 36 h stimulated the secretion of VEGF protein 1.7-fold in medium and this effect was abrogated in the presence of CPSI-1306 (Figure 2E). Both MIF and CD74 expressions are not only upregulated with stage/grade of cancer (25) but recent studies have also demonstrated that activation of CD74 via MIF initiates downstream signaling events that increase cell proliferation, survival and tumor progression (25,30). The mRNA expression for CD74 receptor was measured in the presence and absence of rhMIF (100ng/ml) ± CPSI-1306 (50nM) in HTB-5 cells treated with these agents for 24h. In one experiment, CD74 mRNA expression was increased from 0.6±0.05 to 0.9±0.01 (1.5-fold) with rhMIF treatment (P < 0.05). In another experiment, there was a trend toward increase (1.3-fold) in CD74 mRNA expression level with rhMIF (from 1.7±0.08 to 2.3±0.19) and this increase was not altered in the presence of CPSI-1306.
In vitro: impact of MIF inhibitor on the effects of endogenous MIF
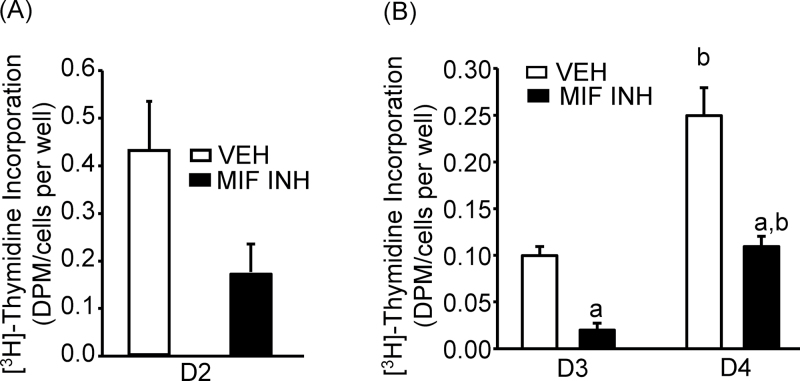
HT-1376 cells were used for the study. In one experiment, HT-1376 cells were treated with the MIF inhibitor CPSI-1306 (500nM) for 2 days. TdR was measured along with the cell counts in parallel cultures. In the presence of MIF inhibitor, TdR, normalized to total cell numbers, decreased, although non-significantly, by 61% compared with vehicle-treated controls (Figure 3A). In another experiment, HT-1376 cells were treated with CPSI-1306 for 4 days. TdR was measured along with the cell counts in parallel cultures on days 3 and 4. The addition of the MIF inhibitor significantly decreased TdR, normalized to total cell numbers, by 80 and 56%, respectively, on days 3 and 4 of culture (Figure 3B).
Fig. 3.
Impact of MIF inhibitor CPSI-1306 (500nM) on the effects of endogenous MIF in HT-1376 cells. (A) Effect on TdR at day 2. (B) Effect on TdR at days 3 and 4. Bars are means ± SEM for n = 3–6 wells of cells. aSignificant effect of CPSI-1306, P < 0.01. bSignificant effect of time, P < 0.01.
In vivo: identification of an effective MIF inhibitor
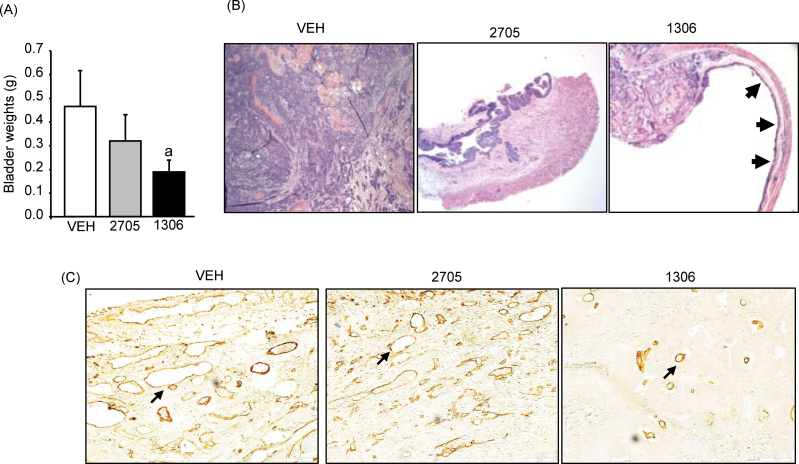
BBN-induced bladder carcinogenesis is a well-established model for studying invasive bladder cancer in rodents (31). Our previous laboratory studies showed that exposure of C57/Bl6 mice to BBN for 22 weeks resulted in reproducible muscle-invasive bladder tumors and that genetic ablation of MIF resulted in non-muscle-invasive tumors compared with wild-type (15). Here, we utilized the BBN model of bladder carcinogenesis to study the efficacy of two oral inhibitors of MIF. All mice were given BBN in drinking water for 22 weeks. Vehicle or MIF inhibitors (CPSI-2705 and CPSI-1306) were given by gavage daily from weeks 16–22, time anticipated for the onset of invasive disease. Prior to initiation of gavage, all animals showed equal weight gain (data not shown) and appeared to be in good health. During gavage therapy, there was 19% weight loss in the vehicle group compared with 14 and 8% in the CPSI-2705- and CPSI-1306-treated groups (not statistically significant), respectively. In general, the group receiving CPSI-1306 appeared healthier (less lethargic, more aggressively resisting gavage) compared with vehicle-treated and CPSI-2705-treated mice. Two animals each from the vehicle and CPSI-2705 groups died (~week 21) prior to completion of the study. Their bladders were harvested within 12 h of death and included in the final analysis. At time of euthanasia, the bladders appeared smaller for both inhibitor groups compared with the vehicle-treated group. Average bladder weights, measured as a surrogate for tumor volume (Figure 4A), were 0.46±0.15, 0.32±0.11 and 0.19±0.05 g for control, CPSI-2705 and CPSI-1306, respectively (P = 0.048, vehicle versus CPSI-1306).
Fig. 4.
Impact of MIF oral inhibitors CPSI-2705 and CPSI-1306 on bladder weights, bladder pathology and MVD in the BBN model of bladder cancer. (A) Bladder weights. Bars are means ± SEM (n = 8–10). aSignificant effect of CPSI-1306 versus vehicle, P = 0.048 (log-transformed t-test). (B) H&E-stained bladder sections (×40) representing infiltrative pT3 cancer replacing normal bladder anatomy in the vehicle-treated group; pT1 disease in the CPSI-2705 group and smaller/focal pT3 with areas of normal mucosa in the CPSI-1306, as depicted by black arrows. (C) Representative pictures of PECAM-1 staining (×40) for bladder specimens. Vascular structures labeled with PECAM-1 stained brown as shown by black arrows.
Pathologically, there was a higher proportion of pT3 disease in the control compared with both inhibitor groups (Table I). Several bladders in the inhibitor treatment arms were noted to have only focal areas of tumor with otherwise normal urothelium (Figure 4B). Tumor grade was generally lower in the inhibitor treatment groups, with more metaplasia than dysplasia noted in the non-tumoral urothelium (data not shown). Representative PECAM-1 staining for MVD measurement is shown in Figure 4C. MVD values were 42±3.6 for vehicle, 42±4.7 for CPSI-2705 and 30±1.9 for CPSI-1306. This was statistically significant (P = 0.04) for the CPSI-1306 versus vehicle group (analysis of variance, Dunnet’s post hoc).
Table I.
Bladder cancer stage in first inhibitor study
| Stage | Treatment | ||
|---|---|---|---|
| Vehicle | CPSI-2705 | CPSI-1306 | |
| pT1 | 1 | 3 | 2 |
| pT2 | 1 | 1 | 2 |
| pT3 | 8 | 6 | 6 |
Pathologic outcomes for groups treated with vehicle or inhibitors showed a trend toward lower stages in the drug groups (n = 10 per group). Tumors in the treatment groups were smaller by weight and tissue involvement.
In vivo: CPSI-1306 dose–response study
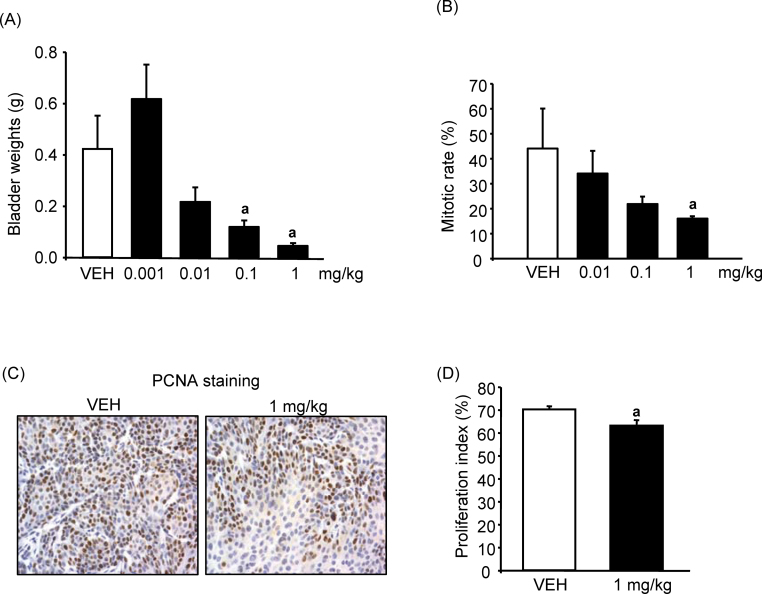
In our first study, as expected, treatment with CPSI-1306 appeared to have the most activity in the BBN carcinogen model. Hence, we performed an additional dose–response study using CPSI-1306. We truncated BBN administration at 16 weeks to mitigate the impact of continual carcinogen exposure on the effects of the inhibitor. As previously noted, this is when carcinoma in situ is anticipated and would more closely parallel administration in a clinical setting. Various doses of CPSI-1306 were given from 16 to 22 weeks. Mean bladder weights ± SE (Figure 5A) at euthanasia for the vehicle, 0.001, 0.01, 0.1 and 1.0mg/kg groups were 0.42±0.13, 0.61±0.13, 0.21±0.05, 0.12±0.02 and 0.05±0.0g, respectively, with the 0.1 and 1.0mg/kg doses being statistically significantly different from control (P = 0.004). A significant number of mice from the higher dose range (10 and 25mg/kg) groups were lost due to fighting prior to drug administration. This made the group size too small to perform statistical analysis.
Fig. 5.
Impact of increasing doses of MIF oral inhibitor CPSI-1306: (A) bladder weights, (B) mitotic body counts, (C) PCNA staining (×100) for bladder specimens to quantify proliferation index and (D) proliferation index. Bars are means ± SEM for n = 4–8. aSignificant effect of drug, p < 0.05.
Pathologic stage trended lower across the statistically significant doses with less pT3 and more pT1 tumors (Table II). No difference was observed in either the status of acute or chronic inflammation between control versus 0.1 or 1mg/kg dose (data not shown). Mitotic rate on H&E-stained slides trended lower across dosing with the 1mg/kg dose being statistically significant (P < 0.05) compared with vehicle (Figure 5B). To confirm the mitotic activity data, a proliferation index was performed on the 1mg/kg dose versus vehicle after quantifying the PCNA positive staining as described in Materials and methods (Figure 5C). The proliferation index significantly (P = 0.046) decreased from 70.2±1.4 (vehicle group) to 63.2±2.4 (1 mg/kg) (Figure 5D). To assess MIF protein expression after administration of CPSI-1306, sections of tumors from control and 1mg/kg dose bladders were labeled with MIF antibody. No difference was seen in either the pattern or intensity of MIF staining between control and 1mg/kg inhibitor group (data not shown) as would be expected given the mechanism of action of the inhibitors. Taken together, these results indicate that the MIF inhibitor CPSI-1306 was effective at decreasing tumor stage, proliferation and angiogenesis in mice exposed to BBN.
Table II.
Bladder cancer stage in the dose escalation study
| Stage | Treatment | ||||
|---|---|---|---|---|---|
| Vehicle | 0.001 | 0.01 | 0.1 | 1.0 | |
| pT1 | 0 | 0 | 1 | 2 | 1 |
| pT2 | 3 | 0 | 0 | 1 | 3 |
| pT3 | 3 | 3 | 4 | 3 | 0 |
Pathologic outcome observed in mice treated with vehicle or doses of CPSI-1306 (n = 3–7 per group). Tumors in the treatment groups were smaller by weight and tissue involvement.
Discussion
Treatment of high-grade bladder cancer has remained essentially unchanged for decades. If disease is superficial, such as carcinoma in situ or T1, treatment options include intravesical therapy or radical surgery. Although the use of intravesical chemotherapy may increase the time to recurrence, it is clear that this approach has little to no impact on disease progression (32). Bacillus Calmette–Guerin intravesical immunotherapy increases time to first recurrence compared with chemotherapeutic agents and is currently the standard of care therapy. However, a question remains regarding its value in reduction of progression. Several meta-analyses have attempted to address this issue. In the largest meta-analysis of bacillus Calmette–Guerin treatment, the expected relative risk reduction in disease progression is championed at 27%, yet the absolute risk reduction is only 4% (13.8 to 9.8%) (32). Despite the risk reduction in progression, bacillus Calmette–Guerin therapy was not shown to have an impact on either disease specific or overall survival. Therefore, there is a desperate need for the development of novel therapies that will prove ultimately effective in the prevention of disease progression. We provide evidence indicating small-molecule inhibitors of the MIF pathway, such as CPSI-1306, may have therapeutic utility for the management of high-grade bladder cancer.
The first reported link between MIF and cancer came from xenograft mouse model studies using 38C13 B-cell lymphoma and G361 melanoma cells (33,34). Tumor-bearing animals were administered a neutralizing anti-MIF antibody, which resulted in reduced tumor growth and angiogenesis. Mechanisms underlying MIF-dependent tumor promotion are still being investigated and have been shown to involve activation of MAPK/ERK and PI3K/AKT signaling pathways, which are important for cellular proliferation and survival (27,28). MIF has also been reported to inhibit p53 tumor suppressor activity in RAW264.7 cells and peritoneal macrophages resulting in cell growth and preventing cell-regulated apoptosis (35). MIF has been shown to increase cyclin D transcription leading to increased phosphorylation of pRb resulting in cellular proliferation (36,37). Recent studies have also suggested that MIF expression can lead to HIF-1α activation under hypoxic conditions, which serves to enhance a proangiogenic transcriptional event integral to cancer growth, invasion and metastasis (12,38,39).
Previously published data from our laboratory and several others strongly suggest that increased MIF expression plays an important role in promoting oncogenesis and tumor progression in a variety of malignancies (6–21). In addition, MIF has been described as a potential predictive biomarker for disease progression in hepatocellular and non-melanoma skin carcinoma (40). Taken together, these findings suggest that targeting MIF with the use of specific inhibitors may represent an important strategy for treating various cancers. The MIF inhibitor CPSI-1306 is an isoxazoline compound that has been shown to inhibit the tautomerase activity of MIF. In binding to the enzymatic pocket, it alters the three-dimensional protein structure thereby preventing its interaction with the putative receptor CD74 (41). Due to its cytostatic rather than cytotoxic activity, CPSI-1306 has great potential for clinical translation. It has been used in other non-cancer inflammation models of disease with positive results. In an experimental autoimmune encephalomyelitis animal model of multiple sclerosis, the use of CPSI-1306 and CPSI-2705 resulted in a reduction in the severity of clinical signs of disease and also reduced relapses of disease in established and relapsing/remitting models of experimental autoimmune encephalomyelitis, respectively (17). Likewise, the use of CPSI-1306 in the streptozotocin-induced non-insulin-dependent diabetes mellitus mouse model resulted in a significant lowering of blood glucose and inflammatory cytokine levels (18). Studies using other MIF inhibitors serve as further support for the suitability of inhibiting this inflammatory cytokine in a malignant setting. For example, intraperitoneal administration of the small-molecule MIF inhibitor ISO-1 in an orthotopic model of colorectal cancer resulted in decreased tumor volume and decreased tumor-associated angiogenesis (42). In spite of promising results with ISO-1, the route of administration and in vivo toxicity suggest that ISO-1 is not suitable for human use (43). Although the mechanism by which MIF inhibition slows tumor progression is not completely understood, other isoxazoline-based MIF inhibitors were shown to inhibit MIF-mediated proinflammatory activities, including macrophage chemotactic migration, nuclear factor-kappaB pathway activity and release of proinflammatory cytokines such as IL-1α, IL-1β, IL-6 and tumor necrosis factor-α both in vitro and in vivo (44).
We describe the use of an oral inhibitor of MIF in the setting of carcinogen-induced bladder cancer. Similar to other studies of inflammatory diseases utilizing these inhibitors, we found a significant improvement in disease status. There was a reduction in tumor burden following carcinogen exposure, as well as a relative stage migration to lower stage disease following treatment with both oral inhibitors. CPSI-1306 had a greater impact in the two drug protocol, with nearly a two-thirds reduction in tumor volume relative to vehicle. Dose escalation studies resulted in a reduction of tumor volume in a dose-dependent manner with the most effective dosing of CPSI-1306 at 1.0mg/kg with the 0.1mg/kg dose also having statistically significant effects. Interestingly, 1.0mg/kg is the same dose at which an effect, noted above, was seen in the two other published reports utilizing this small molecule (17,18). Based on both in vivo and in vitro findings, we show increased proliferation via phosphorylation of ERK as one pathway of mechanistic action by which MIF enhances bladder cancer progression. Analysis of the proliferation index and mitotic activity in carcinogen-exposed mice, as well as in vitro cell counts and TdR, supports this conclusion. MIF has been reported to be an inducible, delayed response gene critical for cell division, as well as to function as a mediator of growth-factor-dependent proliferation (45). MIF monoclonal antibodies reportedly decrease proliferation in T lymphocytes by 80% (46) and MIF knockdown reduced colon cancer (47) and melanoma (33) cell growth by 40 and 60%, respectively, following growth factor treatment. Other small-molecule inhibitors have been tested in various models of tumor growth with similar findings of growth attenuation. For example, Meyer-Siegler et al. (14) reported that ISO-1 significantly reduces prostate cancer cell proliferation in vivo. Most recently, Winner et al. (48) showed that a new small-molecule inhibitor of MIF, 4-iodo-6-phenylpyrimidine, can significantly inhibit lung cancer anchorage-independent growth in culture. Therefore, several independent studies indicate that MIF activates cell proliferation, and MIF inhibition acts to decrease tumor burden and/or progression via decreasing cell proliferation.
Our data and that of others indicate that MIF inhibition decreases tumor angiogenesis. For example, Ren et al. (12) reported that exogenous MIF stimulated both VEGF and IL-8 expression in neuroblastoma cell lines in a dose-dependent manner, and MIF-induced increases in these promoters of angiogenesis could be abrogated by the addition of a MIF monoclonal antibody. In addition, the use of ISO-1 resulted in a significant reduction in neovascularity of colon cancers (42). Our results further support a role for MIF in tumor-associated angiogenesis, as we report MIF inhibitor treatment resulted in reduced PECAM expression in association with decreased angiogenesis, in bladder tumors arising in BBN-exposed mice. This is consistent with our earlier report of decreased angiogenesis in MIF knockout mice exposed to BBN (15). Our in vitro data suggest this could be a result of VEGF expression as rhMIF was able to increase VEGF expression 2-fold.
Conclusions
We report that the use of an oral inhibitor of MIF effectively decreases the growth and progression of bladder cancer in vivo. These compounds effectively inhibit MIF-driven proliferation and expression of angiogenic factors. Combined with the results of our previous study, we suggest that inhibition of MIF may prove to be an effective treatment for patients with the diagnosis of high-grade, superficial disease and may help slow tumor progression. Larger studies will need to be performed to confirm our findings with the goal of moving to human trials.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
American Cancer Society (MRSG 08-270-01-CCE to J.A.T.); National Institutes of Health (R01DK48361, R01AR060286 to C.C.P.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- BBN
N-butyl-N-(4-hydroxybutyl)-nitrosamine
- ERK
extracellular signal-regulated kinase
- H&E
hematoxylin and eosin
- MIF
macrophage migratory inhibitory factor
- mRNA
messenger RNA
- MVD
microvessel density
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
- PECAM-1
platelet/endothelial cell adhesion molecule 1
- rhMIF
recombinant human MIF
- TBST
Tris-buffered saline containing 0.05% Tween 20
- TdR
3H-thymidine incorporation
- VEGF
vascular endothelial growth factor.
References
- 1. American Cancer Society (2012). Cancer Facts and Figures. American Cancer Society, Atlanta, GA; [Google Scholar]
- 2. Ries L., et al. (eds) (2008) SEER Cancer Statistics Review, 1975–2005. National Cancer Institute, Bethesda, MD: http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER website. [Google Scholar]
- 3. Coussens L.M., et al. (2002). Inflammation and cancer. Nature, 420, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. David J.R. (1966). Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl Acad. Sci. USA, 56, 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calandra T., et al. (1997). Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit. Rev. Immunol., 17, 77–88 [DOI] [PubMed] [Google Scholar]
- 6. Tomiyasu M., et al. (2002). Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin. Cancer Res., 8, 3755–3760 [PubMed] [Google Scholar]
- 7. Bando H., et al. (2002). Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn. J. Cancer Res., 93, 389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer-Siegler K., et al. (1996). Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology, 48, 448–452 [DOI] [PubMed] [Google Scholar]
- 9. Meyer-Siegler K.L., et al. (2004). Substance P induced release of macrophage migration inhibitory factor from rat bladder epithelium. J. Urol., 171, 1698–1703 [DOI] [PubMed] [Google Scholar]
- 10. Taylor J.A., et al. (2006). Null mutation in macrophage migration inhibitory factor prevents muscle cell loss and fibrosis in partial bladder outlet obstruction. Am. J. Physiol. Renal Physiol., 291, F1343–F1353 [DOI] [PubMed] [Google Scholar]
- 11. Meyer-Siegler K.L., et al. (2004). Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer, 4, 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ren Y., et al. (2006). Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene, 25, 3501–3508 [DOI] [PubMed] [Google Scholar]
- 13. Sun B., et al. (2003). Induction of macrophage migration inhibitory factor by lysophosphatidic acid: relevance to tumor growth and angiogenesis. Int. J. Mol. Med., 12, 633–641 [PubMed] [Google Scholar]
- 14. Meyer-Siegler K.L., et al. (2006). Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J. Immunol., 177, 8730–8739 [DOI] [PubMed] [Google Scholar]
- 15. Taylor J.A., 3rd, et al. (2007). Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer, 7, 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell R.A., et al. (2000). Tumor growth-promoting properties of macrophage migration inhibitory factor (MIF). Semin. Cancer Biol., 10, 359–366 [DOI] [PubMed] [Google Scholar]
- 17. Kithcart A.P., et al. (2010). A small-molecule inhibitor of macrophage migration inhibitory factor for the treatment of inflammatory disease. FASEB J. 24, 4459–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchez-Zamora Y., et al. (2010). Macrophage migration inhibitory factor is a therapeutic target in treatment of non-insulin-dependent diabetes mellitus. FASEB J., 24, 2583–2590 [DOI] [PubMed] [Google Scholar]
- 19. Bucala R. (1994). Identification of MIF as a new pituitary hormone and macrophage cytokine and its role in endotoxic shock. Immunol. Lett., 43, 23–26 [DOI] [PubMed] [Google Scholar]
- 20. Taylor J.A., 3rd, et al. (2009). Regulation of the prostaglandin pathway during development of invasive bladder cancer in mice. Prostaglandins Other Lipid Mediat., 88, 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edge S.B., et al. (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol., 17, 1471–1474 [DOI] [PubMed] [Google Scholar]
- 22. Lopez-Beltran A., et al. (2004). Tumors of the urinary system. In Eble, J.N. et al (eds) World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press, Lyon, France: [Google Scholar]
- 23. Lende T.H., et al. (1991) In patients younger than age 55 years with lymph node-negative breast cancer, proliferation by mitotic activity index is prognostically superior to adjuvant!. J. Clin. Oncol. 29, 852–858 [DOI] [PubMed] [Google Scholar]
- 24. Weidner N., et al. (1991). Tumor angiogenesis and metastasis. —correlation in invasive breast carcinoma. N. Engl. J. Med., 324, 1–8 [DOI] [PubMed] [Google Scholar]
- 25. Zheng Y.X., et al. (2012). CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J. Gastroenterol., 18, 2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi J.W., et al. (2012) CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int. J. Urol., 20, 251–255 [DOI] [PubMed] [Google Scholar]
- 27. Tawadros T., et al. (2012) Release of macrophage migration inhibitory factor by neuroendocrine-differentiated LNCaP cells sustains the proliferation and survival of prostate cancer cells. Endocr. Relat. Cancer, 20, 137–149 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell R.A., et al. (1999). Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem., 274, 18100–18106 [DOI] [PubMed] [Google Scholar]
- 29. Lue H., et al. (2007). Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene, 26, 5046–5059 [DOI] [PubMed] [Google Scholar]
- 30. Binsky I., et al. (2007). IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc. Natl Acad. Sci. USA, 104, 13408–13413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lubet R.A., et al. (2005). 4-Hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers in mice: characterization of FHIT and survivin expression and chemopreventive effects of indomethacin. Carcinogenesis, 26, 571–578 [DOI] [PubMed] [Google Scholar]
- 32. Sylvester R.J., et al. (2002). Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol., 168, 1964–1970 [DOI] [PubMed] [Google Scholar]
- 33. Shimizu T., et al. (1999). High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem. Biophys. Res. Commun., 264, 751–758 [DOI] [PubMed] [Google Scholar]
- 34. Chesney J., et al. (1999). An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol. Med., 5, 181–191 [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell R.A., et al. (2002). Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl Acad. Sci. USA, 99, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrenko O., et al. (2003). Macrophage migration inhibitory factor deficiency is associated with altered cell growth and reduced susceptibility to Ras-mediated transformation. J. Biol. Chem., 278, 11078–11085 [DOI] [PubMed] [Google Scholar]
- 37. Petrenko O., et al. (2005). Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol. Cell, 17, 225–236 [DOI] [PubMed] [Google Scholar]
- 38. Hagemann T., et al. (2005). Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol., 175, 1197–1205 [DOI] [PubMed] [Google Scholar]
- 39. Winner M., et al. (2007). Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res., 67, 186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grieb G., et al. (2010). Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect., 23, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aroca P., et al. (1991). Specificity of dopachrome tautomerase and inhibition by carboxylated indoles. Considerations on the enzyme active site. Biochem. J., 277(Pt 2)393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He X.X., et al. (2009). Macrophage migration inhibitory factor promotes colorectal cancer. Mol. Med., 15, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conroy H., et al. (2010). Inflammation and cancer: macrophage migration inhibitory factor (MIF. )—the potential missing link. QJM., 103, 831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alam A., et al. (2011). Synthesis and bio-evaluation of human macrophage migration inhibitory factor inhibitor to develop anti-inflammatory agent. Bioorg. Med. Chem., 19, 7365–7373 [DOI] [PubMed] [Google Scholar]
- 45. Bernhagen J., et al. (1993). MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature, 365, 756–759 [DOI] [PubMed] [Google Scholar]
- 46. Bacher M., et al. (1996). An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl Acad. Sci. USA, 93, 7849–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takahashi N., et al. (1998). Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol. Med., 4, 707–714 [PMC free article] [PubMed] [Google Scholar]
- 48. Winner M., et al. (2008). A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res., 68, 7253–7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.