Abstract
Prostate cancer (PCa) is the second leading cause of cancer-related death in American men and many PCa patients develop skeletal metastasis. Current treatment modalities for metastatic PCa are mostly palliative with poor prognosis. Epidemiological studies indicated that patients receiving the diabetic drug metformin have lower PCa risk and better prognosis, suggesting that metformin may have antineoplastic effects. The mechanism by which metformin acts as chemopreventive agent to impede PCa initiation and progression is unknown. The amplification of c-MYC oncogene plays a key role in early prostate epithelia cell transformation and PCa growth. The purpose of this study is to investigate the effect of metformin on c-myc expression and PCa progression. Our results demonstrated that (i) in Hi-Myc mice that display murine prostate neoplasia and highly resemble the progression of human prostate tumors, metformin attenuated the development of prostate intraepithelial neoplasia (PIN, the precancerous lesion of prostate) and PCa lesions. (ii) Metformin reduced c-myc protein levels in vivo and in vitro. In Myc-CaP mouse PCa cells, metformin decreased c-myc protein levels by at least 50%. (iii) Metformin selectively inhibited the growth of PCa cells by stimulating cell cycle arrest and apoptosis without affecting the growth of normal prostatic epithelial cells (RWPE-1). (iv) Reduced PIN formation by metformin was associated with reduced levels of androgen receptor and proliferation marker Ki-67 in Hi-Myc mouse prostate glands. Our novel findings suggest that by downregulating c-myc, metformin can act as a chemopreventive agent to restrict prostatic neoplasia initiation and transformation.
Summary: Metformin, an old antidiabetes drug, may inhibit prostate intraepithelial neoplasia transforming to cancer lesion via reducing c-MYC, an ‘old’ overexpressed oncogene. This study explores chemopreventive efficacy of metformin in prostate cancer and its link to cMYC in vitro and in vivo.
Introduction
Prostate cancer (PCa) is the second most prevalent form of cancer that affects American men (1). It is estimated that 241 740 new cases of PCa were diagnosed in the USA alone and 28 170 men died of PCa in 2012 (2). Current treatment modalities for metastatic disease and hormone refractory prostate cancer (HRPC) are mostly palliative with poor prognosis (3). Early diagnosis and prevention are critical to limit the impact of PCa.
PCa develops through a progressive transition from normal prostatic epithelial cells to prostatic intraepithelial neoplasia, which is also commonly referred as premalignant lesions (i.e. prostate intraepithelial neoplasia, PIN), followed by invasive adenocarcinoma and finally distant metastatic disease (4–6). Several diagnostic morphological features, such as nuclear enlargement and alterations to chromatin structure, characterize the transformation of prostate epithelial cells from normal to PIN and adenocarcinoma. Importantly, several genes are altered at the onset of PIN including the overexpression of the proto-oncogene c-MYC (7–9). The c-MYC oncogene is present on chromosome 8q24 and encodes a master transcription factor, c-myc, which plays a vital role in regulation of cell proliferation, apoptosis and metabolism (9–13). Amplification of the c-MYC oncogene is a key event at the precancer stage (i.e. PIN) of PCa development (14–16). Thus, regulation of c-myc is considered an important and effective therapeutic target in PCa prevention and treatment.
Metformin (1,1-dimethylbiguanide hydrochloride) is one of the most commonly prescribed drugs for Type II diabetes. It reduces glucose levels through activation of the AMP-activated protein kinase (AMPK) pathway and inhibition of hepatic gluconeogenesis (17). Accumulating epidemiological evidence suggests the potential antineoplastic effects of metformin (18). There is also evidence that metformin induces activation of the tumor suppressor gene liver kinase B1 (LKB1), an established regulator of AMPK (17), and thereby inhibit the mTOR (mammalian target of rapamycin) pathway (19). Targeting the mTOR signaling pathway has been suggested as the main mechanism mediating metformin antitumor efficacy (20–24). Sahra et al. (25,26) demonstrated a direct antiproliferative effect of metformin on PCa cells. However, previous studies on metformin and PCa are from cell culture or subcutaneous tumor xenografts in athymic mice, both represent established tumors. These experiments do not determine the efficacy of metformin in preventing the onset of PCa or the transition from PIN to PCa. It is important to investigate whether and how metformin prevents the early neoplastic transformation of prostate tissue to malignancy. Studies in a mouse PCa model, which recapitulates PCa progression from early neoplasia, are therefore very critical.
In this study, Hi-Myc transgenic mice that overexpress c-myc in response to androgen by a prostate-specific probasin promoter, ARR2PB, were used (14). These mice develop PIN as early as 2 weeks old and with rising levels of androgen in puberty and progression into invasive adenocarcinomas at ~6 months (14). Hi-Myc mouse PIN is characterized by enlarged nucleoli and increased nuclear size, features that are analogous to the human diseases, closely resembling the transformation of PIN to PCa in humans (8). Thus, the Hi-Myc mouse model is ideal to study the early stages of PCa development.
Considering that metformin reduces the risk of PCa in patients and c-myc plays an important role in the onset of PCa, we hypothesize that metformin may regulate c-myc expression thereby inhibiting PCa initiation and progression.
Materials and methods
Mice treatment and dissection
Hi-Myc mice were housed and bred in the Laboratory Animal Facility at New York University College of Dentistry. The mice were kept in polypropylene cages (30×21×10cm) under standard light/dark regimen (12h light:12h darkness) at 22±2°C and received standard laboratory chow and water. Mice were treated daily from 4 to 5 weeks old for 4 weeks in drinking water with or without metformin (200 mg/kg). Blood glucose and weight of each mouse were recorded weekly. Following treatment, urogenital organs were isolated and prostates were microdissected in a petri dish containing 10ml of cold phosphate-buffered saline (PBS) under a dissecting microscope. Adipose tissue surrounding the mouse prostates was cleared using forceps and shears. All animal studies were approved by the New York University Committee on the Use and Care of Animals.
Chemicals and materials
The antidiabetic drug, 1,1-dimethylbiguanide hydrochloride (metformin), was purchased from Sigma (St Louis, MO). Cycloheximide (CHX), 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR) and compound C were purchased from Calbiochem (EMD Biosciences, Inc). Stock solutions (1 M) were prepared in PBS and stored at 4°C. Trypsin, penicillin/streptomycin solution and all media were obtained from Invitrogen Corp. (Carlsbad, CA). The cell culture plastic wares and additional chemicals were obtained from Corning Incorporated (Corning, NY) or BD Biosciences (Franklin Lakes, NJ). Antibodies were purchased from Santa Cruz Biotechnology [actin, p21, p27, p53, c-myc and androgen receptor (AR)], Cell Signaling Technology (AMPK, p-AMPK, cyclin D1 and CDK4) and Vector (Ki-67).
Cell lines.
The Myc-CaP cell line derived from prostate carcinoma of a Hi-Myc transgenic mouse (27) was a gift from Charles Sawyers (Howard Hughes Medical Institute and Memorial Sloan-Kettering Cancer Center) and was grown in Dulbecco's modified Eagle's medium (high glucose 4.5 g/l) with 10% fetal bovine serum and 1% penicillin/streptomycin. Cell lines, C4-2b, derived from metastatic PCa lesions in the spine of an athymic mouse (28,29) and PC-3, bone metastasis of a grade IV prostatic adenocarcinoma from a 62-year-old male Caucasian (30), were gifts from Laurie McCauley (University of Michigan). LNCaP, an androgen-responsive human PCa cell line (31), was a gift from Peng Lee (New York University). C4-2b, LNCaP and PC-3 cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cell lines were maintained at 37°C in a humid atmosphere containing 5% CO2.
Cells were treated with metformin or vehicle at indicated concentration for up to 24, 48 and 72h. After 24h exposure to vehicle, metformin, AICAR or compound C, cell lysates were collected for western blot analysis.
Cell count assays
Cells were plated in 96-well plates at a density of 6000 cells/well (Myc-CaP) or 8000 cell/well (C4-2b/LNCaP) and allowed to adhere overnight. The cells were then treated with vehicle (PBS) or metformin as indicated concentration for 24, 48 and 72 h before Alamar-Blue reagent (Invitrogen) was added into the cell cultures. Optical density units of absorbance at 570 nm were measured as the indicator of cell density according to the manufacturer’s instruction.
Western blot analysis
Total cell lysates were prepared using radioimmunoprecipitation assay buffer supplemented with protease inhibitor and phosphatase inhibitor (phenylmethanesulfonyl fluoride and sodium fluoride). The protein concentrations in total cell extracts were determined using a BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Samples were stored at −20°C until assays were performed. Briefly, western blots were performed using actin as a loading control in each sample and the final signal was detected using the Bio-Rad ChemiDoc™ XRST with Image Lab™ Software.
Flow cytometric analysis for cell cycle and apoptosis
Cells were washed twice with cold PBS to remove floating cells before lifted with trypsin and stained with propidium iodide (PI) or the TACS® Annexin V-FITC Apoptosis Detection Kit (Trevigen, Gaithersburg, MD). Cells also were treated with ribonuclease A before staining with PI for cell cycle analysis. Fluorescein isothiocyanate-conjugated annexin V and PI staining were evaluated with a flow cytometric analyzer Calibur (BD Biosciences, San Jose, CA). Raw data were analyzed using FlowJo Version 9.5.2 flow cytometry analysis software (Tree Star, Ashland, OR).
Immunohistochemistry
c-myc, Ki-67 and AR expressions in mouse prostate were determined using immunohistochemical analysis. In brief, formalin-fixed, paraffin-embedded prostate sections were deparaffinized and rehydrated using a series of alcohol washes. For antigen retrieval, slides were steamed for 20 min in 1× citrate buffer solution. Prostate sections were then probed with primary antibodies and incubated at 4°C overnight at the following dilutions: c-Myc (1:100), Ki-67 (1:500) and AR (1:1000). Secondary antibody incubation was done at room temperature for 30 min. All the antibodies were diluted with DAKO antibody dilute solution. Staining was visualized using 3,3′-diaminobenzidine (source) and slides were counterstained with hematoxylin. Percentage of stained cells was measured using ImageJ Version 1.46 processing program.
Statistical analysis
Student’s t-test for independent analysis was applied to evaluate differences, and Fisher’s exact test was applied to compare the incidence rate of PIN lesion development using the GraphPad Instat Software Program (GraphPad Software, San Diego, CA) as described previously (32). Analysis of variance (ANOVA) was applied when need and Bonferroni (Dunn) t-tests were used after ANOVA. A value of P < 0.05 was considered statistically significant.
Results
Metformin reduced PIN and PCa lesions in Hi-Myc mice
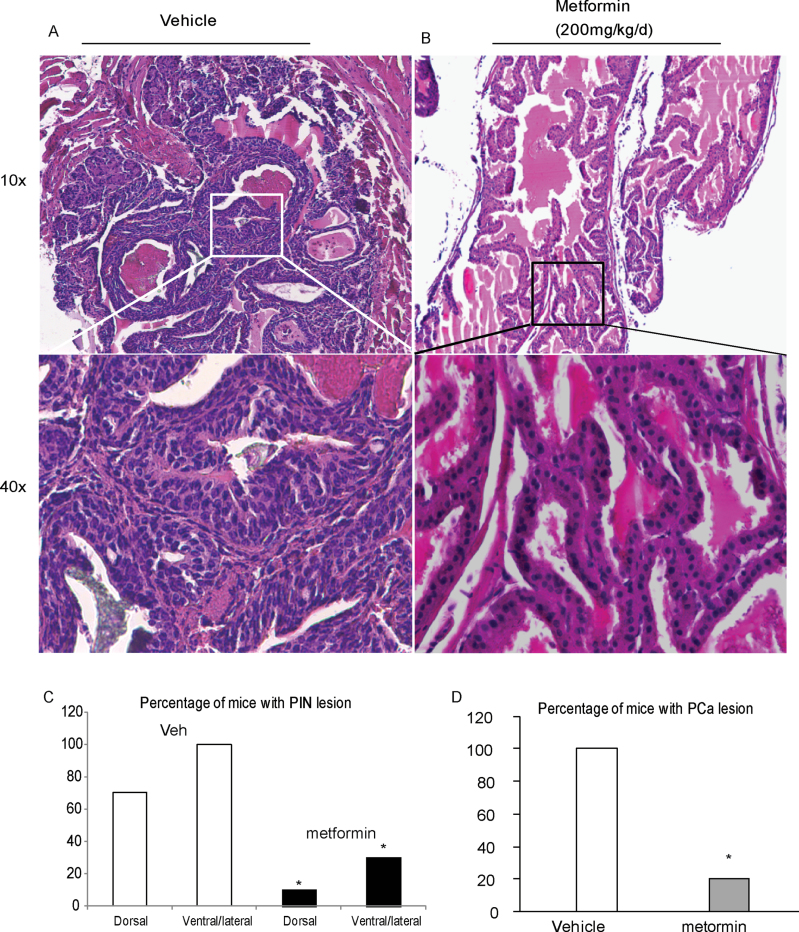
Metformin reduces PCa risk and it is anticipated that metformin will inhibit the transformation from PIN to PCa, or even inhibit the onset of PIN. To determine whether metformin can reduce PIN lesion development, 5-week-old Hi-Myc mice (10 animals/group) were treated with 200 mg/kg metformin or vehicle (water without the drug), daily through drinking water. After 4 weeks of treatment, the incidence of PIN lesions in prostrate tissue was drastically decreased (~80% in total) in mice treated with metformin compared with mice in the vehicle group (Figure 1A and B). PIN development, assessed by enlargement of nuclei and irregular, atypical growth of epithelial cells, was reduced in the metformin treatment group. PIN development in ventral/lateral lobes was observed only in 30% (3/10) mice of the metformin-treated group compared with 100% (10/10) mice of the vehicle-treated group. Although dorsal lobes had less percentage of PIN development, only 10% (1/10) mice in the metformin group developed PIN as against 70% (7/10) mice in the vehicle group (Figure 1C). No changes in body weight and blood glucose level were detected in metformin-treated mice over 4 weeks (Supplementary Figure 1, available at Carcinogenesis Online). To examine whether the attenuation in PIN by metformin treatment leads to reductions in PCa lesions, Hi-Myc mice were administrated with daily metformin treatment for 24 weeks. At the end of the treatment, only 20% (2/10) metformin-treated mice were detected with PCa lesions as against 100% (10/10) mice of the vehicle-treated group (Figure 1D). These results strongly suggest that metformin prevents tumor development in Hi-Myc PCa mice.
Fig. 1.
Metformin decreases PIN formation in prostrates of Hi-Myc mice. Prostate tissues were dissected from mice that were treated orally with metformin (200 mg/kg/day) from 5 weeks old. Representative hematoxylin and eosin staining of paraffin-embedded prostate tissues at lower magnification (×10) and higher magnification (×40) from vehicle-treated (A) and metformin-treated group (B). (C) Incidence of PIN lesions in dorsal or ventral/lateral lobes of mouse prostates with or without metformin treatment for 4 weeks. (D) Incidence of PCa lesions in ventral/lateral lobes of mouse prostates with or without metformin treatment for 24 weeks. n = 10, *P < 0.05 compare to same lobe in vehicle group by Fisher’s exact test.
Metformin decreases c-myc protein levels
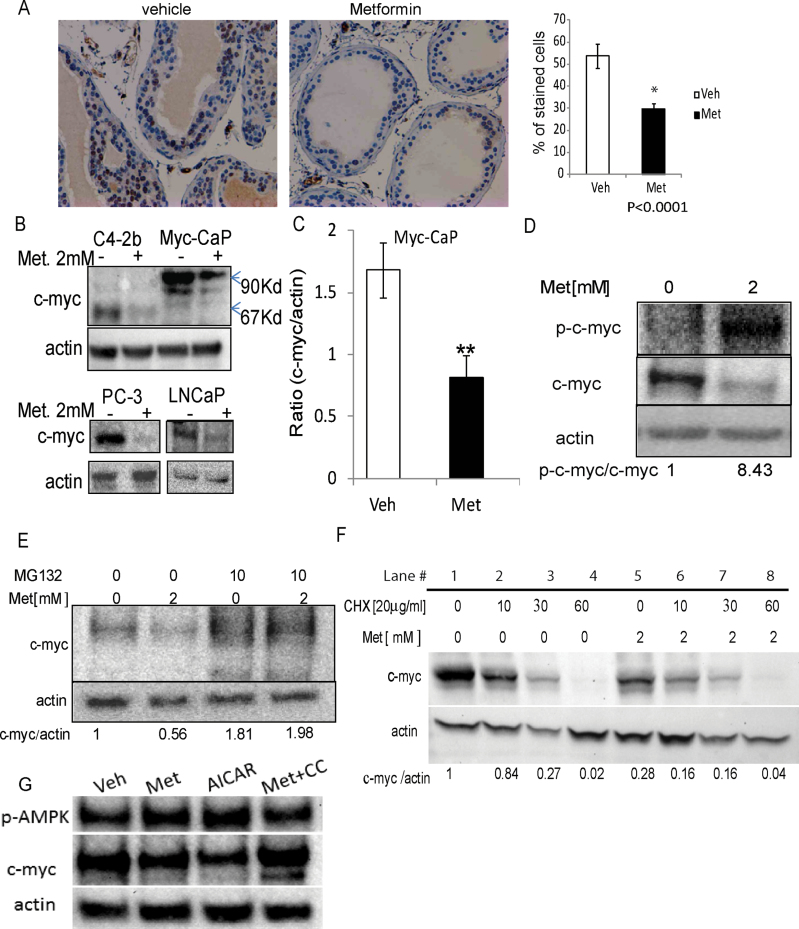
The Hi-Myc mouse model demonstrates early stages of PCa by developing PIN due to overexpression of c-myc. To determine whether c-myc expression is associated with the reduction in PIN by metformin, we examined c-myc protein levels in the prostate glands of metformin- and vehicle-treated Hi-Myc mice by immunohistochemistry. As expected, metformin significantly decreased the number of c-myc positive cells by an average of 24% (Figure 2A). Our in vivo finding that metformin reduces c-myc expression was validated in variety of mice and human PCa cells, Myc-CaP, C4-2b, PC-3 and LNCaP cells (Figure 2B). The endogenous c-myc is 67 kDa and the levels decreased in C4-2b, PC-3 and LNCaP cells after metformin treatment. Interestingly, the endogenous c-myc levels in Myc-CaP cells are too low to detect, so only the exogenous c-myc protein with a molecular weight of 90 kDa was detectable. As mentioned earlier, that Myc-CaP cells were derived from Hi-Myc mouse prostate tumor overexpressing a human c-MYC translated to a 90 kDa c-myc protein. Quantitative analysis of the western blots from Myc-CaP cells demonstrated at least 50% reduction in c-myc protein levels after exposure to 2 mM metformin for 24 h. The ratio of myc/actin protein expression was 1.683±0.219 in vehicle-treated versus 0.821±0.170 in metformin-treated cells (Figure 2C).
Fig. 2.
Metformin reduces c-Myc protein levels in vivo and in vitro. (A) Expression of c-Myc in prostates (8–9 weeks, n = 5) from mice that were treated orally with metformin (200 mg/kg/day) for 4 weeks. Percentage of stained cells was measured using the ImageJ processing program. (B–D) PCa Myc-CaP, C4-2b, PC-3 and LNCaP cells were treated with 2 mM metformin or vehicle (PBS), for 24h. Following treatment, cells were harvested and the amount of phosphorylated c-myc (p-c-myc), total c-myc and actin protein in each sample was determined using western blot analysis. (E) Myc-CaP cells were treated with 2mM metformin or PBS for 24h, in the presence or absence of the proteasome inhibitor MG132. Western blot analysis was used to measure the levels of c-myc and actin protein in treated cells. (F) Myc-CaP cells were treated with PBS or 2 mM metformin for 24h. Posttreatment cells were treated with 20mg/ml CHX at the indicated time points (0–60min) or left untreated. Lysates were prepared and analyzed by western blotting for c-Myc and actin loading control. (G) Myc-CaP cells were treated with vehicle, metformin (2 mM), AICAR (2 mM) or compound C (10 µM) + metformin (2 mM) for 24h. c-myc and actin protein levels were measured by western blots. *P < 0.05, **P < 0.01, two-tailed Student's t-test.
As Hi-Myc mice are engineered to overexpress c-myc due to an additional human c-MYC complementary DNA driven by probasin promoter, which was not affected by metformin at messenger RNA levels in Myc-CaP cells (Supplementary Figure 2, available at Carcinogenesis Online), we hypothesized that the decrease observed in c-myc protein expression with metformin is posttranslational. It is known that phosphorylation of c-myc at the conserved amino acid (T58) significantly decreases c-myc protein stability. To detect phospho-c-myc, we used an antibody that specifically recognizes the Thr58 (as well as Thr58/Ser62 phosphorylated c-myc) phosphorylated forms of c-myc protein. In metformin-treated Myc-CaP cells, phosphorylation of c-myc at Thr58 (p-c-myc) was dramatically stimulated (Figure 2D). Since phosphorylation of c-myc protein at Thr58 triggers ubiquitination and ultimately proteasomal degradation, we treated Myc-CaP cells with the proteasomal inhibitor MG132. The presence of the protease inhibitor blocked metformin-induced c-Myc protein reduction, suggesting that metformin regulates c-myc protein degradation (Figure 2E) and hence, myc ubiquitination. However, there was no significant difference in the ubiquitinated-c-myc proteins in Myc-CaP cells treated with metformin for 24 h or vehicle in the presence of MG132 (Supplementary Figure 3, available at Carcinogenesis Online). We further measured the half-life of c-Myc in the presence of CHX, an inhibitor of de novo protein synthesis. c-myc expression in metformin-treated cells was significantly reduced by 70% (Figure 2F, compare lane 5 versus lane 1), and after protein synthesis was blocked by CHX, an additional 50% reduction was observed with an estimated half-life of 10min (Figure 2F, compare lane 6 versus lane 5) compared with a 20–30min half-life of c-myc in control-treated cells (Figure 2F, compare lanes 2 and 3 versus lane 1). To investigate whether metformin 2 mM reduced c-myc levels in metformin-treated cells occurs through AMPK activation, the effects of AICAR (an AMPK activator) and compound C (an AMPK inhibitor) on c-myc protein were tested in Myc-CaP cells. AICAR reduced c-myc protein, whereas the presence of AMPK inhibitor compound C (10 µM) blocked metformin reduction of c-myc protein levels (Figure 2G). Combined, these data show that metformin reduces c-myc protein levels in vivo in prostate glands and in vitro in PCa cells.
Metformin specifically decreases the growth of PCa cells
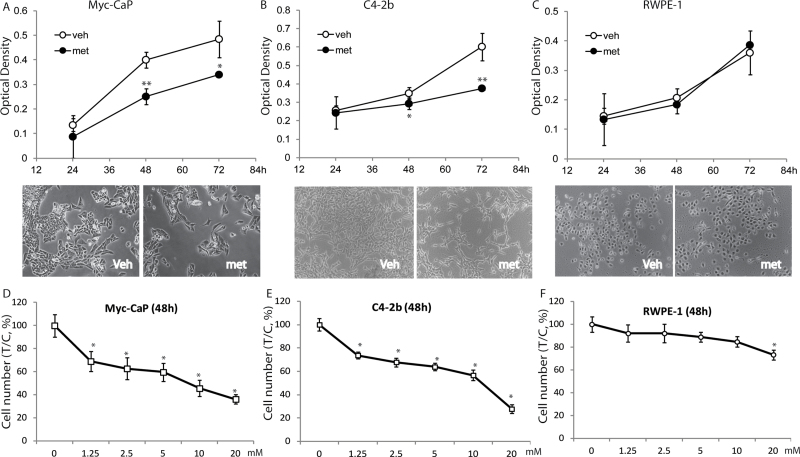
Human (C4-2b) and mouse (Myc-CaP) PCa cell lines as well as normal prostate epithelial cell line RWPE-1 cells were studied to determine metformin regulation of cell growth. A significant decrease in cell numbers of both Myc-CaP and C4-2b was observed after metformin treatment (Figure 3A and B). Metformin at 2 mM significantly and specifically inhibited proliferation of Myc-CaP and C4-2b cells at 48 and 72h, whereas there was no effect on RWPE-1 cells (Figure 3C). A dose response assay using metformin from 2 to 20 mM was performed for 48h in all three cell lines. RWPE-1 cells were resistant to metformin treatment up to 10 mM. Even at the highest dose of 20 mM metformin, RWPE-1 cell numbers were reduced only by 20% in contrast to its suppression of Myc-CaP and C4-2b cells by 80% (Figure 3D–F). These data indicate that metformin specifically inhibits PCa cell growth, whereas normal prostate epithelial cells are more tolerant to metformin.
Fig. 3.
Metformin inhibits growth of PCa cells. (A–C) Myc-CaP, C4-2b and RWPE-1 cells were 2mM metformin for up to 72 h or (D–F) at indicated concentrations for 48h or left untreated (control, PBS vehicle). Following treatment, cell density was measured using the Alamar-Blue assay as described in Materials and methods. Each bar represents the mean ± SD of three independent samples. *P < 0.05, **P < 0.01 when compared with vehicle. Representative photos of each cell type treated with metformin or vehicle are shown.
Metformin causes cell cycle arrest and apoptosis in PCa cells
Decreases in cell numbers can result from an arrest in cell cycle progression or a stimulation of apoptosis. Therefore, we examined whether metformin alters PCa cell cycle distribution and/or apoptosis using flow cytometry.
Metformin arrests cells in G1 phase.
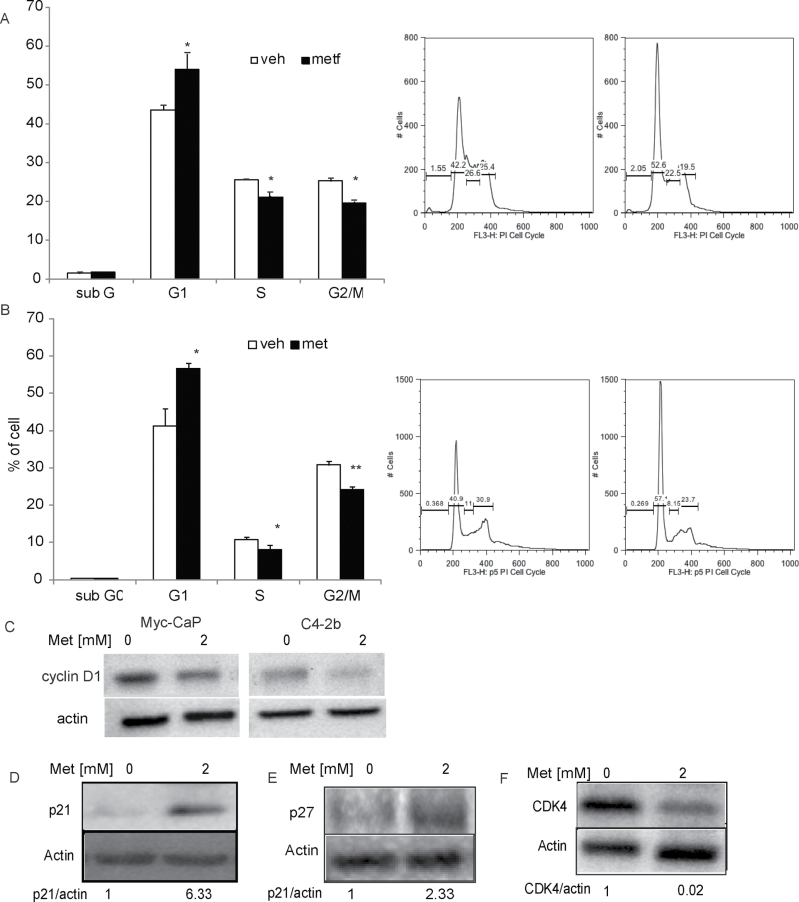
Flow cytometric analysis of C4-2b and Myc-CaP cells treated with metformin for 24h clearly demonstrated a G1 phase arrest (Figure 4A and B) and a significant decrease in the percentage of cells in S and G2/M phases. Cell entry from G1 to S phase is regulated by the activated cyclin D1/CDK4 complex and cyclin-dependent kinase inhibitors, e.g. p21 and p27. Therefore, the molecular events underlying the observed changes in cell cycle profile were examined. A reduction of cyclin D1 expression in metformin-treated Myc-CaP and C4-2b cells (Figure 4C) was observed. As demonstrated by western blot analysis (Figure 4D–F), the metformin reduced CDK4 expression in Myc-CaP cells was associated with an increase in p21 and p27 levels. The changes in the expression of these cell cycle regulators are consistent with the cell cycle arrest at the G1 phase by metformin treatment in PCa cells.
Fig. 4.
Metformin induces G1 cell cycle arrest. (A) Myc-CaP and (B) C4-2b cells were treated with 2mM metformin or PBS for 24 h. The percentage of cells within the different phases of the cell cycle was determined using flow cytometry as described in Materials and methods. *P < 0.01, **P < 0.001, Bonferroni (Dunn) t-tests after ANOVA. Each bar represents the mean ± SD of three independent samples. (C–F) Myc-CaP cells were treated with PBS or 2mM metformin for 24h and proteins were harvested. (C) Cyclin D1, (E) p21 and (F) p27 protein levels were determined using western blot analysis. Membranes were stripped and reblotted for actin as a loading control.
Metformin stimulates apoptosis in PCa cells.
In order to further determine the fate of the arrested cells upon metformin treatment, we examined cell death in these cells. Myc-CaP and C4-2b cells were treated with metformin or PBS (vehicle) for 48h and stained by apoptosis markers PI and annexin V. Flow cytometry analysis demonstrated a significant increase in annexin V positive cells (early apoptotic cells) and annexin V/PI double positive cells (late apoptotic cells) in metformin-treated cells compared with the vehicle-treated cells (Figure 5A and B). We further determined that metformin regulates the expression of Bcl-2 family containing Bcl-2 homology domains (BH) that regulate apoptosis. The expression of a proapoptosis protein Bak was stimulated in both Myc-CaP and C4-2b PCa cells (Figure 5C) and antiapoptotic protein Bcl-2 level was reduced in Myc-CaP cells in a dose-dependent manner (Figure 5D). These data further support the stimulation of apoptosis in PCa cells by metformin. Taken together, these data indicate that metformin inhibits PCa cell growth through cell cycle arrest and stimulation of apoptosis.
Fig. 5.
Metformin increases apoptosis in PCa cells. (A) Myc-CaP and (B) C4-2b cells were treated with PBS or 2mM metformin for 48 h before analyzing apoptosis using an annexin V–fluorescein isothiocyanate apoptosis detection kit as described in Materials and methods. *P < 0.05 and **P < 0.01, Bonferroni (Dunn) t-tests after ANOVA. Representative experiments are shown. (C and D) Myc-CaP and/or C4-2b cells were treated with PBS or metformin at indicated concentration for 24h and proteins from whole cell lysate were harvested. (C) Bak and (D) Bcl-2 protein levels were determined using western blot analysis. Membranes were stripped and reblotted for actin as a loading control.
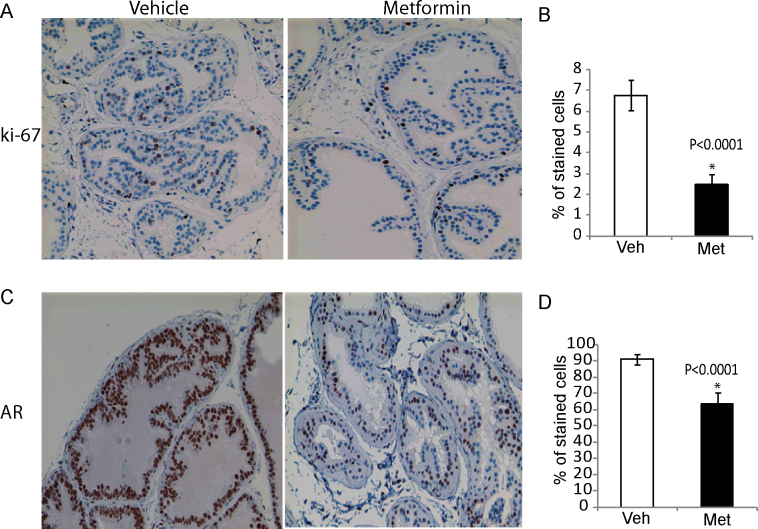
Metformin reduces PIN formation associated with reduction in proliferation and AR levels in vivo
The previous experiments demonstrated that metformin inhibits PCa cell proliferation in vitro. We therefore examined whether the reduction of cell proliferation is associated with the reduction of premalignant lesions formation (i.e. PIN) in Hi-Myc prostate glands upon metformin treatment. Ki-67 was used as a proliferation marker to examine prostate glands from mice treated with metformin or vehicle was assessed by immunohistochemistry analysis. After 4 weeks of treatment with metformin, Ki-67 positive cells were significantly reduced by an average of 4.31% (Figure 6A and B). PCa, especially at the early stages, is an androgen-dependent disease and ARs play an important role in PCa progression. Importantly, Hi-Myc mice express abundant AR in the prostate glands. Therefore, we investigated whether metformin regulates AR expression to prevent PIN in Hi-Myc mice. As anticipated, we observed reduced AR levels (27.39%) with metformin treatment (Figure 6C and D). Together, these changes indicate that metformin prevents the precancerous lesion development in Hi-Myc mice associated with reduction of AR and cell proliferation.
Fig. 6.
Metformin reduces proliferation and AR expression in vivo. Immunohistochemistry of prostates (8–9 weeks, n = 5) from mice that were treated orally with metformin (200 mg/kg/day) for 4 weeks on (A and B) cell proliferation marker Ki-67 and (C and D) AR. Percentage of stained cells was measured using the ImageJ processing program. *P < 0.0001, one-tailed Student's t-test.
Discussion
Although earlier epidemiological studies strongly suggest a role for metformin in reducing PCa risk, direct evidence linking metformin and PCa onset was lacking. Previous studies of metformin in PCa cell cultures or subcutaneous prostate tumor xenografts successfully confirmed metformin inhibitory effects (25,26,33) in PCa but were unable to evaluate its ability to prevent PCa onset. Furthermore, the mechanism by which metformin prevents the early neoplastic transformation of prostate remained elusive. This is the first study to address this question using a mouse PCa model that recapitulates PCa early progression. Hi-Myc mice develop PIN formation as early as 2 weeks old and develop PCa around 6 months. Both PIN and PCa developments are triggered by c-MYC overexpression in Hi-Myc mice. Therefore, anything to inhibit the onset of PIN in this model must be able to reverse c-myc signaling. Here, we clearly demonstrate that metformin treatment decreases the incidence of PIN lesion formation and inhibits the onset of early stage PCa through c-myc reduction.
The oncogene c-MYC plays an important role during the preneoplastic and malignant stages of PCa growth, which makes it an ideal prevention and therapeutic target. Our study demonstrated that c-myc protein levels were reduced by metformin treatment in vivo and in vitro. Further, we provide evidence of activation of AMPK pathway by metformin that downregulates c-myc, a novel mechanism of its antineoplastic activity.
In addition, c-MYC amplification may alter prostate tumor sensitivity to mTOR inhibitors (rapamycin and its analogs) (34,35), which displayed efficacy in inhibiting cancer growth in mouse prostate tumors due to constant activation of AKT (36). However, monotherapy with mTOR inhibitors was not successful in patients (35). The secondary genetic alterations in c-MYC, which often co-occurs with AKT activation in human tumors (34), could be one of the reasons for mTOR inhibitors’ failure in treating PCa patients. This is supported by the preclinical data in transgenic mouse PCa models. It was demonstrated that mTOR inhibitors are effective for PCa associated with PI3K/AKT/mTOR upregulation only but not in PCa involving c-MYC amplification such as Hi-Myc mice (34). Therefore, the efficacy of metformin in PCa early prevention in Hi-Myc mice will not only support metformin as a chemopreventive agent but also provide an alternative therapeutic agent for PCa associated with c-myc overexpression. Considering that metformin itself inhibits mTOR signaling, its effects could cover a broad spectrum of PCa including malignancy due to AKT/mTOR activation and/or c-MYC. Our novel finding may lay the ground work to evaluate the efficacy of metformin in PCa patients as a monotherapy and/or in combination with mTOR inhibitors.
In the present study, reduction of c-myc expression by metformin was confirmed in all PCa cells that were tested regardless of AR status: Myc-CaP (AR-positive), C4-2b (AR-mutant), PC-3 (AR-negative) and LNCaP (AR-mutant). Our results are consistent with the previous study which showed that metformin inhibited PCa DU145 and PC-3 cell growth at 5 mM in vitro and in LNCaP xenograft tumors (37). Taking together, these results suggested that metformin antitumor effect was not obstructed by AR signaling in PCa cells. Metformin also inhibits cellular proliferation in hormone refractory human C4-2b and mouse Myc-CaP PCa cells lines. The fact that metformin had minimal effect on RWPE-1 cells suggests that its inhibitory effect is specific to malignant cells. This is consistent with previous reports that demonstrate metformin selectively inhibits MCF-7 breast cancer cells but not the normal MDA-MB-231 or MCF10A normal breast epithelial cell lines (38). It is likely that downregulation of c-myc expression may be the key event that mediates its antitumor efficacy in vivo and in vitro.
The reduction of c-myc expression levels by metformin has been clearly demonstrated in in vitro and in vivo PCa systems in this study, and the reduction of c-myc at both messenger RNA and protein levels in all tested human PCa cells indicates that the regulation by metformin in these cells is at both transcriptional and translational. However, the reduction of c-myc in Myc-CaP cells is mainly at translational and posttranslational levels. Our data suggest that the metformin-induced decrease in c-myc protein levels occurs through proteasome-mediated degradation since MG 132 blocked the reduction of c-myc in Myc-CaP cells. This is consistent with a similar finding in acute promyelocytic leukemia with metformin (39). It is known that several regions of c-myc participate in its basal proteasomal regulation. Especially the phosphorylation pattern of Thr58 and Ser62 determines c-myc stability. Although phosphorylation of c-myc at Ser62 increases protein stability, the subsequent phosphorylation of c-myc at Thr58 decreases stability of the protein (40,41). Thus, metformin elevated the Thr58-phosphorylated c-myc protein indicates a marked reduction in c-myc protein stability. Even so, there was no significant increase in the ubiquitinated-c-myc proteins levels with 24 h metformin treatment. It is possible that metformin stimulates ubiquitinated c-myc at an earlier time point and hence at 24 h time point tested had minimal effect. Moreover, although the half-life of c-Myc in CHX chase assay was reduced to 10min in metformin-treated cells versus a 20–30min in control-treated cells, subsequent time points demonstrated a delayed reduction of c-myc indicating c-myc stabilization. Considering the levels of c-myc in the metformin-treated cells are much lower than that in control cells, later c-myc protein stabilization in metformin-treated cells may be stimulated due to a feedback mechanism to maintain a minimal basal c-myc levels specifically in these Myc-CaP cells, which are used to survive at a high level of overexpressed c-myc. Recently, metformin was also demonstrated to reduce c-myc expression in breast tumor through activation of AMPK (42). Activation of AMPK was suggested to mediate global translational inhibition by metformin in head and neck squamous cell carcinoma (43). Similarly, our study also suggests that downregulation of c-myc in PCa cells relies on AMPK activation and in part reduction in myc protein levels can be attributed to translational inhibition.
A previous study suggested that p53 status affects metformin efficacy in stimulation of apoptosis (26). Accordingly, Myc-CaP and C4-2b cells should express p53 since metformin stimulated apoptosis in both cells. We confirmed that p53 protein expression increased in C4-2b cells after metformin treatment (Supplementary Figure 4, available at Carcinogenesis Online). However, p53 protein levels were not detectable in Myc-CaP cells. This suggests that metformin may induce apoptosis independent of p53. One possibility is that c-MYC amplification supersedes the role of p53 in regulating cell apoptosis in Myc-CaP cells. Also, depending upon the growth conditions, c-myc can have opposite effects where either downregulation (44) or overexpression (45) of c-myc may stimulate apoptosis. Further studies are needed to fully understand the role of c-myc in metformin-induced apoptosis.
As stated earlier, another important finding of our study is that metformin inhibits PCa cell growth and downregulates AR expression regardless of their AR status. We further confirmed that AR reductions are consistently associated with c-myc reduction in LNCaP, C4-2b and Myc-CaP cells (Supplementary Figure 5, available at Carcinogenesis Online). A recent paper demonstrated that c-myc modulates AR signaling (46); it is possible that metformin-mediated reduction in AR signaling is at least partially mediated by c-myc downregulation. Overexpression of AR contributes to PCa resistance to antiandrogens and facilitates the progression of HRPC (47). Accumulating evidence indicates that ablation of AR signaling is vital in suppressing HRPC. Metformin’s capability of downregulating tumoral AR expression makes it a promising candidate as a new therapeutic agent for HRPC. A recent study suggested that metformin may enhance the antiproliferative and apoptotic effect of bicalutamide in PCa cells (48). Bicalutamide acts through preventing AR activation and subsequent upregulation of androgen-responsive genes. Taken together with our finding that metformin specifically targets tumor cells and reduces AR levels, a greater reduction in AR signaling and inhibition of PCa cell growth could be achieved by combination of metformin and bicalutamide.
In conclusion, this study reveals a plausible mechanism of how metformin prevents or delays the onset of c-myc-dependent PCa. Here, we demonstrate that metformin downregulates c-myc, causes cell cycle arrest and promotes apoptosis. Moreover, considering the inhibitory effects of metformin on AR-mediated signaling and the mTOR pathway, metformin could also play a beneficial role in restricting cancer initiation and progression in non-c-MYC-dependent PCas. As c-MYC oncogene contributes to the genesis of many human cancers as reviewed by Dang (49) recently, future studies to investigate the possible application of metformin as a safe and effective chemoprevention therapy in high cancer risk population are warranted.
Supplementary material
Supplementary Figures 1–5 can be found at http://carcin.oxford journals.org/
Funding
National Institute of Dental and Craniofacial Research (DE020754); National Cancer Institute (CA172894, CA180277); New York University Research Challenge Fund.
Supplementary Material
Acknowledgements
The authors appreciated Dr J.McCutcheon for proof reading this manuscript. We acknowledge the help provided by R.Bhatt and H.Shinglot (Polytechnic Institute of New York University, New York, NY) for their help in western blots and cell culture.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- AICAR
5-amino-1-β-d -ribofuranosyl-imidazole-4-carboxamide
- AMPK
AMP-activated protein kinase
- ANOVA
analysis of variance
- AR
androgen receptor
- CHX
cycloheximide
- HRPC
hormone refractory prostate cancer
- mTOR
mammalian target of rapamycin
- PBS
phosphate-buffered saline
- PCa
prostate cancer
- PI
propidium iodide
- PIN
prostate intraepithelial neoplasia.
References
- 1. Jemal A., et al. (2010). Cancer statistics, 2010. CA Cancer J. Clin., 60, 277–300 [DOI] [PubMed] [Google Scholar]
- 2. SEER (2012). SEER Stat Fact Sheets: Prostate http://seer.cancer.gov/statfacts/html/prost.html (31 January 2013, date last accessed).
- 3. Moser L., et al. (2008). Hormone-refractory and metastatic prostate cancer—palliative radiotherapy. Front. Radiat. Ther. Oncol., 41, 117–125 [DOI] [PubMed] [Google Scholar]
- 4. Bostwick D.G. (1988). Premalignant lesions of the prostate. Semin. Diagn. Pathol., 5, 240–253 [PubMed] [Google Scholar]
- 5. Colanzi P., et al. (1998). Prostatic intraepithelial neoplasia and prostate cancer: analytical evaluation. Adv. Clin. Path., 2, 271–284 [PubMed] [Google Scholar]
- 6. Shin H.J., et al. (1995). Prostatic intraepithelial neoplasia: a potential precursor lesion of prostatic adenocarcinoma. Yonsei Med. J., 36, 215–231 [DOI] [PubMed] [Google Scholar]
- 7. Bethel C.R., et al. (2006). Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with Gleason score and chromosome 8p deletion. Cancer Res., 66, 10683–10690 [DOI] [PubMed] [Google Scholar]
- 8. Iwata T., et al. (2010). MYC overexpression induces prostatic intraepithelial neoplasia and loss of Nkx3.1 in mouse luminal epithelial cells. PLoS One, 5, e9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenkins R.B., et al. (1997). Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res., 57, 524–531 [PubMed] [Google Scholar]
- 10. Dang C.V., et al. (2009). MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res., 15, 6479–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bova G.S., et al. (1996). Review of allelic loss and gain in prostate cancer. World J. Urol., 14, 338–346 [DOI] [PubMed] [Google Scholar]
- 12. Neel B.G., et al. (1982). Molecular analysis of the c-myc locus in normal tissue and in avian leukosis virus-induced lymphomas. J. Virol., 44, 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi E., et al. (1991). Mapping of the MYC gene to band 8q24.12––q24.13 by R-banding and distal to fra(8)(q24.11), FRA8E, by fluorescence in situ hybridization. Cytogenet. Cell Genet., 57, 109–111 [DOI] [PubMed] [Google Scholar]
- 14. Ellwood-Yen K., et al. (2003). Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell, 4, 223–238 [DOI] [PubMed] [Google Scholar]
- 15. Koh C.M., et al. (2010). MYC and prostate cancer. Genes Cancer, 1, 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurel B., et al. (2008). Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol., 21, 1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaw R.J., et al. (2005). The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science, 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nobes J.P., et al. (2011). A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 109, 1495–1502 [DOI] [PubMed] [Google Scholar]
- 19. Shaw R.J., et al. (2004). The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell, 6, 91–99 [DOI] [PubMed] [Google Scholar]
- 20. Chaudhary S.C., et al. (2012). Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem. Photobiol., 88, 1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi W.Y., et al. (2012). Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis., 3, e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ben Sahra I., et al. (2011). Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res., 71, 4366–4372 [DOI] [PubMed] [Google Scholar]
- 23. Kickstein E., et al. (2010). Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl Acad. Sci. USA, 107, 21830–21835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vazquez-Martin A., et al. (2009). The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle, 8, 88–96 [DOI] [PubMed] [Google Scholar]
- 25. Ben Sahra I., et al. (2010). The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy, 6, 670–671 [DOI] [PubMed] [Google Scholar]
- 26. Ben Sahra I., et al. (2010). Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res., 70, 2465–2475 [DOI] [PubMed] [Google Scholar]
- 27. Watson P.A., et al. (2005). Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res., 65, 11565–11571 [DOI] [PubMed] [Google Scholar]
- 28. Thalmann G.N., et al. (1994). Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res., 54, 2577–2581 [PubMed] [Google Scholar]
- 29. Wu H.C., et al. (1994). Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int. J. Cancer, 57, 406–412 [DOI] [PubMed] [Google Scholar]
- 30. Kaighn M.E., et al. (1979). Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol., 17, 16–23 [PubMed] [Google Scholar]
- 31. Horoszewicz J.S., et al. (1980). The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res., 37, 115–132 [PubMed] [Google Scholar]
- 32. Li X., et al. (2011). Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J. Bone Miner. Res., 26, 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Azoulay L., et al. (2011). Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol. Biomarkers Prev., 20, 337–344 [DOI] [PubMed] [Google Scholar]
- 34. Clegg N.J., et al. (2011). MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS One, 6, e17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sawyers C.L. (2003). Will mTOR inhibitors make it as cancer drugs? Cancer Cell, 4, 343–348 [DOI] [PubMed] [Google Scholar]
- 36. Majumder P.K., et al. (2004). mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med., 10, 594–601 [DOI] [PubMed] [Google Scholar]
- 37. Ben Sahra I., et al. (2008). The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene, 27, 3576–3586 [DOI] [PubMed] [Google Scholar]
- 38. Zhao L., et al. (2011). Metformin induces G1 cell cycle arrest and inhibits cell proliferation in nasopharyngeal carcinoma cells. Anat. Rec. (Hoboken), 294, 1337–1343 [DOI] [PubMed] [Google Scholar]
- 39. Huai L., et al. (2012). Metformin induces differentiation in acute promyelocytic leukemia by activating the MEK/ERK signaling pathway. Biochem. Biophys. Res. Commun., 422, 398–404 [DOI] [PubMed] [Google Scholar]
- 40. Wang X., et al. (2011). Phosphorylation regulates c-Myc’s oncogenic activity in the mammary gland. Cancer Res., 71, 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnold H.K., et al. (2009). The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J., 28, 500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blandino G., et al. (2012). Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun., 3, 865. [DOI] [PubMed] [Google Scholar]
- 43. Sikka A., et al. (2012). Metformin suppresses growth of human head and neck squamous cell carcinoma via global inhibition of protein translation. Cell Cycle, 11, 1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J.W., et al. (1998). Transforming growth factor-beta 1 induces apoptosis through down-regulation of c-myc gene and overexpression of p27Kip1 protein in cervical carcinoma. Gynecol. Oncol., 69, 230–236 [DOI] [PubMed] [Google Scholar]
- 45. Gibson A.W., et al. (1995). Apoptosis induced by c-myc overexpression is dependent on growth conditions. Exp. Cell Res., 218, 351–358 [DOI] [PubMed] [Google Scholar]
- 46. Ni M., et al. (2013). Amplitude modulation of androgen signaling by c-MYC. Genes Dev., 27, 734–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mostaghel E.A., et al. (2011). New hormonal therapies for castration-resistant prostate cancer. Endocrinol. Metab. Clin. North Am., 40, 625–642, –x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colquhoun A.J., et al. (2012). Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis., 15, 346–352 [DOI] [PubMed] [Google Scholar]
- 49. Dang C.V. (2012). MYC on the path to cancer. Cell, 149, 22–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.