Abstract
Cytotoxic lymphocytes kill target cells through polarized release of the content of lytic granules at the immunological synapse. In human natural killer (NK) cells, signals for granule polarization and for degranulation can be uncoupled: Binding of β2 integrin LFA-1 to ICAM is sufficient to induce polarization but not degranulation, whereas CD16 binding to IgG triggers unpolarized degranulation. Here we investigated the basis for this difference. IL-2 expanded human NK cells were stimulated by incubation with plate-bound ligands of LFA-1 (ICAM-1) and CD16 (human IgG). Surprisingly, LFA-1 elicited signals similar to those induced by CD16, including tyrosine phosphorylation of the TCR ζ chain, tyrosine kinase Syk, and phospholipase C (PLC)-γ. Whereas CD16 activated Ca2+ mobilization and LAT phosphorylation, LFA-1 did not, but induced strong Pyk2 and paxillin phosphorylation. LFA-1-dependent granule polarization was blocked by inhibition of Syk, PLC-γ, and PKC, and by paxillin knockdown. Therefore, common signals triggered by CD16 and LFA-1 bifurcate to provide independent control of Ca2+-dependent degranulation and paxillin-dependent granule polarization.
Keywords: NK cells, Adhesion Molecules, Signal Transduction, Cellular Activation, Cytotoxicity
Introduction
Integrins play important roles in immunity, providing the adhesion required for cell conjugate formation as well as extravasation from blood vessels to sites of inflammation (1-3). The β2 integrins are expressed on leukocytes, and are important for anti-bacterial responses as seen in leukocyte adhesion deficiency (LAD) patients, who lack a functional β2 chain (CD18) (4). The αLβ2 (CD11a:CD18) integrin LFA-1 is important for the adhesion of T cells and NK cells to target cells. Antibody blockade of LFA-1 on cytotoxic T lymphocytes (CTL) (5) and NK cells (6) impairs cytotoxicity, as does inhibition of LFA-1 on NK cells with small molecule inhibitors (7). We have previously shown that binding of LFA-1 on NK cells to ICAM-1 on target cells is sufficient to induce the accumulation of perforin-containing cytotoxic granules and the microtubule organizing center (MTOC) at the site of NK cell contact with the target cell (8-9)(10). Cytotoxicity occurs when this polarization signal is combined with a second signal that triggers degranulation. This behavior contrasts with that of LFA-1 in T cells, which requires “inside-out” signaling for its own activation (11). In T cells, perforin polarization requires engagement of the T cell antigen receptor (TCR), and costimulation with LFA-1 greatly enhances polarization of perforin-containing granules (12).
In NK cells, stimulation of LFA-1 results in phosphorylation of Vav1 (13), a guanine nucleotide exchange factor (GEF) for the GTPase Rac1. Vav1 regulates cytoskeletal dynamics (14-16) and is required for NK cell cytotoxicity (17-21). siRNA-mediated knockdown of Vav1 in human NK cells reduces calcium mobilization and degranulation (22), which are responses not induced by LFA-1 in NK cells (9), suggesting that Vav1 signaling controls multiple pathways. In NK cells, β1 integrin engagement results in phosphorylation of both Pyk2 and paxillin (23), molecules that regulate the cytoskeleton. Phosphorylation of paxillin is observed after β2 integrin engagement in neutrophils (24-25), and LFA-1 stimulation in human NK cells induces tyrosine phosphorylation of Pyk2 and possibly paxillin (26).
Signaling by β1 and β2 integrins in macrophages and neutrophils results in degranulation, and is dependent on the immunoreceptor tyrosine-based activation motif (ITAM)-containing adapter proteins DAP12 and FcR γ chain (27). Cells from mice lacking these two adapters failed to respond to integrin ligands, as did cells lacking the tyrosine kinase Syk (28). The dependence of the response on the SH2 domains of Syk suggests binding to the phosphorylated ITAMs, as opposed to the SH2 domain-independent recruitment of Syk to the β2 integrin cytoplasmic tail, which has been described (29-30). ITAM-dependent integrin signaling has been seen in other cell systems (31-33).
We investigated whether LFA-1 in NK cells induces ITAM-dependent signals, and if molecules such as paxillin are involved in LFA-1-induced perforin polarization. In this report, we demonstrate that β2 integrin engagement by ICAM-1 alone leads to tyrosine phosphorylation of the TCR ζ chain, Syk, and PLC-γ1 and PLC-γ2 in human NK cells. Inhibition of Syk and PLC-γ enzymatic activity prevents perforin polarization in response to ICAM-1 expressed on target cells. Comparison to a known ITAM-dependent signaling pathway in NK cells, the CD16 response, reveals surprising similarity in the proximal biochemical responses, despite very different downstream outcomes with regard to perforin polarization and degranulation. Further investigation reveals differential requirement of the adapter proteins paxillin and LAT for responses to LFA-1 and CD16, suggesting the importance of different signaling complex assemblies or compartmentalization of signaling molecules to determine functional outcomes.
Materials and Methods
Cells
Human NK cell populations were isolated from peripheral blood by negative selection using an NK cell isolation kit (Miltenyi Biotec). NK populations were resuspended with irradiated autologous peripheral blood lymphocyte (PBL) feeder cells in IMDM (Invitrogen) supplemented with 10% human serum (Valley Biomedical Inc.), 10% purified IL-2 (Hemagen), 100 units/ml recombinant human IL-2 and 5 μg/ml phytohemaglutinin (PHA, Sigma), and expanded in the same medium without PHA or feeder cells. Alternatively, in experiments including stimulation through CD16, NK cells were cultured with irradiated autologous PBLs as feeder cells in OpTmizer T cell expansion medium (Invitrogen) supplemented with 10% purified IL-2, 100 units/ml recombinant human IL-2 and 5 μg/ml PHA. Subsequent passages of the cells were performed in the same medium, without PHA or feeder cells. All IL-2 expanded NK populations were used between week 3 and 4 of expansion. Culture, maintenance, and transfection of Drosophila Schneider line 2 (S2) cells were performed as described (34-35). 293T cells were cultured in IMDM supplemented with 10% FBS.
Antibodies and reagents
Antibodies against CD56 (Clone B159), CD16 (Clone 3G8), CD11a (Clone HI111), Pyk2 (Clone 11), and ICAM-1 (Clone HA58, PE conjugated) were purchased from BD Biosciences (San Jose, CA). Anti-phosphotyrosine antibody 4G10 and its agarose conjugate, anti-FcR γ (#06-727), paxillin (#05-417) and anti-LAT (#06-807) were from Millipore. TCR ζ (6B10.2), DAP12 (C-20), Syk (4D10), PLC-γ1 (1249), and PLC-γ2 (Q-20) antibodies were purchased from Santa Cruz Biotechnologies. Anti-perforin antibody clone δG9 was acquired from Endogen. Anti-phosphoserine PKC substrate antibody (#2261) was purchased from Cell Signaling. Purified human IgG (I5029) and sodium phenyl phosphate (P7751) were from Sigma Aldrich. Goat anti-mouse IgG F(ab)'2 was from Jackson Immunolabs. Syk Inhibitor II [2-(2-Aminoethylamino)-4-(3-trifluoromethylanilino)-pyrimidine-5-carboxamide], bisindolylmaleimide, U73122, and U73433 were purchased from EMD Biosciences. Celltracker Green, Fluo-4, Fura Red and 4%-20% MOPS SDS-PAGE gels were purchased from Invitrogen.
Production and purification of His-tagged ICAM-1
A cDNA encoding the extracellular domain of the mouse ICAM-1 with a C-terminal 6× His tag was generated by PCR with the following primers: forward- 5′-TCGACGCCACCATGGCTTCAACCCGTGCCAAGCC-3′ and reverse- 5′-TCTAGATCAATGATGGTGGTGATGATGGTTATTTTGAGAGTGGTACAGTACTGTCAGGTAC-3′. The PCR product was ligated in the pCR2.1-Topo vector (Invitrogen), and confirmed by sequencing. The insert was digested with SalI and BamHI, and subcloned into the SalI and BamHI sites of pBabe+CMV-Puro. This plasmid was transfected into 293T cells via Fugene (Roche), and a stable transfectant was selected with 1 μg/ml puromycin (Sigma). Clones were generated by limiting dilution, and were screened for ICAM-1 expression by intracellular flow cytometry. The highest expressing clone was expanded into ten 162 cm2 flasks, and when the cells were near confluence, the medium was replaced with serum-free medium (20 ml per flask). After 5 days, culture supernatants were harvested, cell debris was removed by centrifugation, and the supernatant was dialyzed against PBS. The dialyzed solution was flowed over a nickel-NTA column (Invitrogen), which was washed with PBS. Bound protein was eluted with 500 mM imidazole in PBS. The buffer was exchanged with PBS through repeated concentration with a 15 ml Centriprep concentrator (Millipore).
Stimulation of NK cells for immunoprecipitation
10 cm Petri dishes were incubated overnight, at 37°C, with 5 ml of a 50 mM sodium carbonate solution (pH 9.6) containing 10 μg/ml of either purified ICAM-1 or purified human IgG, or 15 ml of sodium carbonate solution containing no protein. Plates were washed twice with PBS, and blocked, at 4°C for 30 minutes, with 5 ml 1% BSA in PBS. Plates were then washed again, twice with PBS. NK cells were harvested, washed in PBS, and resuspended in cold, serum free IMDM at approximately 4 × 106 cells/ml. 5 ml (approximately 20 × 106 cells) were added to each coated and blocked plate. The plates were placed at 4°C for 15 minutes, and then moved to 37°C for 20 minutes. The medium and unbound cells were removed from each plate, placed into a 15 ml conical tube, and the unbound cells were pelleted. The pelleted cells were lysed in 1 ml lysis buffer (50 mM Tris-HCl (pH 7.6), 10 mM sodium pyrophosphate, 150 mM NaCl, 0.5% Triton X-100, 1 mM sodium orthovanadate, 1 mM phenylmethanesulfonylfluoride, 10 mM sodium fluoride), and this 1 ml of lysate was added to the plate from which the cells were harvested. Plates were placed on a rocker at 4°C for 10 minutes, the lysates were moved to 1.5 ml Eppendorf tubes, and nuclei were pelleted at 16,100 × g. In experiments including pharmacological inhibitors, NK cells were pretreated with the inihibitor, in their standard culture medium, for 45 minutes at 37°C, and the inhibitor was included in the stimulation. Anti-phosphotyrosine immunoprecipitations were performed with 30 μl of agarose-conjugated 4G10 for 1 hour at 4°C. IPs were washed in lysis buffer, and bound proteins were eluted with 40 μl of 100 mM sodium phenyl phosphate in PBS. Eluates were diluted with 2× Laemmli buffer containing 10% 2-mercaptoethanol. Other IPs were performed with 1 μg of antibody and protein A agarose, and resolved by standard SDS-PAGE.
Perforin Polarization Assays
Perforin polarization induced by target cells was performed as described (8-9), with modifications. Target cells were pre-labeled with 1 μg/ml Celltracker Green for 30 minutes at room temperature in their standard culture medium. In experiments including pharmacological inhibitors, NK cells were pretreated with the inihibitor, in their standard culture medium, for 45 minutes at 37°C. Inhibitors were included in the assay. After fixation and permeablization, intracellular perforin was stained with 3 μg/ml anti-perforin antibody and a 1:2000 dilution of Alexa 568 labeled goat-anti-mouse secondary antibody. Complete z-stacks of 40X fields were acquired by confocal microscopy. Three dimensional reconstruction of the interfaces and visual analysis of the z-stacks were used to determine if intracellular perforin was concentrated at the point on NK cell contact with the target cell in each conjugate pair.
CD107a surface expression assay
The CD107a assay was performed at described (9), with some modifications. 2 × 105 IL-2 expanded NK cells were mixed with an equal number of target cells, in V-bottom 96 well plates, in 200 μl of IMDM + 10% FBS in the presence of 6 μg/ml monensin and 5 μl of FITC-labled anti-CD107a antibody (BD Biosciences). Cells were mixed by pipeting and incubated for 2 hours at 37°C. The cells were pelleted at 300 × g, and resuspended in 200 μl of FACS buffer (PBS + 2 % FBS) containing 2 μl of PECy7-labeled anti-CD56 antibody (Clone NCAM16.2, BD Biosciences) per well. Staining was performed at 4°C for 30 minutes and cells were washed twice with FACS buffer. The CD107a expression of CD56+ NK cells was analyzed by flow cytometry.
Determination of intracellular calcium mobilization
NK cells were labeled for 30 minutes at 37°C in their standard culture medium with 1 μg/ml Fluo-4 and 1 μg/ml Fura Red. For experiments involving antibody stimulation, cells were washed twice with PBS, and resuspended at 10 × 106 cells/ml in cold, serum free IMDM. 2 × 106 cells (200 μl) were stained with 1 μg of the indicated stimulating antibody for 30 minutes on ice. Cells were washed twice with IMDM, resuspended in 1 ml IMDM, and transferred to FACS tubes. Cells were warmed at 37°C for 5 minutes, and then placed on the flow cytometer. Data were acquired for 30 seconds, the cells were removed from the flow cytometer, and 4 μg crosslinking goat-anti-mouse F(ab)'2 was added. Cells were vortexed and replaced on the flow cytometer, and data were acquired for a total of 5 minutes. For experiments involving stimulation with target cells, a protocol used for monitoring calcium flux after antigen presenting cell stimulation of T cells was adapted (36). 1 × 106 labeled NK cells were placed into a FACS tube along with an equal number of target cells in 800 μl warm, serum-free IMDM. The tubes were placed on the flow cytometer, and data were acquired for 30 seconds to establish a baseline. Tubes were removed from the flow cytometer, and cells were pelleted at 100 × g for 30 seconds. After centrifugation, the cells were gently resuspended with a micropipet, and the tubes placed back on the flow cytometer. Data were acquired for a total of 5 minutes. Analysis was performed in Flowjo (Treestar). Fluorescently labeled NK cells were gated, and the ratio of Fluo-4 to Fura Red was calculated. Due to variability in the baseline value from sample to sample, the results are presented as normalized to the starting value, such that the ratio at time 0 is set to 1.
siRNA Transfections
NK cells expanded in the serum-free OpTmizer T cell expansion medium were nucleofected with 300 pmol of siRNA duplexes in the solution from the human NK cell transfection kit, using nucleofector program U-001 (Lonza). The oligos used to achieve knockdown of paxillin were 5′-UGUGGAGCCUUCUUUGGUUU-3′ and 5′-CCAAAGAAGGCUCCACACUU-3′ for the sense and antisense strands, respectively, as described (37). The siRNA for LAT was part of Trifecta Dicer substrate kit from Integrated DNA Technologies. The oligos used were 5′-CACAUCCUCAGAUAGUUUGUAUCC-3′ and 5′-GAUACAAACUAUCUCUGAGGAUGUGCUG-', for the sense and antisense strands, respectively.
Results
LFA-1 binding to ICAM-1 results in TCR ζ and Syk phosphorylation
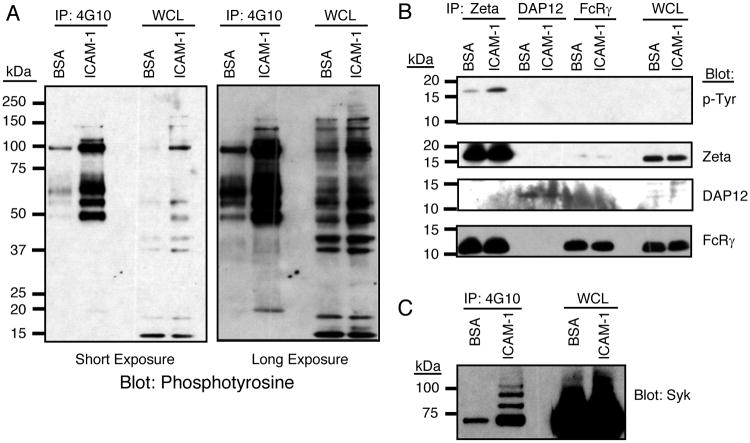
NK cell activation by LFA-1 was determined by stimulating IL-2 expanded human NK cells with purified, plate-bound soluble ICAM-1 tagged with 6 histidines at the end of the extracellular domain. Tyrosine phosphorylated proteins were immunoprecipitated with agarose-conjugated mAb 4G10, eluted with sodium phenylphosphate, resolved by SDS-PAGE along with whole cell lysates, and analyzed by immunoblotting for phosphotyrosine (Fig. 1A). Strong tyrosine phosphorylation of five proteins, with approximate molecular weights of 115 kDa, 105 kDa, 65 kDa, 55 kDa, and 48 kDa, was induced by ICAM-1 stimulation, as these proteins were easily visualized. Longer exposure of the blot revealed several less-heavily phosphorylated proteins with approximate molecular weights of 160 kDa, 140 kDa, and 18 kDa (Fig. 1A).
Figure 1.
Tyrosine phosphorylation of TCR ζ and Syk induced by LFA-1 engagement in IL-2 expanded human NK cells. (A) NK cells were stimulated at 37°C for 20 minutes on plates coated with either purified ICAM-1 or BSA. Cells were lysed, and tyrosine phosphorylated proteins were immunoprecipitated with 4G10-agarose. IPs were resolved by 4-20% SDS-PAGE and immunoblotted with biotinylated anti-phosphotyrosine antibody (4G10). The left and right panels represent short and long exposures of the resulting chemiluminescent reaction. (B) NK cells were stimulated and lysed. TCR ζ, DAP12, and the FcR γ chain were immunoprecipitated, resolved by SDS-PAGE, and immunoblotted for phosphotyrosine. The blot was stripped and probed in succession with anti-TCR ζ, anti-DAP12, and anti-FcR γ antibodies. (C) Anti-phosphotyrosine immunoprecipitations prepared as in Fig. 1A were immunoblotted with an anti-Syk antibody.
Recent reports have demonstrated that integrins in leukocytes, including LFA-1, signal through ITAM-containing adapter molecules (27). We therefore investigated if the small phosphoprotein observed around 18 kDa was one of three ITAM-bearing adapter proteins expressed in NK cells: the TCR ζ chain, the FcR γ chain, and DAP12 (38-40). Immunoprecipitations (IP) were performed from NK cell lysates using antibodies to the TCR ζ chain, the FcR γ chain, and DAP12. Their tyrosine phosphorylation status was determined by immunoblotting with 4G10 mAb (Fig. 1B). TCR ζ, but not FcR γ or DAP12, was detectably phosphorylated. Each IP was further immunoblotted for all 3 chains, as controls for the IP and to test for heterodimer formation (Fig. 1B). Interestingly, the TCR ζ IPs contained substantial amounts of FcR γ, consistent with the existence of a ζ:γ heterodimer known to be expressed in NK cells. Curiously, it appears that only the TCR ζ chain in those IPs is phosphorylated, as tyrosine phosphorylation of the FcRγ chain was not observed in TCR ζ IPs and TCR ζ phosphorylation was not observed in FcRγ IPs. This suggests a preferential phosphorylation of TCR ζ homodimers. Although DAP12 was difficult to detect by immunoblot, phosphorylation could be detected in DAP12 IPs following pervanadate stimulation of NK cells (data not shown). Given that ITAM-mediated signaling is known to proceed through the recruitment of the Syk/ZAP70 family of kinases (41), we next examined Syk and ZAP70. ICAM-1 stimulation induced phosphorylation of Syk (Fig. 1C), but not ZAP70 (data not shown). In several experiments, in which the stimulation was particularly potent, a ladder of higher bands appeared in Syk blots, consistent with the ubiquitination of Syk observed upon activation in other cell systems (42-44).
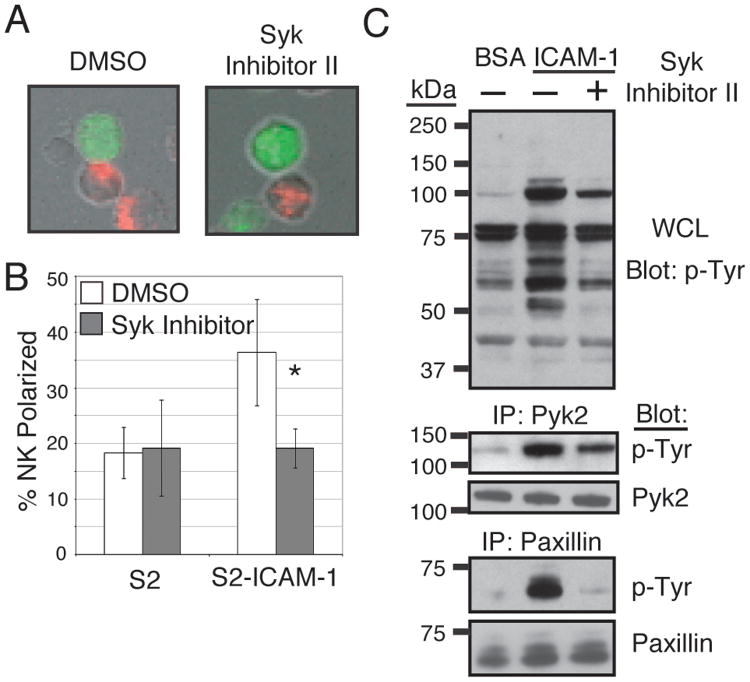
Syk inhibition blocks LFA-1-induced perforin polarization
We have previously demonstrated that engagement of LFA-1 by ICAM-1 expressed on Drosophila S2 cells causes polarization of perforin-containing granules in the NK cell toward the site of cell–cell contact (8-9)(10). To test for the importance of Syk in this response, the same assay was employed in the presence of a selective inhibitor of Syk, Syk inhibitor II (IC50 = 41 nM), which is reportedly effective against ZAP70 (IC50 = 11.2 μM) and other kinases only at much higher concentrations (IC50 = 5.1 μM to 22.6 μM). Preincubation of NK cells with 1 μM Syk inhibitor II reduced binding of NK cells to ICAM-1 (Table 1), as determined in a bead binding assay using an ICAM-1-Fc fusion protein that carries a mutation in the CD16 binding binding site of the Fc (9), although considerable binding to ICAM-1 remained. As the polarization assay scores only NK cells that are in definite contact with target cells, and therefore favors the NK cells that retain binding to ICAM-1 (Fig. 2A), it is likely that inhibition of polarization would reflect genuine inhibition of the polarization response, and not the consequence of inhibition of adhesion. Upon mixing of NK cells with S2 cells expressing ICAM-1, the Syk inhibitor completely blocked polarization of perforin to the site of contact with ICAM-1-expressing S2 cells (Fig. 2B). Western blotting also revealed the importance of Syk in the LFA-1 signaling response, as much of the tyrosine phosphorylation seen after ICAM-1 stimulation was blocked or reduced by pretreatment with the Syk inhibitor (Fig. 2C). Two proteins, Pyk2 (115 kDa) and paxillin (65 kDa), were identified as tyrosine phosphorylated following stimulation, and correspond to bands observed in Fig. 1A. Inhibition of Syk significantly reduced phosphorylation of Pyk2 and nearly abolished phosphorylation of paxillin. With the complete block of perforin polarization observed with the Syk inhibitor, the differential effects of the inhibitor on these proteins may reflect a greater importance of paxillin in LFA-1-induced polarization.
Table I.
Binding of NK cells to ICAM-1-coated beads following treatment with pharmacological inhibitors or siRNA transfection. NK cells were either pretreated with the indicated inhibitors, or transfected with the indicated siRNA oligos. Cells were incubated with ICAM-1-coated beads for 20 minutes at 37°C and fixed with 4% paraformaldehyde. Binding of NK cells to beads was monitored by flow cytometry.
| Treatment | % NK cells Bound to ICAM-1‐Fc Beads |
|---|---|
|
| |
| DMSO | 62.1 ± 0.3 |
| Syk Inhibitor II | 37.2 ± 12.9 |
| U73433 | 62.3 ± 2.6 |
| U73122 | 19.9 ± 11.6 |
| Bisindolylmaleimide | 33.7 ± 15.4 |
|
| |
| Control siRNA | 75.9 ± 8.0 |
| LAT siRNA | 79.1 ± 9.6 |
| Paxillin siRNA | 75.5 ± 12.3 |
Figure 2.
LFA-1 dependent responses are ablated by inhibition of the Syk kinase. (A) NK cells pretreated for 45 minutes at 37°C in culture medium with 1 μM Syk inhibitor II or DMSO carrier were mixed with Celltracker Green labeled S2 cells or S2 cells expressing ICAM-1 for 20 min at 37°C, adhered to poly-D-lysine coated slides, fixed, permeablized, and stained with an anti-perforin antibody and Alexa-568 goat-anti-mouse secondary antibody. A representative image of treated or untreated NK cells in contact with S2-ICAM-1 cells is shown. (B) Treated or untreated NK cells in conjugate with S2 or S2-ICAM-1 cells were scored for polarization of perforin toward the target cell interface. Error bars represent S.D. of three experiments conducted with NK cells from different donors. *p < 0.05 relative to DMSO-treated NK cells + S2-ICAM-1 cells. (C) NK cells were pretreated for 45 minutes at 37°C in culture medium with 1μM Syk inhibitor II (Calbiochem) or DMSO carrier. Cells were then stimulated with purified ICAM-1 on plates for 20 minutes at 37°C and lysed. Pyk2, CasL, and paxillin immunoprecipitations and whole cell lysates (WCL) were resolved by SDS-PAGE and immunoblotted for phosphotyrosine.
LFA-1 and CD16 induce ITAM-based signals but different responses
Integrin engagement in neutrophils induces degranulation and release of reactive oxygen species in an ITAM- and Syk-dependent manner (27). We have previously shown that while engagement of LFA-1 on freshly isolated NK cells induces perforin polarization, it does not induce degranulation (9). In contrast, in freshly isolated NK cells, the ITAM-utilizing low affinity receptor for IgG, CD16, induces sustained calcium flux (45) and degranulation (9). If both LFA-1 and CD16 use ITAM-bearing adapters and the Syk kinase to transduce signals, why are the downstream responses so different?
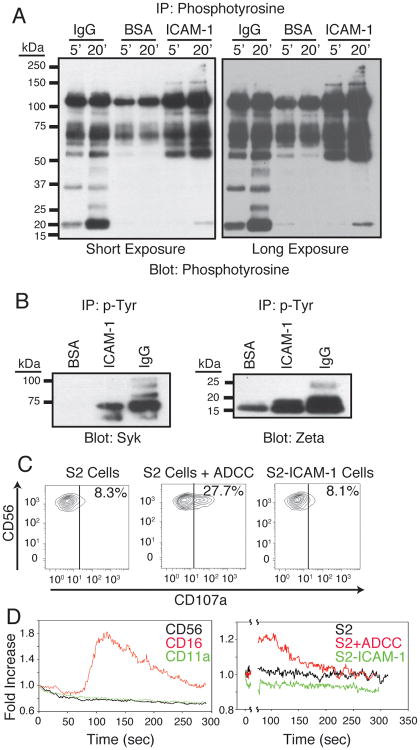
To investigate the signaling pathways induced by these receptors, the tyrosine phosphorylation response of NK cells was monitored following stimulation with plate-bound ICAM-1 or purified human IgG (Fig. 3A). The most prominent tyrosine phosphorylation induced by both receptors, as detected by 4G10-agarose IP and 4G10 immunoblotting, and observed in a short exposure of the blot, is surprisingly similar, with major bands around 105 kDa, 65 kDa, and 55 kDa. A prominent band seen around 18 kDa following CD16 stimulation is likely the TCR ζ chain. Other bands uniquely induced in the CD16 response were observed around 34 kDa and 26 kDa. Weaker bands, observed upon longer exposure, were seen around 45 kDa and 25 kDa only after CD16 stimulation. LFA-1 stimulation produced weaker phosphorylation of the 18 kDa band (possibly TCR ζ), as well as weak phosphorylation of higher molecular weight proteins (150 kDa and above) that were not observed following CD16 stimulation.
Figure 3.
LFA-1 and CD16 both induce phosphorylation of Syk and TCR ζ, but provoke different cellular responses. (A) NK cells were stimulated with purified ICAM-1 or purified human IgG on plates for either 5 minutes or 20 minutes at 37°C and lysed. Lysates were immunoprecipitated with 4G10 agarose, and immunoprecipitates were resolved by SDS-PAGE and immunoblotted for phosphotyrosine. (B) 4G10-agarose immunoprecipitates from NK cells stimulated as in Fig. 3C for 20 minutes were immunoblotted for Syk and TCR ζ. (C) NK cells were mixed with S2 cells, S2 cells precoated with a polyclonal antiserum (S2 + ADCC), or S2-ICAM-1 cells in the presence of FITC-labeled anti-CD107a antibody and 6 μM monensin for 2 hours at 37°C. Cells were then stained with a PECy7-labeled anti-CD56 antibody at 4°C for 30 minutes. Cells were analyzed by flow cytometry. CD56+ NK cells were gated and examined for CD107a staining. (D) NK cells were labeled with Fluo-4 and Fura Red and stimulated (as described in Methods) with either antibodies against CD56, CD16 and CD11a (left panel) or S2 cells, S2 cells + antiserum, or S2-ICAM-1 cells (right panel). Fluo-4 and Fura Red intensities were monitored for 5 minutes total, the ratio of Fluo-4 to Fura Red was calculated, and the ratios for each time course were normalized to the starting ratio at Time=0. Data are expressed as fold changes in the ratio from the starting time point.
Phosphorylation of TCR ζ and Syk was confirmed following both stimuli (Fig. 3B), with stronger phosphorylation after CD16 stimulation. The multiple bands in the Syk blot following IgG stimulation are likely due to Syk ubiquitination. Changes in TCR ζ mobility occur following tyrosine phosphorylation, with distinct molecular weights corresponding to phosphorylation at specific sites (46). The largest band observed in the IgG stimulation after 4G10-agarose IP and TCR ζ immunoblot is likely the fully phosphorylated p23 form of TCR ζ.
Although TCR ζ and Syk phosphorylation were stronger following CD16 stimulation in comparison to LFA-1 stimulation, the two responses displayed similar kinetics, with greater phosphorylation after 20 minutes (Supplemental Fig. 1). In both cases, phosphorylation was reduced by an inhibitor of Syk (Supplemental Fig. 2). These results are consistent with the known ability of Syk to phosphorylate ITAM sequences (47). The reduction in TCR ζ phosphorylation was perhaps more pronounced following LFA-1 stimulation, potentially indicating a greater reliance of LFA- signaling on positive feedback.
The similarity in much of the biochemical responses to LFA-and CD16 led us to examine the inability of LFA-1 to induce degranulation, previously observed in freshly isolated NK cells, in the IL-2 expanded NK cells used in this report. Degranulation was monitored through the surface expression of CD107a by NK cells after mixing with target cells (Fig. 3C). Stimulation with Drosophila S2 cells coated with a polyclonal rabbit anti-serum, which engages CD16, induced significant CD107a surface expression. In contrast, ICAM-1-expressing S2 cells induced no degranulation, as seen previously in freshly isolated cells. To further highlight the differences in CD16- and LFA-1-mediated signaling, calcium flux induced by both receptors was determined. Calcium mobilization was observed following antibody crosslinking of CD16, but not LFA-1 (Fig. 3D, left panel). To rule out the possibility that the ligand ICAM-1 would stimulate cells in a manner different than antibody crosslinking, NK cells were stimulated with S2 cells using a protocol previously utilized to observe calcium mobilization following stimulation of T cells with antigen-loaded antigen presenting cells (36). Consistent with the antibody crosslinking, stimulation of NK with antibody-coated S2 cells induced calcium flux, while ICAM-1-expressing S2 cells did not (Fig. 3D, right panel). Determination of calcium flux via flow cytometry may not reveal transient calcium fluxes, such as those observed in platelets following integrin engagement (48), leaving the possibility that LFA-1 engagement in NK cells induces transient calcium oscillations. However, our results contrast with published data in isolated primary T cells, in which LFA-1 stimulation causes a TCR-dependent, slow, and sustained calcium mobilization (49).
Both LFA-1 and CD16 engagement induce PLC-γ1 and PLC‐γ2 phosphorylation
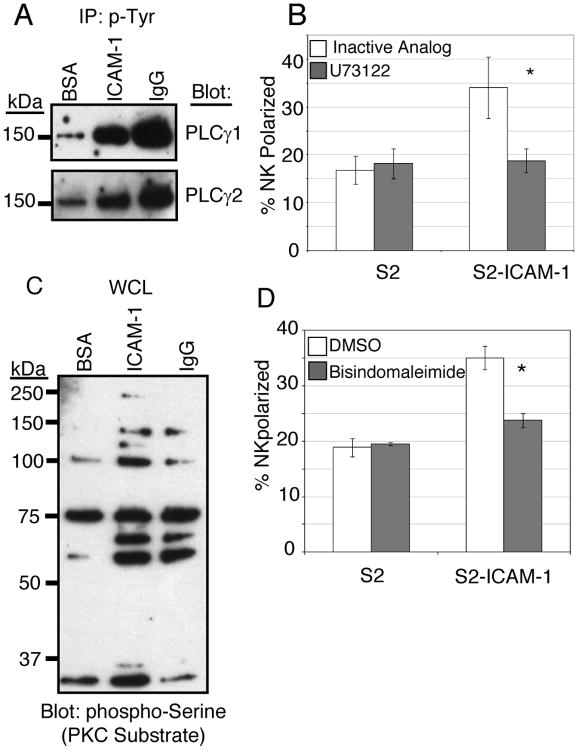
PLC-γ1 or PLC-γ2 activity is required for calcium flux and degranulation following CD16 engagement in freshly isolated NK cells (45, 50). PLC-γ activity is also recruited to ITAM-dependent immune receptors in a variety of cell types (51), and in most cases calcium mobilization is induced as a result of IP3 production (52). We therefore hypothesized that the Syk-dependent pathways used by LFA-1 and CD16 might diverge at the level of PLC-γ involvement. Surprisingly, both PLC-γ1 and PLC-γ2 were tyrosine phosphorylated following engagement of LFA-1 or CD16 (Fig. 4A). PLC-γ activation following LFA-1 stimulation is surprising, given the lack of calcium flux following LFA-1 stimulation. Inhibition of PLC activity resulted in a block in perforin polarization in response to ICAM-1 (Fig. 4B), indicating that PLC-γ1/2 are involved in this response. Treatment of the NK cells with the PLC inhibitor also reduced binding to ICAM-1 (Table 1), although some binding remained.
Figure 4.
PLC-γ and PKC are required for perforin polarization in response to LFA-1 engagement. (A) NK cells were stimulated at 37°C for 20 minutes on plates coated with BSA, purified ICAM-1, or purified human IgG. Cells were lysed, and tyrosine phosphorylated proteins were immunoprecipitated with 4G10-agarose. IPs were resolved by 4-20% SDS-PAGE and immunoblotted for PLC-γ1 and PLC-γ2. (B) NK cells were pretreated in culture medium for 45 minutes with 50 nM PLC inhibitor (U73122) or its inactive analog (U73343). Perforin polarization was analyzed as in Fig. 2B. Error bars represent S.D. of three experiments conducted with NK cells from different donors. *p < 0.05 relative to control U73343-treated NK cells + S2-ICAM-1 cells. (C) Whole cell lysates from cells stimulated as in Fig. 4A were resolved by SDS-PAGE and immunoblotted with an anti-PKC substrate (anti-phospho-serine motif) antibody. (D) NK cells were pretreated in culture medium for 45 minutes with 5 μM bisindolylmaliemide or DMSO carrier at 37°C. Perforin polarization was analyzed as in Fig. 2B. Error bars represent S.D. of three experiments conducted with NK cells from different donors. *p < 0.05 relative to control DMSO-treated NK cells + S2-ICAM-1 cells.
The second product of PLC-γ activity is diacylglycerol (DAG). Since DAG production is known to stimulate members of the protein kinase C (PKC) family, we tested the involvement of PKC in the response to LFA-1. Stimulation of NK cells with ligands for either LFA-1 or CD16 induced serine phosphorylation that is detected by an antibody directed against a motif that corresponds to preferred substrates of PKC (Fig. 4C). Several bands were induced by LFA-1 or CD16 engagement, and at least three bands were induced only by LFA-1. These proteins have not been identified. Inhibition of PKC activity reduced perforin polarization in response to LFA-1 engagement (Fig. 4D), suggesting that PKC activity induced by DAG production was involved in the LFA-1 response. Treatment with the PKC inhibitor reduced binding to ICAM-1 (Table 1), but again substantial binding remained.
Role of paxillin and LAT in the responses to LFA-1 and CD16
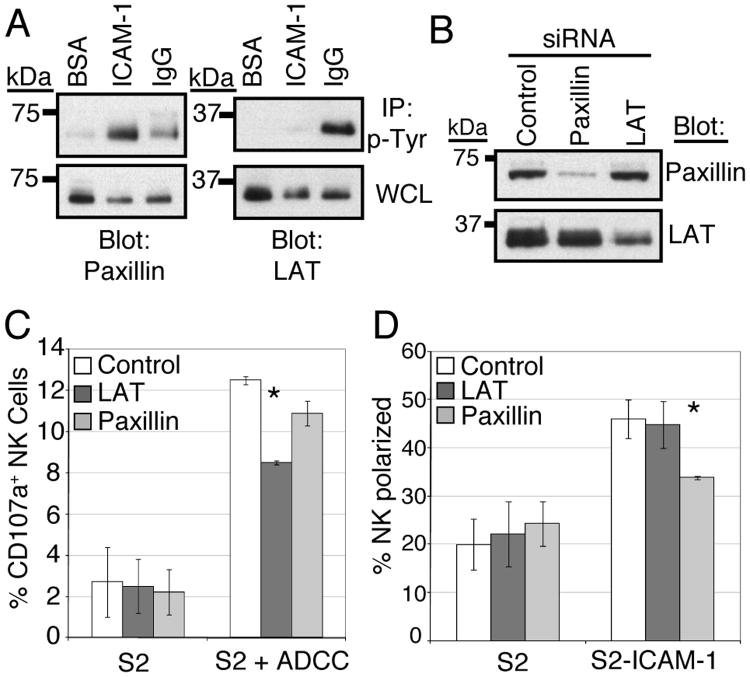
We next chose to investigate potential differences in signaling that might explain the differing outcomes of LFA-1 and CD16 engagement. We assayed for the phosphorylation status of proteins known to be involved in integrin signaling and ITAM-mediated signaling in different systems. As shown in Fig. 2A, Syk-dependent paxillin phosphorylation is induced by LFA-1 engagement. Stimulation through CD16 resulted in a lesser amount of paxillin phosphorylation than LFA-1 stimulation (Fig. 5A). In comparison, LAT, a protein involved in TCR signaling (53), is phosphorylated following CD16 stimulation and very weakly phosphorylated by LFA-1 stimulation (Fig. 5A). To determine if the differential patterns of phosphorylation correlated with differential involvement in functional outcomes, CD16-mediated degranulation and LFA-1-mediated perforin polarization were determined after siRNA-mediated knockdown of paxillin and LAT (Fig. 5B). Knockdown of LAT reduced CD16 induced degranulation by a statistically significant degree (by approximately 1/3, Fig. 5C). Knockdown of paxillin reduced degranulation to a lesser extent, which did not achieve statistical significance. Degranulation of NK cells after control siRNA transfection was generally less than degranulation seen in untransfected NK cells (compare Fig. 3A and Fig. 5C). This reduction was consistent across multiple siRNA oligos, and appears to be a result of nucleofection (data not shown). Perforin polarization was reduced by a statistically significant amount following paxillin knockdown (Fig. 5D), and was unaffected by LAT knockdown. Therefore, we conclude that paxillin is involved in LFA-1-mediated polarization, while LAT-dependent signaling is required for CD16-mediated degranulation but dispensable for LFA-1-mediated polarization.
Figure 5.
Paxillin and LAT are differentially involved in the LFA-1 and CD16 responses. (A) Anti-phosphotyrosine immunoprecipitates from NK cells stimulated as in Fig. 4A were resolved by SDS-PAGE and immunoblotted for paxillin or LAT. (B) NK cells were nucleofected with 300 pmols of siRNA targeting either paxillin or LAT, or a control siRNA. 48 hours after nucleofection, whole cell lysates from 2.5 × 105 cells were resolved by SDS-PAGE and immunoblotted for paxillin or LAT. (C) NK cells transfected with control, paxillin, or LAT siRNA mixed with either S2 cells or S2 cells precoated with a polyclonal antiserum (S2 + ADCC) were analyzed for CD107a surface expression (degranulation) as in Fig. 3A. Error bars represent S.D. of three experiments conducted with NK cells from different donors. *p < 0.05 relative to control siRNA transfected NK cells + S2+ADCC. (D) NK cells transfected with control, paxillin, or LAT siRNA mixed with either S2 or S2-ICAM-1 cells were analyzed for perforin polarization as in Fig. 2B. Error bars represent S.D. of three experiments conducted with NK cells from different donors. *p < 0.05 relative to control siRNA transfected NK cells + S2-ICAM-1 cells.
Discussion
In this report we have described a signaling network utilized by the integrin LFA-1 to induce perforin polarization in NK cells. We have used protein biochemistry, pharmacological inhibitors, and siRNA-mediated protein knockdown to demonstrate the phosphorylation of the TCR ζ chain, Syk, and PLC-γ1/2 and a requirement for paxillin in this signaling pathway. By stimulating NK cells with natural ligands for LFA-1 or CD16, we have shown that LFA-1 and CD16 share signaling properties (TCR ζ, Syk and PLC-γ phosphorylation), yet initiate different functional outcomes. The basis for this difference was explored. Knockdown of protein expression by siRNA transfection showed that paxillin contributed to LFA-1-dependent perforin polarization, while LAT contributed to CD16-mediated degranulation.
A requirement for ITAM-containing adapter molecules in integrin signaling was reported in macrophages and neutrophils (27). These cells normally express DAP12 and the FcR γ chain, and in the absence of these two adapters the response to both fibrinogen, a ligand for integrin αMβ2, and ICAM-1 was defective. Deletion of Syk also abrogated these integrin-dependent responses. In comparison, NK cells express three ITAM-containing adapters: TCR ζ, FcR γ, and DAP12. We show that engagement of LFA-1 resulted in detectable tyrosine phosphorylation of only the TCR ζ chain. NK cells are known to express a heterodimer of TCR ζ and FcR γ (39). Even in conditions where this heterodimer was detected in our IPs, we detected phosphorylation of only the TCR ζ chain (Fig. 1B), suggesting that homodimers of TCR ζ are utilized preferentially in LFA-1 signaling. Complete ablation of integrin responses in myeloid cells required the deletion of both DAP12 and FcR γ, and it is possible that other ITAM-containing adapters could replace TCR ζ in integrin signaling in NK cells despite our inability to detect phosphorylation of these molecules in normal human NK cells.
Previous reports have established a link between LFA-1 and TCR signaling chains. In Jurkat T cells, clustering of the integrin β2 cytoplasmic tail results in calcium flux, and this response is defective in cells lacking the TCR ζ chain (54). This study was performed using overexpressed fusion proteins, rather than intact integrin chains, but it highlights a possible functional tie between β2 integrins and the TCR ζ chain in T cells. Additionally, crosslinking and capping LFA-1 on primary CTL induces a capping of CD3 signaling chains (55), which was interpreted as LFA-1 favoring the formation of the immunological synapse in T cells. Despite the evidence linking integrins and ITAMs, no protein-protein interaction between an integrin chain and an ITAM-containing adapter has been demonstrated, and ITAM signaling by integrins remains somewhat mysterious (33).
In human NK cells, we have previously shown that LFA-1 engagement results in polarization of perforin containing granules, but does not induce degranulation (8)(9). Conversely, CD16 engagement induces degranulation, but does not induce perforin polarization (9). Interestingly, in mouse NK cells, stimulation through LFA-1 alone is not sufficient to induce perforin polarization, but polarization occurs following simultaneous engagement of LFA-1 and NKG2D (56). This difference between mouse and human NK cell responses to LFA-1 may reflect genuine differences between species, or the fact that NK cells in humans may have been primed in vivo.
We have shown previously (and confirmed here) that CD16 stimulation induces calcium flux in human NK cells. In contrast, crosslinking of LFA-1 by antibodies or engagement with ICAM-1 on target cells induces no degranulation or calcium mobilization (Fig. 3). The lack of degranulation and calcium flux in response to LFA-1 engagement on NK cells stands in contrast to previous reports in other cells types. LFA-1 engagement in neutrophils induces degranulation and release of antimicrobial products such as reactive oxygen species (27). β2 integrin Mac-1-mediated phagocytosis of opsonized particles initiates calcium mobilization in human neutrophils (57), and antibody crosslinking of the β2 chain in primary human CD4+ T cells induces robust calcium flux (49). Perhaps this represents true cell-type specific differences, although these details remain to be elucidated. Certainly, calcium mobilization is considered a typical response in ITAM-dependent signaling (51), and the involvement of TCR ζ and Syk in a signaling pathway that does not induce calcium, as reported here, is novel.
CD16 engagement induces TCR ζ and Syk phosphorylation, along with calcium flux and degranulation, but without perforin polarization. The similarities in proximal intracellular signaling downstream of LFA-1 and CD16 are striking given the obvious difference in the ultimate downstream outcomes. Interestingly, PLC-γ1 and PLC-γ2 are tyrosine phosphorylated in response to both stimuli. The phosphorylation of PLC-γ1 and PLC-γ2 following LFA-1 engagement is surprising, given the role of the PLC-γ product IP3 in calcium flux (58). However, LFA-1 induced no calcium flux in NK cells. These observations suggest an unknown bifurcation in the signaling pathways used by LFA-1 and CD16. This could be a threshold effect, as the observed biochemical responses to CD16 stimulation were stronger (particularly with TCR ζ, Syk, and PLC-γ1/2) than the responses to LFA-1. Alternatively, there may be qualitative differences, including different kinetics, in the responses. However, the point at which signals by LFA-1 and CD16 diverge is unknown, and the reason for the failure of LFA-1 to initiate calcium flux remains to be elucidated.
PLC-γ phosphorylation following integrin engagement has been observed in other cell types: PLC-γ1 following LFA-1 engagement in T cells (49) and PLC-γ2 in neutrophils following β2 crosslinking (59). A recent report revealed that the MTOC in T cell moves to sites of localized DAG production (60). The MTOC movement in response to TCR stimulation is PLC-γ dependent, as DAG is also a product of PLC-γ activity. Our results with PLC-γ1 and PLC-γ2 in LFA-1 signaling are consistent with these observations.
Signaling pathways that influence integrin affinity are referred to as inside-out signals (61)(62). Binding of integrins to their ligands is controlled by the activation status of the integrin, with distinct conformations of the integrin ranging from low to high affinity states (63-64). In turn, engagement of integrins by ligands initiates outside-in signaling, which leads to biological responses. We have previously shown, in freshly isolated human NK cells, that LFA-1 binding to ICAM-1 leads to further activation of LFA-1, such that outside-in signaling by LFA-1 results in its own inside-out activation, and that stimulation by IL-2 leads to activation of LFA-1 (34). However, the pharmacological inhibitors used here in several biochemical experiments with IL-2 expanded NK cells caused a reduction in binding to ICAM-1 (Table 1). The residual binding was sufficient to score for polarization in an assay that considers only cells that are in contact with target cells. Our results with siRNA transfections, in which paxillin knockdown inhibited perforin polarization without reduction of binding to ICAM-1, indicate that it is possible to separate integrin activation and outside-in signaling by using tools of sufficient specificity. In this respect, NK cells offer a unique tool to study outside-in signaling by LFA-1, given that binding to ICAM-1 is not completely dependent on inside-out signaling.
To identify molecules that could explain the disparate functional outcomes of LFA-1 and CD16 engagement, we investigated the activation of other downstream signaling molecules. Pyk2 and paxillin were preferentially phosphorylated downstream of LFA-1, as compared to CD16. LAT was almost exclusively phosphorylated after CD16 engagement. We therefore tested their potential roles in LFA-1 signaling for perforin polarization and in CD16 signaling for degranulation in IL-2 expanded human NK cells. Knockdown of LAT by siRNA transfection reduced CD16-induced degranulation, but had no effect on LFA-1-induced polarization. Knockdown of paxillin significantly reduced polarization through LFA-1, and had a lesser effect on CD16-mediated degranulation. The role of LAT downstream of the ITAM-dependent CD16 signaling was expected. LAT is best known for its role in T cell receptor signaling, in which it serves downstream of ITAMs as a tyrosine phosphorylated docking platform for the assembly of a large, multi-molecular complex, including PLC-γ1 and Vav1 (65). Paxillin is an adapter protein that interacts with actin binding proteins (66-67) and proteins with actin regulating functions (68-69). It is phosphorylated downstream of integrins in human NK cells (23). Paxillin associates with Pyk2 (70-74), which was tyrosine phosphorylated downstream of LFA-1 in NK cells (Fig. 2A). Paxillin localized to the MTOC in T lymphoblasts (75) and a complex of Pyk2, paxillin, and the MTOC localizes to cytotoxic synapses in NK cells (76), creating a direct link between this adapter protein and a crucial component of the cytotoxic granule polarization machinery. We have shown a selective role of paxillin downstream of LFA-1 in perforin polarization. While further understanding will require a more detailed analysis of paxillin-containing protein complexes formed following LFA-1 stimulation, our results are a first step towards understanding the signaling pathways utilized by LFA-1 in perforin polarization in NK cells.
Supplementary Material
Supplemental Figure 1: Syk and TCR ζ tyrosine phoshorylation in response to LFA-1 and CD16 stimulation. NK cells were stimulated with purified ICAM-1 or purified human IgG on plates for 5 and 20 minutes at 37°C and lysed. Lysates were immunoprecipitated with 4G10 agarose, and immunoprecipitates were resolved by SDS-PAGE and immunoblotted for Syk (top panel) or TCR ζ chain (bottom panel).
Supplemental Figure 2: NK cells were pretreated for 45 minutes at 37°C in culture medium with 1 μM Syk inhibitor II (Calbiochem) or DMSO carrier. Cells were then stimulated with purified ICAM-1 or purified human IgG on plates for 20 minutes at 37°C and lysed. Lysates were immunoprecipitated with 4G10 agarose, and immunoprecipitates were resolved by SDS-PAGE and immunoblotted for Syk or TCR ζ as indicated. Both a short and long exposure of the TCR ζ blot are shown.
3Abbreviations used in this paper
- ITAM
Immunoreceptor Tyrosine-based Activation Motif
- S2 cells
Schneider 2 cells
- APC
Antigen Presenting Cell
- SH2
Src Homology 2
- DAG
diacylglycerol
- IP
immunoprecipitation
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 2.Hogg N, Smith A, McDowall A, Giles K, Stanley P, Laschinger M, Henderson R. How T cells use LFA-1 to attach and migrate. Immunol Lett. 2004;92:51–54. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Harlan JM. Leukocyte adhesion deficiency syndrome: insights into the molecular basis of leukocyte emigration. Clin Immunol Immunopathol. 1993;67:S16–24. doi: 10.1006/clin.1993.1079. [DOI] [PubMed] [Google Scholar]
- 5.van Kooyk Y, de Vries-van der Zwan A, de Waal LP, Figdor CG. Efficiency of antibodies directed against adhesion molecules to prolong skin graft survival in mice. Transplant Proc. 1994;26:401–403. [PubMed] [Google Scholar]
- 6.Schmidt RE, Bartley G, Levine H, Schlossman SF, Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985;135:1020–1025. [PubMed] [Google Scholar]
- 7.Weitz-Schmidt G, Chreng S, Riek S. Allosteric LFA-1 inhibitors modulate natural killer cell function. Molr Pharmacol. 2009;75:355–362. doi: 10.1124/mol.108.051169. [DOI] [PubMed] [Google Scholar]
- 8.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 9.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 12.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantrell D. Lymphocyte signalling: a coordinating role for Vav? Curr Biol. 1998;8:R535–538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- 15.Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid1 M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 16.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opinion Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Billadeau DD, Brumbaugh KM, Dick CJ, Schoon RA, Bustelo XR, Leibson PJ. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella M, Fujikawa K, Tassi I, Kim S, Latinis K, Nishi S, Yokoyama W, Colonna M, Swat W. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J Exp Med. 2004;200:817–823. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colucci F, Rosmaraki E, Bregenholt S, Samson SI, Di Bartolo V, Turner M, Vanes L, Tybulewicz V, Di Santo JP. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J Exp Med. 2001;193:1413–1424. doi: 10.1084/jem.193.12.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galandrini R, Palmieri G, Piccoli M, Frati L, Santoni A. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J Immunol. 1999;162:3148–3152. [PubMed] [Google Scholar]
- 21.Graham DB, Cella M, Giurisato E, Fujikawa K, Miletic AV, Kloeppel T, Brim K, Takai T, Shaw AS, Colonna M, Swat W. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Das A, Gross CC, Bryceson YT, Long EO. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32:175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase-2 activation by beta 1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol. 1997;159:4729–4736. [PubMed] [Google Scholar]
- 24.Graham IL, Anderson DC, Holers VM, Brown EJ. Complement receptor 3 (CR3, Mac-1, integrin alpha M beta 2, CD11b/CD18) is required for tyrosine phosphorylation of paxillin in adherent and nonadherent neutrophils. J Cell Biol. 1994;127:1139–1147. doi: 10.1083/jcb.127.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuortes M, Jin WW, Nathan C. Beta 2 integrin-dependent tyrosine phosphorylation of paxillin in human neutrophils treated with tumor necrosis factor. J Cell Biol. 1994;127:1477–1483. doi: 10.1083/jcb.127.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gismondi A, Jacobelli J, Mainiero F, Paolini R, Piccoli M, Frati L, Santoni A. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272–2276. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 27.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 29.Woodside DG, Obergfell A, Leng L, Wilsbacher JL, Miranti CK, Brugge JS, Shattil SJ, Ginsberg MH. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr Biol. 2001;11:1799–1804. doi: 10.1016/s0960-9822(01)00565-6. [DOI] [PubMed] [Google Scholar]
- 30.Woodside DG, Obergfell A, Talapatra A, Calderwood DA, Shattil SJ, Ginsberg MH. The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J Biol Chem. 2002;277:39401–39408. doi: 10.1074/jbc.M207657200. [DOI] [PubMed] [Google Scholar]
- 31.Boylan B, Gao C, Rathore V, Gill GC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakus Z, Fodor S, Abram CL, Lowell CA, Mocsai A. Immunoreceptor-like signaling by beta 2 and beta 3 integrins. Trends Cell Biol. 2007;17:493–501. doi: 10.1016/j.tcb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Barber DF, Long EO. Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J Immunol. 2003;170:294–299. doi: 10.4049/jimmunol.170.1.294. [DOI] [PubMed] [Google Scholar]
- 35.March ME, Gross CC, Long EO. Use of transfected Drosophila S2 cells to study NK cell activation. Methods Mol Biol. 2010;612:67–88. doi: 10.1007/978-1-60761-362-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rethi B, Detre C, Gogolak P, Kolonics A, Magocsi M, Rajnavolgyi E. Flow cytometry used for the analysis of calcium signaling induced by antigen-specific T-cell activation. Cytometry. 2002;47:207–216. doi: 10.1002/cyto.10086. [DOI] [PubMed] [Google Scholar]
- 37.Sanders MA, Basson MD. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2005;280:23516–23522. doi: 10.1074/jbc.M413165200. [DOI] [PubMed] [Google Scholar]
- 38.Lanier LL, Yu G, Phillips JH. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 39.Letourneur O, Kennedy IC, Brini AT, Ortaldo JR, O'Shea JJ, Kinet JP. Characterization of the family of dimers associated with Fc receptors (Fc epsilon RI and Fc gamma RIII) J Immunol. 1991;147:2652–2656. [PubMed] [Google Scholar]
- 40.Mason LH, Willette-Brown J, Anderson SK, Gosselin P, Shores EW, Love PE, Ortaldo JR, McVicar DW. Characterization of an associated 16-kDa tyrosine phosphoprotein required for Ly-49D signal transduction. J Immunol. 1998;160:4148–4152. [PubMed] [Google Scholar]
- 41.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 42.Dangelmaier CA, Quinter PG, Jin J, Tsygankov AY, Kunapuli SP, Daniel JL. Rapid ubiquitination of Syk following GPVI activation in platelets. Blood. 2005;105:3918–3924. doi: 10.1182/blood-2004-09-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paolini R, Molfetta R, Beitz LO, Zhang J, Scharenberg AM, Piccoli M, Frati L, Siraganian R, Santoni A. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of Fcepsilon RI and Syk in RBL cells. J Biol Chem. 2002;277:36940–36947. doi: 10.1074/jbc.M204948200. [DOI] [PubMed] [Google Scholar]
- 44.Paolini R, Molfetta R, Piccoli M, Frati L, Santoni A. Ubiquitination and degradation of Syk and ZAP-70 protein tyrosine kinases in human NK cells upon CD16 engagement. Proc Natl Acad Sci U S A. 2001;98:9611–9616. doi: 10.1073/pnas.161298098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitcher LA, Young JA, Mathis MA, Wrage PC, Bartok B, van Oers NS. The formation and functions of the 21- and 23-kDa tyrosine-phosphorylated TCR zeta subunits. Immunol Rev. 2003;191:47–61. doi: 10.1034/j.1600-065x.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 47.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, Reth M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057–1069. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 48.Pelletier AJ, Bodary SC, Levinson AD. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992;3:989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanner SB, Grosmaire LS, Ledbetter JA, Damle NK. Beta 2-integrin LFA-1 signaling through phospholipase C-gamma 1 activation. Proc Natl Acad Sci U S A. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upshaw JL, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. The isoforms of phospholipase C-gamma are differentially used by distinct human NK activating receptors. J Immunol. 2005;175:213–218. doi: 10.4049/jimmunol.175.1.213. [DOI] [PubMed] [Google Scholar]
- 51.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 54.Sirim P, Zeitlmann L, Kellersch B, Falk CS, Schendel DJ, Kolanus W. Calcium signaling through the beta 2-cytoplasmic domain of LFA-1 requires intracellular elements of the T cell receptor complex. J Biol Chem. 2001;276:42945–42956. doi: 10.1074/jbc.M103224200. [DOI] [PubMed] [Google Scholar]
- 55.Marwali MR, MacLeod MA, Muzia DN, Takei F. Lipid rafts mediate association of LFA-1 and CD3 and formation of the immunological synapse of CTL. J Immunol. 2004;173:2960–2967. doi: 10.4049/jimmunol.173.5.2960. [DOI] [PubMed] [Google Scholar]
- 56.Mace EM, Monkley SJ, Critchley DR, Takei F. A dual role for talin in NK cell cytotoxicity: activation of LFA-1-mediated cell adhesion and polarization of NK cells. J Immunol. 2009;182:948–956. doi: 10.4049/jimmunol.182.2.948. [DOI] [PubMed] [Google Scholar]
- 57.Dewitt S, Hallett MB. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelke M, Engels N, Dittmann K, Stork B, Wienands J. Ca(2+) signaling in antigen receptor-activated B lymphocytes. Immunol Rev. 2007;218:235–246. doi: 10.1111/j.1600-065X.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 59.Hellberg C, Molony L, Zheng L, Andersson T. Ca2+ signalling mechanisms of the beta 2 integrin on neutrophils: involvement of phospholipase C gamma 2 and Ins(1,4,5)P3. Biochem J. 1996;317(Pt 2):403–409. doi: 10.1042/bj3170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 61.Kishimoto TK, Larson RS, Corbi AL, Dustin ML, Staunton DE, Springer TA. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- 62.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 63.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 66.Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. The Journal of cell biology. 2000;151:1435–1448. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiregowdara D, Avraham H, Fu Y, London R, Avraham S. Tyrosine phosphorylation of the related adhesion focal tyrosine kinase in megakaryocytes upon stem cell factor and phorbol myristate acetate stimulation and its association with paxillin. J Biol Chem. 1997;272:10804–10810. doi: 10.1074/jbc.272.16.10804. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 72.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salgia R, Avraham S, Pisick E, Li JL, Raja S, Greenfield EA, Sattler M, Avraham H, Griffin JD. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271:31222–31226. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 74.Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herreros L, Rodriguez-Fernandez JL, Brown MC, Alonso-Lebrero JL, Cabanas C, Sanchez-Madrid F, Longo N, Turner CE, Sanchez-Mateos P. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem. 2000;275:26436–26440. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- 76.Sancho D, Nieto M, Llano M, Rodriguez-Fernandez JL, Tejedor R, Avraham S, Cabanas C, Lopez-Botet M, Sanchez-Madrid F. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249–1262. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Syk and TCR ζ tyrosine phoshorylation in response to LFA-1 and CD16 stimulation. NK cells were stimulated with purified ICAM-1 or purified human IgG on plates for 5 and 20 minutes at 37°C and lysed. Lysates were immunoprecipitated with 4G10 agarose, and immunoprecipitates were resolved by SDS-PAGE and immunoblotted for Syk (top panel) or TCR ζ chain (bottom panel).
Supplemental Figure 2: NK cells were pretreated for 45 minutes at 37°C in culture medium with 1 μM Syk inhibitor II (Calbiochem) or DMSO carrier. Cells were then stimulated with purified ICAM-1 or purified human IgG on plates for 20 minutes at 37°C and lysed. Lysates were immunoprecipitated with 4G10 agarose, and immunoprecipitates were resolved by SDS-PAGE and immunoblotted for Syk or TCR ζ as indicated. Both a short and long exposure of the TCR ζ blot are shown.