Abstract
Objective
To investigate the effect of Vitamin D3 on physical performance in patients with HF.
Background
HF is associated with functional decline and frailty. Vitamin D deficiency is associated with loss of muscle strength and poor outcomes in patients with HF. Methods: Sixty-four patients participated in a 6-month parallel design double blind RCT to test the hypothesis that oral vitamin D3 would improve physical performance. Vitamin D3 50,000 IU or placebo was given weekly; all received daily calcium. Patients were included regardless of EF and 25OHD ≤ 37.5 ng/ml. The primary outcome was peak VO2, and secondary outcomes were the 6MW, TGUG and knee isokinetic muscle strength. Between group comparisons were made using ANCOVA models that adjust for baseline measures.
Results
Patients were age 65.9 ± 10.4 years old, 48% women, 64% African American, EF 37.6±13.9, 36% NYHA III, the remainder NYHA II. At baseline the vitamin D group 25OHD was 19.1 ± 9.3 ng/ml and increased to 61.7 ± 20.3 ng/ml; in the placebo group baseline 25OHD was 17.8 ± 9.0 ng/ml and decreased to 17.4 ± 9.8 ng/ml at 6 months (between groups p<0.001). There was no significant change from baseline to 6 months in peak VO2, 6MW, TGUG or isokinetic muscle strength.
Conclusions
Vitamin D3 did not improve physical performance for patients with HF despite a robust increase in serum 25OHD. Vitamin D repletion in patients with HF should conform to standard adult guidelines for vitamin D supplementation.
Keywords: trial, vitamin D, heart failure
INTRODUCTION
HF is a complex syndrome which results in exercise intolerance and disability. Unraveling the mechanisms for the loss of physical function is complex since the failing heart results in a cascade of neurohormonal and peripheral muscle effects. HF adversely impacts function and strength of peripheral skeletal muscle and is strongly associated with frailty.(1,2) Strategies to improve the function of peripheral skeletal muscle is important for older adults with HF to improve aerobic capacity (3)and maintain independence.
Vitamin D deficiency is known to cause muscle weakness and myopathy.(4) In older adults with and without HF, vitamin D deficiency is associated with reduced physical performance and frailty.(5–8) Vitamin D repletion has shown improvement in strength, balance and reduced risk of falls.(9–11) Higher vitamin D concentrations are associated with improved lower extremity function including skeletal muscle function and strength.(9,12,13) HF and vitamin D deficiency are both common in older adults (14,15). It is unknown if these two conditions are additive in worsening functional decline. Repletion of vitamin D may have particular benefit in patients with HF who are at risk for muscle weakness and falls.(16)
Vitamin D deficiency is common in those with HF.(17) A recent trial randomized HF patients to vitamin D or placebo, without change in physical performance (18). Although this trial was neutral, two questions remain: First, if the achieved serum 25OHD was higher would an effect on physical performance been identified? Second, if the vitamin D was administered over a longer period of time, would the patients have had improvements in physical function? To address these 2 questions, we report a randomized trial of patients with HF to 50,000 IU D3 or weekly placebo for 6 months. The 6 month time period was chosen since this would be more than adequate time to increase serum 25OHD to ≥ 40 ng/ml. We hypothesized that achieving 2 5OHD concentrations well into the normal range, would improve functional capacity measured by peak VO2, the 6MW and knee isokinetic muscle strength.
METHODS
Trial Design
This is a parallel design randomized double-blind placebo controlled trial from an academic medical center. Sample size planning included 30 subjects per group plus 20% inflation for attrition. At the time of planning for the study, there were no trials of vitamin D treatment in patients with HF. Therefore, the sample size was based on standard deviation estimates from a study of exercise in HF by Belardinelli et. al.(19) in a similar HF population for the ventilatory threshold endpoint and on an expected standardized effect size of 0.8.
The IRB at University Hospitals, Case Medical Center approved the trial. Eligible subjects were consented and randomly assigned in a 1:1 ratio to receive vitamin D3, 50,000 IU or matching placebo. Both groups received calcium citrate 400 mg twice daily.
Randomization and Allocation
Patients were randomized in a permuted block scheme according to race, age and sex. Group assignment remained concealed from study staff, participants, and investigators until data collection was complete.
Patients
Patients’ age ≥ 50 years, regardless of EF and NYHA Class II-IV were recruited. Patients were on maximum tolerated doses of evidence-based HF medications per their cardiologist. Serum 25OHD required was ≤37.5 ng/ml. Exclusion criteria: primary hyperparathyroidism, sarcoid, hypercalcemia, nephrolithiasis, osteoporosis, creatinine of > 2.5mg/dL, vitamin D > 400 IU daily, corticosteroids, PTH, androgen or estrogen, current illicit drug use or ≥ 3 alcoholic drinks daily, advanced cancer, or myocardial infarction in preceding 6 months. Use of medications known to lower serum 25OHD or the bioavailability of oral vitamin D: ketoconazole, colestipol, cholestyramine, mineral oil, phenobarbitol, and phenytoin.
Primary Outcome
CPX
Aerobic capacity was measured at baseline and 6 months using a Modified Naughton protocol. Breath by breath on-line gas measurements (Medical Graphics, St Paul, MN) were obtained at rest and throughout exercise. Peak VO2 was defined as the highest VO2 in the last minute of symptom-limited exercise. Ventilatory threshold was determine using the V-slope method(20). The Borg Rating of Perceived Exertion (RPE) was used to assess patient effort. Patients were encouraged to exercise to a RPE >15 (hard)(21) and a respiratory exchange ratio (RER= VCO2/ VO2) > 1.05. The ECG was continuously monitored and blood pressure was obtained at rest and at the end of each exercise stage.
Secondary Outcomes
TGUG
The TGUG was performed at baseline and 6 months. It is a reliable and valid test of basic mobility maneuvers including balance, gait speed, strength and functional ability (22). Subjects were instructed to rise from a chair, walk 3 meters, turn around, return to the chair. The test was timed. If subjects normally use a walking aid (cane or walker), they were encouraged to use the aid during the test.
6MW
A 6MW was performed at baseline, month 3 and 6 in a pre-measured hallway (23). Patients were permitted to use a walking aid and instructed to walk at their own pace from the start point to the turnaround point, as many times as possible in 6 minutes. Patients were permitted to slow their pace or rest as needed, but were encouraged to resume walking when possible.
Isokinetic Muscle Testing
Isokinetic muscle strength was measured by peak torque in Newton-meters (Nm) and peak torque/body weight in Nm/kg at baseline and 6 months in the dominant leg using the Biodex System 3 pro isokinetic dynamometer (Biodex, Shirley, NY). The protocol was designed for the older adult. Each patient was instructed on how to breathe during testing to avoid valsalva breath-holding. Patients sat upright on the Biodex chair and secured using torso, pelvic, thigh, and ankle straps to avoid extraneous movements and provide stability. The lateral femoral epicondyle was used as the bony landmark for matching the axis of rotation of the knee joint with the axis dynamometer resistance adapter. All tests were performed using the same positioning standards for reproducibility. Ranges of motion were determined prior to warm-up. Gravity correction was obtained by measuring the torque applied to the resistance arm with the leg completely relaxed at the terminal extension position. Patients were familiarized with the dynamometer resistance adapter and given a chance to perform 10 repetitions of a warm-up set before performing the test followed by 5 minute recovery period before starting the test protocol. Quadriceps strength during knee extension and hamstring strength during knee flexion were measured over a range of motion of ~90° using a speed of 60°s−1 and 120°s−1. Verbal encouragement was given to achieve maximal efforts.
Serum Analysis
Blood was stored at 2–8°C. Serum 25OHD was measured by chem-illuminescence immunoassay (ARUP Salt Lake City, Utah) with an intra-assay CV of 3 and 6% and a between assay variability of 6 to 11%. Parathyroid Hormone was measured by chemiluminometric technology (Siemens Dimension Vista Systems, Newark, DE) by University Hospitals clinical laboratory). Creatinine, BUN, albumin and calcium were measured at University Hospitals clinical laboratory. High sensitivity C-reactive protein was measured by nephelometry (Siemens Dimension Vista Systems, Newark, DE by University Hospitals clinical laboratory).
Statistical Analyses
Baseline demographics were summarized by means and standard deviations for continuous variables and frequency and proportions for categorical variables within study groups. VO2 was reported by gender due to expected differences in performance between men and women. The treatment effect (vitamin D versus placebo) was evaluated for each endpoint using an ANCOVA model that adjusts for baseline endpoint. An extended model was considered that additionally included race, sex, and ejection fraction as covariates (results not shown). A separate extension of the simple ANCOVA model additionally included baseline serum vitamin D level as a covariate. Rates of adverse events were compared using a test of incidence rates between the treatment groups for each category of adverse event. All statistical analyses were performed using R (Vienna, Austria).
Results
Recruitment, Retention, and Adherence
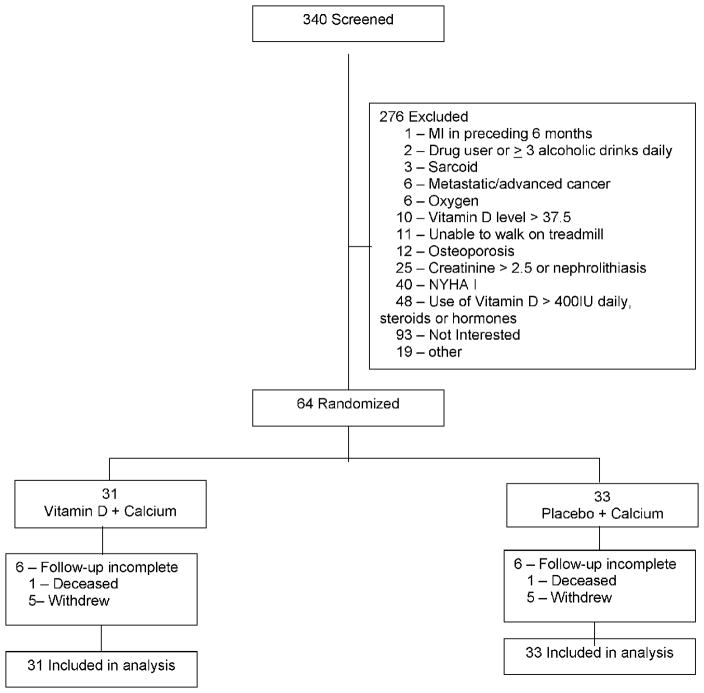
Patients were recruited from May 2007 to April 2011. Three hundred forty patients were screened for study inclusion and 276 did not meet entry criteria. (Figure 1) Sixty-four were randomized and data included in the analysis. Based on monthly pill counts, adherence in the vitamin D group was 100% and 99.5% in the placebo group. The vitamin D group had 90.7% and placebo group 90.8% adherence to the calcium pills.
Figure 1. Flow Diagram.
Flow diagram of research design.
Patient Characteristics
Mean age for the participants in intervention group was 65.8 ± 10.6, 51.6% women and 61.3% African American. For the placebo group 66.0 ± 10.4, 45.5% women and 66.7% African American. (Table 1)
Table 1.
Baseline Demographics
| Characteristic | n, if different | Vitamin D (n=31) | Placebo (n=33) | p-value |
|---|---|---|---|---|
| Age (years), mean (SD)* | 65.8 ± 10.6 | 66.0 ± 10.4 | .9 | |
| Women n, (%) | 16 (51.6) | 15 (45.5) | .6 | |
| African American n, (%) | 19 (61.3) | 22 (66.7) | .6 | |
| BMI† (kg/m2), mean (SD) | 30, 33 | 34.8 ± 7.2 | 31.3 ± 6.9 | .05 |
| Ischemic Etiology n, (%) | 8 (25.8) | 10 (30.3) | .7 | |
| Ejection fraction mean (SD) | 39.2 ± 13.2 | 36.1 ± 14.5 | .4 | |
| NYHA Class | .1 | |||
| II n, (%) | 17 (55) | 24 (73) | ||
| III n, (%) | 14 (45) | 9 (27) | ||
| Hypertension n, (%) | 26 (83.9) | 28 (84.8) | .9 | |
| Hyperlipidemia n, (%) | 26 (83.9) | 25 (75.8) | .4 | |
| Diabetes n, (%) | 16 (51.6) | 14 (42.4) | .5 | |
| Pulmonary disease‡ n, (%) | 16 (51.6) | 16 (48.5) | .8 | |
| Depression n, (%) | 10 (32.3) | 10 (30.3) | .9 | |
| Medications§ | ||||
| ACE Inhibitor, n (%) | 20 (64.5) | 22 (66.7) | .9 | |
| Enalapril EQ|| dose (mg) | 20, 22 | 40, 32.8 (10, 80) | 40, 30.2 (5, 80) | |
| Angiotensin Inhibitor Blocker, n (%) | 8 (25.8) | 9 (27.3) | .9 | |
| Valsartan EQ dose (mg) | 8, 9 | 240, 215.0 (40, 320) | 240, 202.2 (40, 320) | |
| Beta Blocker, n (%) | 29 (93.5) | 28 (84.8) | .3 | |
| Metoprolol EQ dose (mg) | 28, 28 | 150, 130.4 (25, 200) | 175, 139.3 (25, 200) | |
| Loop Diuretic, n (%) | 22 (71.0) | 26 (78.8) | .5 | |
| Furosemide EQ dose (mg) | 22, 26 | 40, 62.4 (6, 200) | 40, 70.0 (10, 400) | |
| Aldosterone Antagonist, n (%) | 7 (23) | 13 (39) | .1 | |
|
| ||||
| Baseline Outcome Measures | n, if different | Vitamin D (n=31) | Placebo (n=33) | |
|
| ||||
| Cardiopulmonary Stress Test, mean (SD, min, max) | ||||
| Peak VO2 (ml/kg/min) | 30, 31 | 13.4 ± 3.5 (7.8, 22.5) | 14.0 ± 4.1 (7.5, 27.9) | .6 |
| Men | 14, 16 | 14.1 ± 3.6 (10.0, 22.5) | 14.4 ± 4.8 (7.5, 27.9) | |
| Women | 16, 15 | 12.7 ± 3.3 (7.8, 17.1) | 13.4 ± 3.4 (9.4, 20.2) | |
| Exercise Duration (min) | 30, 32 | 6.9 ± 3.4 | 7.8 ± 4.1 | .3 |
| Peak exercise VE/VCO2 | 30, 31 | 32.8 ± 6.3 | 35.3 ± 7.0 | .1 |
| Respiratory Exchange Ratio | 30, 31 | 0.98 ± 0.12 | 0.98 ± 0.09 | .9 |
| Labs, mean (SD) | ||||
| Serum 25OHD (ng/ml) | 19.1 ± 9.3 | 17.8 ± 9.0 | .6 | |
| Serum PTH (pg/ml) | 30, 33 | 62.3 ± 44.3 | 72.8 ± 40.2 | .3 |
| Get-Up-and-Go (sec) | 31, 31 | 11.1 ± 4.1 | 10.1 ± 5.7 | .5 |
| Six minute walk (meters) | 30, 31 | 337.7 ± 108.5 | 341.4 ± 12.2 | .9 |
| Isokinetic Muscle Strength mean (SD) | ||||
| Peak Torque¶ (Newton-Meters/kg) | ||||
| Extension 60°s-1 | 30, 30 | 101.0 ± 44.5 | 111.8 ± 30.7 | .3 |
| 120°s-1 | 30, 30 | 81.9 ± 31.4 | 90.6 ± 24.7 | .2 |
| Flexion 60°s-1 | 30, 30 | 45.9 ± 19.0 | 50.4 ± 16.9 | .3 |
| 120°s-1 | 30, 30 | 43.7 ± 17.0 | 48.5 ± 18.7 | .3 |
Standard Deviation
Body Mass Index
Includes COPD, emphysema, asthma, sleep apnea
For each medication the first line shows the number of patients on the medication followed by the percentage. On the second line is the median, mean and range of the equivalency medication dose.
Equivalency dose
Adjusted for body weight
Serum Vitamin D Concentrations
At 6 months, the serum 25OHD increased by 42.3 ± 16.4 ng/ml in the treatment group and by 0.2 ± 6.6 ng/ml in the placebo group (between groups p<0.001). Serum PTH declined in the treatment group by 23.1 ± 40.0 pg/ml and by 3.1 ± 38.1pg/ml in the placebo group(p=.014).
Aerobic Capacity and Muscle Strength
The change in peak VO2, 6MW, TGUG, and isokinetic muscle testing did not differ between groups. (Table 2) Adjustment for race, sex and ejection fraction had no effect on outcomes (results not shown). Further analysis was performed to assess if a low baseline serum 25OHD was associated with more improvement in peak VO2, 6MW or TGUG. Data show that those with higher baseline 25OHD had more improvement in peak VO2 than those with low baseline regardless of group (i.e. for each 5 IU increase in 25OHD the VO2 increased by 0.3 ml/kg/min) although this did not reach statistical significance (p=.06). There was no association found between baseline 25OHD and improvement in either the 6MW or the TGUG. (p=0.7 for both) (Figure 2)
Table 2.
Change in Aerobic Capacity and Muscle Strength Vitamin D vs. Placebo
| 6 month change in endpoint | ANCOVA model | ||||
|---|---|---|---|---|---|
| Endpoint | N’s (if diff) | Vitamin D (N=31) | Placebo (N=33) | VitD – Placebo | p-value |
| Peak VO2* | 24, 23 | −0.17 ± 2.07 | −0.68 ± 1.85 | 0.56 | 0.3 |
| Exercise Duration | 24, 24 | 12.0 ± 86.7 | 9.7 ± 88.6 | 2.3 | 0.9 |
| Peak VE/VCO2 | 24, 23 | 0.00 ± 3.11 | −1.65 ± 6.79 | 0.45 | 0.7 |
| Timed Get Up Go | 25, 25 | −0.2 ± 3.3 | −1.0 ± 3.3 | 0.8 | 0.3 |
| 6MW (baseline to 3 mos) | 26, 27 | 9 ± 160 | 27 ± 150 | −18 | 0.7 |
| 6MW (baseline to 6mos) | 24, 26 | 59 ± 282 | 36 ± 228 | 20 | 0.8 |
| Peak Torque† (Newton-Meters/kg) | |||||
| Extension 60°s-1 | 24, 24 | 5.2 ± 29.7 | 3.5 ± 17.1 | 0.8 | 0.9 |
| 120°s-1 | 24, 24 | −0.2 ± 26.4 | 6.5 ± 13.2 | −7.2 | 0.2 |
| Flexion 60°s-1 | 24, 24 | 1.6 ± 17.2 | 5.1 ± 8.8 | −3.8 | 0.3 |
| 120°s-1 | 24, 24 | −1.0 ± 14.9 | 2.9 ± 8.9 | −4.7 | 0.2 |
no change in results with additional adjustment for RER
adjusted for body weight
Figure 2. Outcomes by Treatment Group according to Baseline Serum 25OHD.
Scatterplots of trial participants according to baseline serum 25 hydroxyvitamin D (25[OH]D) and physical performance outcomes: (A) change in peak oxygen uptake (V02), (B) change in 6-min walk distance (6MWD), and (C) change in timed get up and go (TGUG). Solid lines represent the vitamin D group and dotted lines represent the placebo group. Change in peak VO2 neared significance regardless of group (p = 0.06). Change in 6MWD and TGUG by baseline 25(OH)D level were both nonsignificant (both p = 0.70).
Adverse Effects
Vitamin D and calcium were well tolerated. Adverse events were not significantly different between groups, except for an increased number of infections in the vitamin D group compared with placebo. (Table 3)
Table 3.
Adverse Events
| Adverse Event | Vitamin D | Placebo | p-value* |
|---|---|---|---|
| Hosp† for HF/ Chest Pain | 3 | 0 | 0.2 |
| Arrhythmia | 0 | 2 | 0.5 |
| Respiratory Arrest (Fatal) | 0 | 1 | 1.0 |
| Worsening HF Symptoms** | 55 | 59 | 1.0 |
| Decreased Appetite | 10 | 13 | 0.8 |
| Nausea / Vomiting | 6 | 10 | 0.5 |
| Constipation | 19 | 14 | 0.4 |
| Diarrhea | 3 | 3 | 0.7 |
| Muscle / Joint Pain | 11 | 6 | 0.3 |
| Infection | 18 | 6 | 0.02 |
| Headache/dizziness | 3 | 1 | 0.6 |
| Fall | 1 | 1 | 0.5 |
| Anxiety/Depression | 1 | 2 | 1.0 |
| Elevated BP/HR | 0 | 2 | 0.5 |
| Hematuria | 0 | 1 | 1.0 |
| COPD exacerbation | 0 | 1 | 1.0 |
| Total Adverse Events | 130 | 122 | 0.3 |
Due to small numbers, p-value less reliable except for category “Worsening HF Symptoms”
Symptoms included any of the following: shortness of breath, fatigue, swelling, decreased appetite, and cough.
Hospitalization
Discussion
Older adults with HF who were treated with high dose vitamin D3 and calcium for 6 months had no change in aerobic capacity or skeletal muscle strength when compared to those taking placebo plus calcium. This finding is noteworthy since those in the vitamin D group increased 25OHD by an average of 45% with an appropriate decline in PTH. Our trial was unique in that it included a diverse study population with a strong representation of women, predominantly African American, and with either reduced or preserved systolic failure. Patients were receiving appropriate HF medications as per evidence based guidelines.(24) Both types of HF were included under the premise that skeletal muscle is affected and deconditioning occurs regardless of ejection fraction.
In 2006 Schleithoff et. al.(33) randomized 123 systolic failure patients to 2000 IU of vitamin D3 daily vs. placebo, both with calcium 500 mg for 9 months. The 25OHD concentrations increased by a median of 26.8 ng/ml; significantly more than the 25OHD increase in the placebo group of 3.6 ng/ml. Peak VO2 as a secondary outcome (pro-inflammatory cytokines were the primary outcome) was not significantly different between the vitamin D and placebo groups. Witham et al.(18) gave two doses of 100,000 IU of vitamin D2 (baseline and 10 weeks) to patients with systolic HF and also showed no significant change in the 6MW distance or the TGUG at both 10 and 20 weeks. Our trial with confirmed high serum levels of 25OHD adds to the growing body of evidence that therapy with vitamin D does not affect aerobic capacity/functional performance in patients with HF. Our duration of therapy was shorter than in the Schleithoff trial but longer than that in the Witham trial. We achieved the highest serum concentration for 25OHD published to date, but with no measurable effect.
Baseline 25OHD concentrations, regardless of group, showed a relationship with the change in peak VO2, although not statistically significant. This finding mirrors what has been seen in observational studies that an association of worse physical performance with low 25OHD has been reported (6,8,25). This may indicate that vitamin D is a marker of poor function rather than a modulator. Alternatively since both groups received calcium we cannot rule out that calcium affected both groups equally, regardless of 25OHD.
Results from clinical trials of vitamin D treatment which measured muscle strength have been mixed with some demonstrating benefit (9,11,26) while others have not (27,28). In a meta-analysis which examined physical performance with vitamin D, smaller, more frequent dosing (800–1000 IU daily), and vitamin D with calcium had the most consistent benefit. However, benefit was only shown for the TGUG and tests of balance, not for distance walked.(12) In our trial vitamin D and calcium were given, but it is possible that the dose given was too high when compared to these other trials. Optimal dosing and the target for serum 25OHD has been under intense debate especially since a one time high dose of vitamin D3 increased the risk of falls in older women.(29) Optimal dosing, frequency and serum target to decrease events and modulate different disease states requires further study.
The myopathy of vitamin D deficiency is hallmarked by muscle weakness and fatigue. Skeletal muscle biopsies of those who are severely deficient in vitamin D, show selective atrophy of type II fibers, and fat infiltration and fibrosis.(30) The change in fiber type is in contrast to the myopathy of HF where there is an increase of type II skeletal muscle fibers and a loss of type I fibers. The vitamin D receptor (VDR) present on skeletal muscle has many polymorphisms and variable expression with aging. There are both genomic and non-genomic pathways which influence protein transcription and calcium metabolism.(31) If vitamin D has an opportunity to improve physical performance in those with HF, further characterization of the VDR and intrinsic muscle changes may help to target the population of patients who may benefit the most.
Adverse events in our patients were expected and equal between groups. One exception, however, was the report of more infections in the vitamin D group than placebo, which was statistically significant. This finding is contrary to the expected higher risk of infection in those who are deficient in serum 25OHD since vitamin D is theorized to reduce risk of infection and inflammation (32). Additionally, this finding may be spurious due to small sample size or due to multiple comparisons.
Limitations
The study was limited by a short duration of 6 months and a relatively small sample size which may have affected our ability to show a difference between groups (Type II error).
Conclusion
The findings of this study do not support the use of vitamin D + calcium to improve functional performance in older adults with HF. Vitamin D may still be a good candidate to help break the cycle of frailty and functional decline in patients with HF since benefit has been demonstrated in older adults in non-diseased base studies (12). A trial of exercise combined with vitamin D as the intervention may have the greatest chance to demonstrate benefit from vitamin D for patients with HF.
Acknowledgments
Funding Sources
Dr. Boxer and this work are supported by the NIH KL2RR024990 and in part by the AHA Scientist Development Grant 0635055N and the Joan C. Edwards Fund, Cleveland, OH. This publication was made possible by the CTSC of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CPX
Cardiopulmonary Stress Test
- EF
Ejection Fraction
- IU
International Units
- NYHA
New York Heart Association Class
- PTH
Parathyroid Hormone
- Peak VO2
peak oxygen uptake
- TGUG
Timed Get Up and Go
- 25OHD
25 Hydroxyvitamin D
- 6MW
6 Minute Walk Distance
Footnotes
Clinical Trial: ClinicalTrials.gov NCT01125436
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol. 1996;28:2255–62. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 3.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 4.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 5.Zamboni M, Zoico E, Tosoni P, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57:M7–11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 6.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56:454–61. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 8.Boxer RS, Kenny AM, Cheruvu VK, Vest M, Fiutem JJ, Pina Serum 25-hydroxyvitamin D concentration is associated with functional capacity in older adults with heart failure. American heart journal. 2010;160:893–9. doi: 10.1016/j.ahj.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010;58:2063–8. doi: 10.1111/j.1532-5415.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18:343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 11.Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20:315–22. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 12.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. 2009;64:599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–4. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 18.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin d supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 19.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 20.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–23. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 21.Borg G. Borg’s Perceived Exertion and Pain Scale. Champaign, Illinois: Human Kinetics; [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Shane E, Mancini D, Aaronson K, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 26.Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Annals of nutrition & metabolism. 2009;54:291–300. doi: 10.1159/000235874. [DOI] [PubMed] [Google Scholar]
- 27.Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc. 2003;51:1762–7. doi: 10.1046/j.1532-5415.2003.51561.x. [DOI] [PubMed] [Google Scholar]
- 28.Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 30.Ceglia L. Vitamin D and its role in skeletal muscle. Current opinion in clinical nutrition and metabolic care. 2009;12:628–33. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton B. Vitamin D and human skeletal muscle. Scandinavian journal of medicine & science in sports. 2010;20:182–90. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection and immunity. 2008;76:3837–43. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]