Abstract
Congenital analbuminemia is a rare autosomic recessive inherited disorder characterized by low plasma albumin and hypercholesterolemia, which may increase cardiovascular risk. Patients are essentially asymptomatic, apart from ease of fatigue, minimal ankle oedema and hypotension. There is no accepted strategy for safely treating both hypercholesterolemia and analbuminemia in order to eventually decrease the atherosclerotic risk. We report a case of congenital analbuminemia (1.0 g/dL)
in a 38-year-old male with hypercholesterolemia (range: 406-475 mg/dL) and severe arterial dysfunction [no brachial artery flow-mediated dilation (FMD)]. Long-term, cholesterol-lowering treatment with atorvastatin was associated with the appearance of peripheral edema. Two-months of infusion with albumin improved FMD (7%) and reduced serum cholesterol (273 mg/dL), supporting the hypothesis of a compensatory role of hypercholesterolemia. Statin treatment, together with periodical albumin infusions, may contribute to the safe reduction of cardiovascular risk.
Keywords: Analbuminemia, Hypercholesterolemia, Atorvastatin, Albumin infusion, Endothelial dysfunction
Core tip: Congenital analbuminemia is characterized by low plasma albumin and compensatory hypercholesterolemia, which may increase cardiovascular risk. We report a case of congenital analbuminemia (1.0 g/dL) in a 38-year-old male with hypercholesterolemia (range: 406-475 mg/dL) and severe arterial dysfunction [no brachial artery flow-mediated dilation (FMD)]. Long-term, cholesterol-lowering treatment with atorvastatin was associated with the appearance of peripheral edema. Two-months of infusion with albumin improved FMD (7%) and reduced serum cholesterol (273 mg/dL). Statin treatment, together with periodical albumin infusions, may contribute to the safe reduction of cardiovascular risk.
INTRODUCTION
Congenital analbuminemia is a rare autosomic recessive inherited disorder in which the subject has no or little plasma albumin[1-3]. Analbuminemia is attributable to defects in the gene coding for albumin which is located on chromosome 4 and is split into 15 exons by 14 intervening introns[4,5]. Multiple mutations causing analbuminemia have been identified in the homozygous state[6-8]. The estimated prevalence is less than one in a million and to date, fewer than 50 cases have been reported worldwide. Although it is extremely rare, analbuminemia may teach us important pathophysiological lessons.
Albumin is the major plasma protein and the most important for maintaining plasma colloid oncotic pressure and preventing systemic edema[9]. It is also an important circulating antioxidant and a transporter of many less soluble metabolites[9]. Surprisingly, the absence of albumin is tolerable and most patients are detected fortuitously. In fact, patients are essentially asymptomatic, apart from ease of fatigue, minimal ankle oedema and hypotension[10-13].
In these patients the body is able to compensate for the lack of albumin through the synthesis of immunoglobulins and other serum proteins such as ceruloplasmin, fibrinogen and transferrin, but particularly through an increased secretion of apolipoprotein-B from the liver[14,15]. Patients have enhanced plasma low density lipoprotein-cholesterol (LDL-C) levels, normal or reduced high density lipoprotein-cholesterol (HDL-C) and normal triglycerides, possibly leading to premature atherosclerosis and cardiovascular events[16-18]. Indeed, hypoalbuminemia is strongly associated to cardiovascular disease in patients with nephrotic syndrome and in hemodialysis patients, where an inverse association between albumin and both total and LDL-C is observed[19]. Based on this, congenital analbuminemia should be associated with premature atherosclerosis[20] and thrombotic events although, no data on atherosclerotic risk have been reported so far.
Consequently, there is no accepted strategy for safely treating both hypercholesterolemia and analbuminemia in order to eventually decrease the atherosclerotic risk.
CASE REPORT
Previous medical history
A 38-year-old Italian man was admitted with severe hypercholesterolemia and congenital analbuminemia. Born in 1973 from healthy and non-sanguineous parents, he received replacement therapy with human serum albumin for the presence of mild oedema in his lower limbs and eyelids[21]. At 1 mo old he was admitted to the children’s hospital where laboratory examinations revealed a reduced serum total protein of 3.2 g/dL and almost undetectable albumin but with normal urinary protein excretion and a remarkable increase in serum cholesterol (222 mg/dL). During hospitalization, he received replacement therapy with human albumin and was discharged at 6 mo of age, free of oedemas and in good general condition. He was followed for 8 years with periodic laboratory check ups, which showed an absence of the albumin peak in protein electrophoresis, thus confirming the initial diagnosis of congenital analbuminemia associated with severe hypercholesterolemia (total cholesterol > 400 mg/dL). Evaluation of the child’s family revealed no members with hypoalbuminemia or familial hypercholesterolemia.
Clinical presentation
After 21 years of life without any major clinical complication, the patient was referred to our outpatient clinic for the treatment of hypercholesterolemia. His past clinical history was unremarkable except for some lipotymic events and the presence of mild ankle oedema during the hot season after standing for a long time. He had taken no medications regularly. The physical examination was within normal limits with no signs of oedema of the lower limbs, and no pleuric or ascitic effusion. His arterial blood pressure was 110/70 mmHg, with a body weight of 80 kg and a body mass index of 24.7 kg/m2. Resting and exercise electrocardiogram, arterial blood pressure monitoring, mono/bidimensional echocardiogram, echo-color doppler studies of the carotids and of peripheral arteries were without pathological findings and confirmed the absence of clinical signs of atherosclerotic complications. However, on several occasions the brachial artery flow-mediated dilation (FMD) test[22], a surrogate marker of atherosclerotic disease, confirmed the existence of severe arterial dysfunction (absence of post-ischemic arterial dilatation). No tendon xanthomas were detected.
Repeated laboratory examination revealed serum total protein ranging from 5.0 to 5.2 g/dL and serum albumin from 1.0 to 1.2 g/dL. Cellulose-acetate electrophoresis revealed the absence of an albumin peak. Renal function tests, urinalysis and urinary protein excretion were normal. The patient showed a remarkable elevation in serum total-cholesterol (range, 406-475 mg/dL), LDL-C (range, 317-379 mg/dL) and apolipoprotein-B (> 200 mg/dL) with normal HDL-cholesterol and triglyceride levels. Serum lipoprotein(a), assessed by monoclonal-based enzyme-linked immuno-absorbent assay, had the remarkably high value of 90.5 mg/dL (normal < 30 mg/dL). No corneal arcus or other signs of dyslipidemia were present.
Our patient is the first case of congenital analbuminemia attributable to compound heterozygosity for 2 new mutations in the exons 10 and 11 of albumin gene[5].
Response to atorvastatin treatment
The patient received a American Heart Association step-1 low-cholesterol, low-saturated-fat diet on his first visit, without any significant decrease in serum lipids over a 5-mo period (total cholesterol from 409 to 397 mg/dL; LDL-C from 336 to 328 mg/dL, apolipoprotein-B from 225 to 203 mg/dL). Treatment with atorvastatin was then started at the initial daily dose of 10 mg, increased to 20 mg at week 5 and to 40 mg at week 21[23]. Baseline and on-treatment lipid and lipoprotein data are reported in Table 1. After 6 mo of drug treatment, total and LDL-C dropped by 37.7% and by 50.6% respectively and HDL-C increased by 13.4%. Moreover, apolipoprotein-B and lipoprotein(a) were decreased by 18.7% and 19.7% respectively while apolipoprotein-A1 was increased by 65.0%. The treatment was safe and well tolerated with no increases in creatine-kinases or in liver enzymes. Total and LDL-C, apolipoprotein-B and lipoprotein(a) reached pre-treatment values only 2 wk after stopping atorvastatin. Based on the above favourable results, long-term treatment with atorvastatin 40 mg was prescribed.
Table 1.
Changes in serum lipid profile during treatment with atorvastatin at increasing dosages
| Time | Treatment | TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | Apo A1 (mg/dL) | Apo B (mg/dL) | Lp (a) (mg/dL) | ALT (UI) | AST (UI) | CK (U/L) | Alb (g/L) |
| Week 20 | Diet | 409 | 97 | 53 | 336 | 225 | 20 | 26 | 195 | 1.1 | ||
| Week 0 | Diet | 397 | 85 | 52 | 328 | 124 | 203 | 90.5 | 18 | 18 | 162 | 0.8 |
| Week 1 | At 10 mg | 384 | 144 | 45 | 310 | 25 | 30 | 193 | 0.8 | |||
| Week 2 | At 10 mg | 307 | 104 | 52 | 234 | 18 | 20 | 181 | 0.8 | |||
| Week 3 | At 10 mg | 284 | 122 | 45 | 214 | 21 | 22 | 138 | 0.7 | |||
| Week 4 | At 10 mg | 261 | 111 | 44 | 194 | 162 | 151 | 85.3 | 21 | 15 | 147 | 0.7 |
| Week 5 | At 20 mg | 303 | 100 | 60 | 223 | 14 | 20 | 160 | 0.8 | |||
| Week 6 | At 20 mg | 290 | 99 | 61 | 209 | 20 | 20 | 147 | 0.7 | |||
| Week 8 | At 20 mg | 240 | 130 | 49 | 165 | 80.0 | 19 | 21 | 123 | 0.8 | ||
| Week 10 | At 20 mg | 263 | 93 | 45 | 199 | 22 | 15 | 123 | 0.8 | |||
| Week 12 | At 20 mg | 264 | 84 | 36 | 211 | 25 | 20 | 192 | 0.7 | |||
| Week 14 | At 20 mg | 264 | 88 | 47 | 199 | 23 | 17 | 172 | 0.8 | |||
| Week 16 | At 20 mg | 250 | 83 | 49 | 184 | 76.6 | 22 | 17 | 142 | 0.7 | ||
| Week 18 | At 20 mg | 279 | 119 | 42 | 213 | 17 | 17 | 131 | 0.7 | |||
| Week 20 | At 20 mg | 272 | 90 | 57 | 197 | 224 | 160 | 23 | 24 | 195 | 1.0 | |
| Week 21 | At 40 mg | 243 | 90 | 59 | 166 | 199 | 165 | 72.6 | 25 | 30 | 189 | 0.7 |
| Week 26 | Diet | 375 | 113 | 52 | 300 | 89.6 | 19 | 15 | 153 | |||
| Week 28 | Diet | 435 | 124 | 51 | 359 | 224 | 206 | 22 | 10 | 137 | 0.6 |
TC: Total cholesterol; TG: Triglycerides; HDL-C: High density lipoprotein-cholesterol; LDL: Low density lipoprotein-cholesterol; Apo: Apolipoprotein; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; CK: Creatine phosphokinase; Alb: Albumin.
Response to albumin infusions
After 2 years on 40 mg/d atorvastatin treatment, the patient complained of severe bilateral, gravity-dependent, oedema and painless swelling of the ankles and lower legs.
At that time serum cholesterol and albumin were 360 mg/dL and 0.8 g/dL, respectively. Therefore, statin therapy was stopped and oral furosemide (25 mg/d) treatment was prescribed.
In addition, starting 1 mo later, human albumin (20 g) was infused over a period of 8 wk (two infusions during the first week and one per week thereafter). During the treatment course, blood samples were taken immediately before each albumin infusion in order to evaluate long-term changes in serum lipids and albumin. Serum albumin levels rose remarkably from 0.8 to 2.7 g/dL, while a progressive decrease in total and serum cholesterol was observed (total cholesterol from 391 to 273 mg/dL; LDL-C from 312 to 196 mg/dL) (Figure 1). No alterations in liver and kidney function were observed after the peripheral infusion of albumin.
Figure 1.
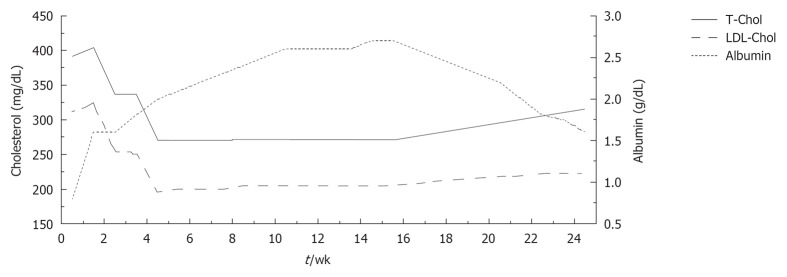
Variation of plasma albumin (g/dL) and of total and low density lipoprotein cholesterol (mg/dL) during albumin infusions and after albumin discontinuation. LDL: Low density lipoprotein; TC: Total cholesterol.
Albumin infusion was also associated with recovery from symptoms of peripheral oedema and an increase in FMD (from 0% to 7%).
During the 4 mo after albumin discontinuation, a mild, progressive increase of total and LDL-C was observed (total cholesterol up to 333 mg/dL; LDL-C up to 249 mg/dL) which was paralleled by a progressive decrease of serum albumin (from 2.7 to 1.4 g/dL).
DISCUSSION
So far, more than 50 analbuminemia-causing homozygote or compound heterozygote mutations of the albumin gene have been reported[24]. The majority of patients do not have major clinical complications and can live fairly normal lives. In fact, the lack of albumin is compensated by a severe hypercholesterolemia which is mainly caused by an increased rate of production of apo-B-containing lipoproteins in the liver and to a lesser extent by a decrease in LDL catabolism[17,18]. Hypercholesterolemia, whether primary or secondary, is recognized as the most important risk factor for premature atherosclerosis and there is overwhelming evidence that lowering cholesterol decreases cardiovascular risk. Indeed, in our patient, the presence of severe arterial dysfunction, as assessed by the absence of post ischemic brachial artery dilation, was demonstrated on several occasions.
There are only two other cases in the literature of short-term treatment of hypercholesterolemia in human analbuminemics[18]. They relate to two South African adult patients who were treated with lipid-lowering diet and simvastatin at up to 40 mg day for a period of 20 wk. based on general experience with simvastatin, one patient responded as anticipated (LDL-C-48.3%), but the other responded less than expected (LDL-C-38.3%). However, both patients experienced a three- to fivefold increase in creatine-kinase.
In our patient a 21-wk period of drug treatment with atorvastatin, with the dose increasing in stages from 10 to 40 mg, resulted in a 50.6% reduction of serum LDL-C, eventually reaching a value close to the goal of 160 mg/dL.
Short-term treatment was safe and well tolerated, and no clinical complaints or biochemical signs of hepatotoxicity, myopathy or dysprotidemia developed. However long-term lipoprotein cholesterol-lowering treatment led to a progressive decrease of oncotic pressure, due to the reduction of the compensatory hypercholesterolemia, with the consequent development of severe swelling of the ankles and lower legs.
Treatment with albumin infusions reversed peripheral edema and decreased serum cholesterol, supporting the hypothesis that severe hypercholesterolemia may represent a compensatory mechanism for the deficit of albumin.
We also observed an improvement in FMD after albumin infusion, suggesting a role for low albumin in eliciting artery dysfunction. As oxidative stress unfavourably influences FMD[25], this favourable change may be interpreted as a combined antioxidant and cholesterol-lowering effect of albumin. Therefore, we suggest a direct correlation between albumin infusion and the improvement in FMD.
In conclusion, we describe a patient with analbuminemia in whom statin reduced serum cholesterol but was associated with the appearance of peripheral edema. Albumin infusions were also effective in reducing serum cholesterol and ameliorating artery dysfunction. Long-term atorvastatin therapy together with periodic albumin infusions may contribute to decreasing cardiovascular risk safely in this clinical setting.
Footnotes
P- Reviewer Tan XR S- Editor Gou SX L- Editor Hughed D E- Editor Zheng XM
References
- 1.Buehler BA. Hereditary disorders of albumin synthesis. Ann Clin Lab Sci. 1978;8:283–286. [PubMed] [Google Scholar]
- 2.Russi E, Weigand K. Analbuminemia. Klin Wochenschr. 1983;61:541–545. doi: 10.1007/BF01486843. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery DA, Neill DW, Dowdle EB. Idiopathic hypoalbuminaemia. Clin Sci. 1962;22:141–154. [PubMed] [Google Scholar]
- 4.Minghetti PP, Ruffner DE, Kuang WJ, Dennison OE, Hawkins JW, Beattie WG, Dugaiczyk A. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11-22 of chromosome 4. J Biol Chem. 1986;261:6747–6757. [PubMed] [Google Scholar]
- 5.Campagna F, Fioretti F, Burattin M, Romeo S, Sentinelli F, Bifolco M, Sirinian MI, Del Ben M, Angelico F, Arca M. Congenital analbuminemia attributable to compound heterozygosity for novel mutations in the albumin gene. Clin Chem. 2005;51:1256–1258. doi: 10.1373/clinchem.2005.048561. [DOI] [PubMed] [Google Scholar]
- 6.Minchiotti L, Galliano M, Kragh-Hansen U, Peters T. Mutations and polymorphisms of the gene of the major human blood protein, serum albumin. Hum Mutat. 2008;29:1007–1016. doi: 10.1002/humu.20754. [DOI] [PubMed] [Google Scholar]
- 7.Watkins S, Madison J, Galliano M, Minchiotti L, Putnam FW. Analbuminemia: three cases resulting from different point mutations in the albumin gene. Proc Natl Acad Sci USA. 1994;91:9417–9421. doi: 10.1073/pnas.91.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnoli M, Rossi A, Palmqvist L, Flisberg A, Niklasson A, Minchotti L, Galliano M. A novel splicing mutation causes an undescribed type of analbuminemia. Biochim Biophys Acta. 2002;1586:43–49. doi: 10.1016/s0925-4439(01)00084-9. [DOI] [PubMed] [Google Scholar]
- 9.Boldt J. Use of albumin: an update. Br J Anaesth. 2010;104:276–284. doi: 10.1093/bja/aep393. [DOI] [PubMed] [Google Scholar]
- 10.Kallee E. Bennhold’s analbuminemia: a follow-up study of the first two cases (1953-1992) J Lab Clin Med. 1996;127:470–480. doi: 10.1016/s0022-2143(96)90064-5. [DOI] [PubMed] [Google Scholar]
- 11.Reynaud JP, Sagniez M, Rousseau A, Nicolas G. [Biological study of a case of analbuminemia] Sem Hop. 1983;59:124–128. [PubMed] [Google Scholar]
- 12.Dammacco F, Miglietta A, D’Addabbo A, Fratello A, Moschetta R, Bonomo L. Analbuminemia: report of a case and review of the literature. Vox Sang. 1980;39:153–161. doi: 10.1111/j.1423-0410.1980.tb01851.x. [DOI] [PubMed] [Google Scholar]
- 13.Berger GM, Stephen CR, Finestone A, Beatty DW. Analbuminaemia. Clinical and laboratory features in a South African patient. S Afr Med J. 1985;67:418–422. [PubMed] [Google Scholar]
- 14.Baldo-Enzi G, Baiocchi MR, Vigna G, Andrian C, Mosconi C, Fellin R. Analbuminaemia: a natural model of metabolic compensatory systems. J Inherit Metab Dis. 1987;10:317–329. doi: 10.1007/BF01799973. [DOI] [PubMed] [Google Scholar]
- 15.Rosipal S, Debreová M, Rosipal R. A speculation about hypercholesterolemia in congenital analbuminemia. Am J Med. 2006;119:181–182. doi: 10.1016/j.amjmed.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Baldo G, Fellin R, Manzato E, Baiocchi MR, Ongaro G, Baggio G, Fabiani F, Pauluzzi S, Crepaldi G. Characterization of hyperlipidemia in two patients with analbuminemia. Clin Chim Acta. 1983;128:307–319. doi: 10.1016/0009-8981(83)90330-3. [DOI] [PubMed] [Google Scholar]
- 17.Maugeais C, Braschi S, Ouguerram K, Maugeais P, Mahot P, Jacotot B, Darmaun D, Magot T, Krempf M. Lipoprotein kinetics in patients with analbuminemia. Evidence for the role of serum albumin in controlling lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 1997;17:1369–1375. doi: 10.1161/01.atv.17.7.1369. [DOI] [PubMed] [Google Scholar]
- 18.Liang K, Vaziri ND. HMG-CoA reductase, cholesterol 7alpha-hydroxylase, LCAT, ACAT, LDL receptor, and SRB-1 in hereditary analbuminemia. Kidney Int. 2003;64:192–198. doi: 10.1046/j.1523-1755.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaysen GA. Albumin turnover in renal disease. Miner Electrolyte Metab. 1998;24:55–63. doi: 10.1159/000057351. [DOI] [PubMed] [Google Scholar]
- 20.Demirsoy E, Sirin G, Ozker E. Coronary artery bypass surgery in a patient with analbuminemia. Tex Heart Inst J. 2011;38:85–87. [PMC free article] [PubMed] [Google Scholar]
- 21.Papi N, Castiglioni A, Reale A. Su di un caso di analbuminemia congenita. Riv Ital Ped. 1983;9:85–87. [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Del Ben M, Burattin M, Arca M, Ceci F, Violi F, Angelico F. Treatment of severe hypercholesterolemia with atorvastatin in congenital analbuminemia. Am J Med. 2004;117:803–804. doi: 10.1016/j.amjmed.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 24. Available from: http://www.albumin.org (accessed 10/09/2012)
- 25.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, et al. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]


