Abstract
Tuberculosis (TB) involving the pancreas are uncommon, especially when present in immunocompetent hosts. Pancreatic TB is more frequently associated with miliary TB or widely disseminated disease. Pancreatic TB may present as cystic or solid pancreatic masses, pancreatic abscess or acute or chronic pancreatitis. Majority of the cases are diagnosed after surgical exploration for presumed pancreatic malignancy and pre-operative diagnosis is quite difficult. However, improvement in imaging techniques and the resulting image-guided interventions gradually can obviate the need for more invasive diagnostic surgical procedures and expedite the planning of therapy. Herein, we report a rare case of isolated pancreatic TB which presented with pancreatic mass lesion in an immunocompetent host. Diagnosis was made by contrast enhanced computed tomography and guided fine needle aspiration of the pancreatic mass which revealed acid-fast bacilli on Ziehl-Neelsen stain. The case was treated successfully with antituberculous drugs. Pancreatic tuberculosis should be considered in the differential diagnosis of a pancreatic mass when the patient is young, residing in the endemic zone of tuberculosis. Every attempt should be made to diagnose the cases to prevent unnecessary operation.
Keywords: Pancreatic tuberculosis, Pancreatic mass, Pre-operative diagnosis, Computed tomography, Fine needle aspiration, Antituberculous drugs
Core tip: Isolated pancreatic tuberculosis is rare, even in countries with a high incidence of tuberculosis. Pancreatic tuberculosis (TB) is more frequently associated with miliary TB or widely disseminated disease. Pancreatic tuberculosis most commonly presents as a solitary lesion with multiple cystic components. The most important differential diagnosis includes pancreatic malignancy. Majority of the cases are diagnosed after surgical exploration for presumed pancreatic malignancy and pre-operative diagnosis is quite difficult. In the present study, we describe a rare case of isolated pancreatic TB in a 24-year-old man, presented with pancreatic mass lesion in an immunocompetent host. Diagnosis was made by contrast enhanced computed tomography (CT) and fine needle aspiration of the pancreatic mass revealed acid-fast bacilli. The case was treated successfully with antituberculous drugs. Pancreatic tuberculosis should be considered in the differential diagnosis of a pancreatic mass when the patient is young, residing in the endemic zone of tuberculosis.
INTRODUCTION
The abdomen is a common site in setting of extrapulmonary tuberculosis (TB). The prevalence of abdominal TB in developing countries has been estimated to be as high as 12%[1]. It commonly affects the intestinal tract, lymph nodes, peritoneum and solid organs in varying combinations. Pancreatic and peripancreatic involvements are rare. Majority of the cases occur as a part of disseminated tuberculosis[2-4]. Isolated involvement of the pancreas is even rarer, probably due to biological resistance imparted by the pancreatic enzymes[5]. The incidence of pancreatic TB is reported to be less than 4.7% worldwide[3]. Pancreatic tuberculosis was first reported by Auerbach[3] in 1944. This review was done as available literature related to pancreatic tuberculosis is mostly in the form of case reports or series and we still lack a complete clinical picture of the disease. Literature search revealed that only nine cases of pancreatic tuberculosis have been reported from India in last 5 years. Clinico-radiologically pancreatic TB closely resembles a pancreatic malignancy. Therefore, most cases of pancreatic TB have been diagnosed after exploratory laparotomy surgery for suspected malignancy. However, with the use of improved imaging techniques computed tomography (CT) or more recently endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and image-guided interventions preoperative diagnosis of pancreatic masses is now possible without going for surgery[6]. We present a case of isolated pancreatic TB presenting as discrete pancreatic mass in the body and tail with mesenteric lymphadenopathy in an immunocompetent host diagnosed without laparotomy and treated successfully with antituberculosis drugs (ATD).
CASE REPORT
A 24-year-old man presented with, abdominal pain, intermittent episodes of bilious vomiting and low-grade fever of three months duration. The pain was located in the upper abdomen without any radiation to the back. He also admitted having a 5 kg weight loss over this period, anorexia and generalised weakness. He denied any hematemesis, melena, jaundice or altered bowel habits. There was no history of alcohol ingestion. He had no history of exposure to tuberculosis had never participated in any sexual activity. Physical examination was unremarkable except mild pallor and epigastric tenderness without guarding.
His laboratory studies showed hemoglobin of 10.6 g/dL, total leukocyte count of 9800/μL with a normal differential count, an elevated erythrocyte sedimentation rate (86 mm in first hour). Serum amylase was 200 IU/L (reference range: 40-140 U/L) and lipase was 82 IU/L. Liver function tests were normal. A chest radiograph did not reveal any abnormality. The Mantoux test was negative and results of human immunodeficiency virusserology were negative. Ultrasound of the abdomen revealed bulky, inhomogeneous pancreas with a 3.19 cm × 2.2 cm hypoechoic space occupying lesion (SOL) with inner necrosis in body and tail of pancreas with few peripancreatic and a mesenteric lymph node with inner necrosis. Contrast-enhanced CT (CECT) of the abdomen demonstrated bulky pancreas, retropancreatic lymphadenopathy and a low-density lesion in body and tail of pancreas (3.5 cm × 2.4 cm) with peripheral enhancement (Figure 1) without any ascites, bile or pancreatic duct compression or dilatation and ileo-caecal or omental thickening. As the patient had ongoing chronic pain with loss of weight and appetite and a bulky head and body of the pancreas, CT-guided fine needle aspiration was carried out to exclude a pancreatic malignancy. The cytological examination of the fine needle aspiration revealed serosanguinous aspirate, microscopical examination of which suggested granulomatous inflammation with epitheloid cells, pancreatic acinar and ductal cells with patchy necrotic material (Figure 2A). Zeil-Neelsen stain revealed abundant acid-fast bacilli (AFB) on the background of proteinaceous material and inflammatory cells (Figure 2B).
Figure 1.

Contrast-enhanced computerized tomography of abdomen showing bulky pancreas (A), retro pancreatic lymphadenopathy (B) and hypodense lesion in the body and tail of the pancreas (3.5 cm × 2.4 cm) with peripheral enhancement.
Figure 2.

CT guided fine-needle aspiration cytology from the pancreatic lesion shows collection of epitheloid cells (inset) forming granuloma along with pancreatic ductal and acinar cells with patchy necrotic material (Giemsa staining) (A), Ziehl-Neelsen stain showing acid fast bacilli in the background of proteinaceous material (B).
The diagnosis of isolated pancreatic tuberculosis was made based on the above findings and subsequently the patient was treated with antituberculous therapy (ATT) for twelve months. Initial two months he was treated with Isoniazide 300 mg/d, Rifampicin 450 mg/d, Ethambutol 800 mg/d and Pyrazinamide 1500 mg/d. Subsequently for next ten months he was treated with rifampicin 450 mg/d and isoniazide 300 mg/d. The patient improved with the therapy and was asymptomatic after completing two months of therapy. Repeat ultrasound revealed significant resolution of pancreatic hypoechoic collection and regression of lymph node. A repeat CECT of the abdomen performed after 9 mo of the therapy revealed a normal pancreas with near complete resolution of hypoechoic lesion (Figure 3). No varices were noted on upper gastrointestinal endoscopy. After three months of follow-up, the patient is asymptomatic.
Figure 3.
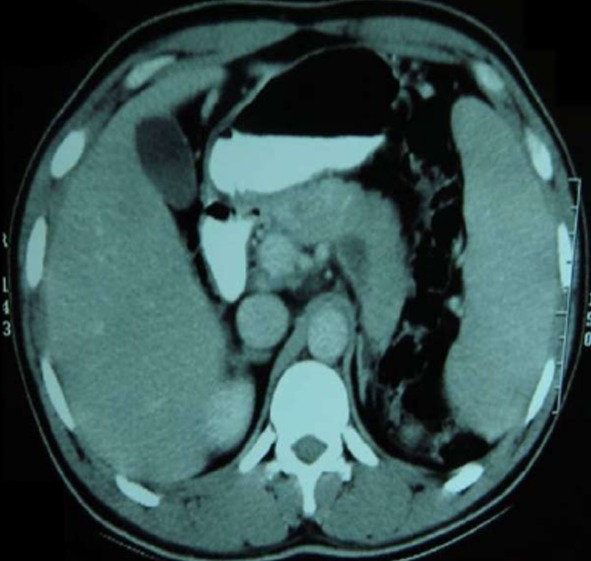
Review Contrast-enhanced computerized tomography of the abdomen performed at 9 mo of ATT showing near complete regression of pancreas size and resolution of hypodense lesion at body and tail.
DISCUSSION
Tuberculosis is a major health problem in developing countries. with an annual incidence of 9.7 million cases of TB; highest incidence being in Asia, South America, Eastern Europe, and most sub-Saharan African countries. Although pulmonary TB is the most common presentation of disease; extrapulmonary TB (EPTB) accounts for nearly 20% of all cases of TB in immunocompetent hosts[7]. Abdominal TB includes infection of varying combinations of the intestinal tract, peritoneum, and solid organs such as the spleen, liver, and pancreas. Isolated pancreatic TB is extremely uncommon, with pancreatic involvement usually occurring in the setting of miliary or widely disseminated TB; often in immunocompromised hosts[8,9]. Pancreatic tuberculosis was first reported by Auerbach in 1944. In his series of 1656 autopsies of tuberculous patients, only 14 cases had pancreatic involvement that may have mimicked neoplasia but did not find any cases of isolated pancreatic tuberculosis. The incidence of pancreatic TB is reported to be less than 4.7% worldwide[3,10]. It has been speculated that tubercular involvement of the pancreas might occur as a result of direct extension, lymphohematogenous dissemination, and reactivation of a previous abdominal focus or immune reaction to generalized tuberculosis[8,11].
Pancreatic tuberculosis usually affects young adults but convincing epidemiological data with regards to age and sex distribution is not available. Nine cases of pancreatic tuberculosis have been reported from India in last 5 years[12-18]. Clinical data are summarized as Table 1. Among this group are 3 men and 6 women with an overall mean age of 33.2 years. The mean age among men was 36 years and mean age among women was 31.83 years. In all of these cases a presumptive diagnosis other than tuberculosis were made reflecting the diagnostic challenge faced when encountered with such cases. The challenge is partly because of rarity of the disease itself and partly due to its nonspecific presentation mimicking pancreatic malignancy.
Table 1.
Summary of reported cases of pancreatic tuberculosis from India in the last 5 years
| Ref. | Age (yr)/sex | Presenting symptoms | Pulmonary tuberculosis | Location | Nature of mass | Lymph nodes | Invasion | Pancreatic duct and bile ducts | Presumptive diagnosis | Confirmation of diagnosis | ATT with duration | Outcome |
| Ray et al[12] | 50/Female | Pain abdomen | - | Head | Cystic | Present | None | Normal | Pancreatic malignancy | CT guided FNAC | HRZE 6 mo | Good |
| 15/Female | Pain abdomen, weight loss | - | Head | Cystic | Present | None | Normal | Pancreatic malignancy | EUS guided FNAC | HRZE | Good | |
| 6 mo | ||||||||||||
| 13/Female | Pain abdomen, weight loss | - | Head | Cystic | Present | None | Normal | Pancreatic malignancy | EUS guided FNAC | HRZE | Good | |
| 6 mo | ||||||||||||
| Arora et al[13] | 48/Male | Pain abdomen, weight loss | - | Head | Cystic | Absent | None | Bile Duct dilatation | Pancreatic malignancy | EUS guided FNAC | HRZE 6 mo | Good |
| Gupta et al[14] | 24/Male | Pain abdomen, jaundice | - | Head | Cystic | Absent | Portal vein, splenic artery, hepatic artery | Biliary radicles and bile duct dilatation | Pancreatic malignancy | EUS guided FNAC | HRZE | Good |
| 8 mo | ||||||||||||
| Rana et al[15] | 40/Female | Pain abdomen, jaundice | - | Head | Cystic | Absent | Portal vein | Pancreatic and common bile duct dilatation | Pancreatic malignancy | EUS guided FNAC | HRZE х 2, HR х 10 | Good |
| Singh et al[16] | 45/Female | Pain abdomen, bilious vomiting | - | Head and uncinate process | Cystic | Present | Duodenu, portal vein | Pancreatic and common bile duct dilatation | Pancreatic malignancy | Laparotomy and Whipple's Pancreati coduodene ctomy | HRZE х 3 until first follow up | Good |
| Pandita et al[17] | 28/Female | Pain abdomen, dyspepsia | - | Head and body | Cystic | Absent | None | Normal | Pancreatic malignancy | CT guided FNAC | HRES | Good |
| 1 yr | ||||||||||||
| Bhatia et al[18] | 36/Male | Epigastric pain abdomen | Past history: 4 yr back | Head and body | Cystic | Absent | Right lobe of liver | Not dilated | Intraductal pancreatic mucinous tumour | EUS guided FNAC | HRZE | Good |
| 8 mo |
CT: Computed tomography; EUS: Endoscopic ultrasound; FNAC: Fine needle aspiration cytology.
In a study by Saluja et al[19], the three most common presenting complaints in patients found to have pancreatic TB were abdominal pain, jaundice, and weight loss. Individuals infected with pancreatic TB may also present with fever, gastrointestinal hemorrhage secondary to splenic vein thrombosis, and anorexia. In our case, there was involvement of the body and tail of the pancreas. Xia et al[20] have summarized characteristic features of pancreatic tuberculosis as follows: (1) mostly occurs in young people, especially female; (2) have past history of tuberculosis or come from endemic zone of tuberculosis; (3) often present with epigastric pain, fever, and weight loss; and (4) ultrasound or CT scan show pancreatic mass and peripancreatic nodules, some with focal calcification. Other reported presentations are obstructive jaundice, acute or chronic pancreatitis, pancreatic abscess, portal vein thrombosis causing portal hypertension, etc. Tuberculin skin testing and an interferon-γ release assay for TB may be negative in patients with abdominal tuberculosis because of poor nutritional status leading to a weak immune response as happened in our case[8,15].
Several imaging methods like transcutaneous ultrasound, CT scan and endoscopic ultrasound are used for assessment of pancreatic pathology. The imaging findings may suggest the possibility of tuberculosis, but none of the findings are pathognomic for pancreatic tuberculosis. Ultrasound is often the first investigation used for diagnosis of pancreatic tuberculosis which may reveal focal hypoechoic mass or cystic lesion of the pancreas mostly situated in the head and uncinate process of the pancreas. CT scan is still regarded as the investigation of choice for pancreatic pathology. CT scan may show hypodense lesion with irregular border in the head of the pancreas, diffuse enlargement of the pancreas or enlarged peripancreatic lymph nodes[21,22]. The presence of hypodense peripancreatic lymph nodes with rim enhancement, ascites and/or mural thickening affecting the ileo-caecal region suggests the pancreatic tuberculosis[22]. Magnetic resonance imaging (MRI) findings of focal pancreatic tuberculosis include a sharply delineated mass in the pancreatic head showing heterogeneous enhancement which is hypointense on fat-suppressed T1-weighted images and show a mixture of hypo- and hyperintensity on T2-weighted images[23]. An important image finding in pancreatic tuberculosis is the normal appearing common bile duct and the pancreatic duct, even if the mass is positioned centrally in the head of the pancreas. This in contrast to pancreatic adenocarcinoma where the pancreatic duct is dilated in centrally located tumors in the head region. The diffuse form of pancreatic tuberculosis is characterized by pancreatic enlargement with narrowing of the main pancreatic duct and heterogeneous enhancement[23]. Bile cytology on endoscopic retrograde cholangiopancreatography (ERCP) may occasionally help in establishing the diagnosis[19,24]. D’cruz et al[25] further suggested that there is no radiographical difference between cystic neoplasm of the pancreas and pancreatic TB abscess formation, as both can present as septated masses with surrounding hypodense lymphadenopathy.
Since there are no clinical, laboratory or radiological features which are specific for pancreatic tuberculosis, histopathological or cytological as well as bacteriological confirmation is necessary for establishing the diagnosis of isolated pancreatic tuberculosis. Percutaneous imaging or endoscopic ultrasound-guided fine needle aspiration of the pancreatic lesion have been reported for establishing a diagnosis of pancreatic tuberculosis[6,26-28]. EUS is preferred for obtaining tissue biopsy because of less chances of needle tract dissemination particularly if the mass seems to be malignant. In a recent series by Song et al[29], EUS-FNA was able to diagnose pancreatic/peri-pancreatic tuberculosis in 76.2% of patients. The microscopic features of tuberculosis are caseation necrosis and presence of acid fast bacilli. Caseating granuloma is seen in 75%-100% of cases, and acid-fast bacilli are identified in 20%-40% of cases[26]. In our case the diagnosis was done by CT guided FNAC and the ZN staining showed plenty of AFB.
Once the diagnosis is made, antituberculous drugs should be started as early as possible. Majority of the patients show symptomatic improvement within two weeks. Because of the rarity of this disease, there are no specific treatment guidelines. The majority of cases of pancreatic tuberculosis respond well to 6-12 mo of anti-tubercular therapy and their prognosis is good (Table 1). In our case ATT was given for 12 mo with a repeat CT scan at 9 mo showing near complete resolution of the pancreatic mass.
In a conclusion, isolated pancreatic tuberculosis is rare, even in countries with a high incidence of tuberculosis. Radiologically, pancreatic tuberculosis presents typically as a solitary lesion located in the body or head with peripancreatic lymph nodes. Therefore, diagnosis is a challenge, calling for a team approach with the goal of making the diagnosis non-invasively. A more recent development includes endoscopic ultrasound-guided fine-needle aspiration for histological and microbiological tuberculosis diagnosis; thereby, major surgery may be avoided. Clinical awareness of pancreatic TB may guide clinicians to appropriate diagnostic studies and management; which may lead to alleviation of symptoms and possible resolution of pancreatic masses with ATT.
Footnotes
P- Reviewer Yilmaz A S- Editor Wen LL L- Editor A E- Editor Lu YJ
References
- 1.Chen CH, Yang CC, Yeh YH, Yang JC, Chou DA. Pancreatic tuberculosis with obstructive jaundice--a case report. Am J Gastroenterol. 1999;94:2534–2536. doi: 10.1111/j.1572-0241.1999.01389.x. [DOI] [PubMed] [Google Scholar]
- 2.Meinke AK. Pancreatic tuberculous abscess. Conn Med. 1989;53:139–141. [PubMed] [Google Scholar]
- 3.Auerbach O. Acute Generalized Miliary Tuberculosis. Am J Pathol. 1944;20:121–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Turan M, Sen M, Koyuncu A, Aydin C, Elaldi N, Arici S. Pancreatic pseudotumor due to peripancreatic tuberculous lymphadenitis. Pancreatology. 2002;2:561–564. doi: 10.1159/000066097. [DOI] [PubMed] [Google Scholar]
- 5.Knowles KF, Saltman D, Robson HG, Lalonde R. Tuberculous pancreatitis. Tubercle. 1990;71:65–68. doi: 10.1016/0041-3879(90)90064-f. [DOI] [PubMed] [Google Scholar]
- 6.Kaushik N, Schoedel K, McGrath K. Isolated pancreatic tuberculosis diagnosed by endoscopic ultrasound-guided fine needle aspiration: a case report. JOP. 2006;7:205–210. [PubMed] [Google Scholar]
- 7.World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: WHO; 2008. [Google Scholar]
- 8.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–353. [PubMed] [Google Scholar]
- 9.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–315. [PubMed] [Google Scholar]
- 10.Bhansali SK. Abdominal tuberculosis. Experiences with 300 cases. Am J Gastroenterol. 1977;67:324–337. [PubMed] [Google Scholar]
- 11.Stock KP, Riemann JF, Stadler W, Rösch W. Tuberculosis of the pancreas. Endoscopy. 1981;13:178–180. doi: 10.1055/s-2007-1021678. [DOI] [PubMed] [Google Scholar]
- 12.Ray S, Das K, Mridha AR. Pancreatic and peripancreatic nodal tuberculosis in immunocompetent patients: report of three cases. JOP. 2012;13:667–670. doi: 10.6092/1590-8577/1108. [DOI] [PubMed] [Google Scholar]
- 13.Arora A, Mukund A, Garg H. Isolated pancreatic tuberculosis: a rare occurrence. Am J Trop Med Hyg. 2012;87:1–2. doi: 10.4269/ajtmh.2012.12-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P, Guleria S, Agarwal S. Role of endoscopic ultrasound guided FNAC in diagnosis of pancreatic TB presenting as mass lesion: a case report and review of literature. Indian J Tuberc. 2011;58:120–124. [PubMed] [Google Scholar]
- 15.Rana SS, Bhasin DK, Rao C, Singh K. Isolated pancreatic tuberculosis mimicking focal pancreatitis and causing segmental portal hypertension. JOP. 2010;11:393–395. [PubMed] [Google Scholar]
- 16.Singh DK, Haider A, Tatke M, Kumar P, Mishra PK. Primary pancreatic tuberculosis masquerading as a pancreatic tumor leading to Whipple’s pancreaticoduodenectomy. A case report and review of the literature. JOP. 2009;10:451–456. [PubMed] [Google Scholar]
- 17.Pandita KK, Dogra S. Isolated pancreatic tuberculosis. Indian J Med Microbiol. 2009;27:259–260. doi: 10.4103/0255-0857.53212. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia V, Garg PK, Arora VK, Sharma R. Isolated pancreatic tuberculosis mimicking intraductal pancreatic mucinous tumor. Gastrointest Endosc. 2008;68:610–611. doi: 10.1016/j.gie.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Saluja SS, Ray S, Pal S, Kukeraja M, Srivastava DN, Sahni P, Chattopadhyay TK. Hepatobiliary and pancreatic tuberculosis: a two decade experience. BMC Surg. 2007;7:10. doi: 10.1186/1471-2482-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia F, Poon RT, Wang SG, Bie P, Huang XQ, Dong JH. Tuberculosis of pancreas and peripancreatic lymph nodes in immunocompetent patients: experience from China. World J Gastroenterol. 2003;9:1361–1364. doi: 10.3748/wjg.v9.i6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagar AM, Raut AA, Morani AC, Sanghvi DA, Desai CS, Thapar VB. Pancreatic tuberculosis: a clinical and imaging review of 32 cases. J Comput Assist Tomogr. 2009;33:136–141. doi: 10.1097/RCT.0b013e31816c82bc. [DOI] [PubMed] [Google Scholar]
- 22.Takhtani D, Gupta S, Suman K, Kakkar N, Challa S, Wig JD, Suri S. Radiology of pancreatic tuberculosis: a report of three cases. Am J Gastroenterol. 1996;91:1832–1834. [PubMed] [Google Scholar]
- 23.De Backer AI, Mortelé KJ, Bomans P, De Keulenaer BL, Vanschoubroeck IJ, Kockx MM. Tuberculosis of the pancreas: MRI features. AJR Am J Roentgenol. 2005;184:50–54. doi: 10.2214/ajr.184.1.01840050. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev A, D’Cruz S, Chauhan S, Thakur R, Kapoor V, Handa U. Pancreaticobiliary tuberculosis diagnosed by endoscopic brushings. JOP. 2006;7:665–669. [PubMed] [Google Scholar]
- 25.D’Cruz S, Sachdev A, Kaur L, Handa U, Bhalla A, Lehl SS. Fine needle aspiration diagnosis of isolated pancreatic tuberculosis. A case report and review of literature. JOP. 2003;4:158–162. [PubMed] [Google Scholar]
- 26.Levine R, Tenner S, Steinberg W, Ginsberg A, Borum M, Huntington D. Tuberculous abscess of the pancreas. Case report and review of the literature. Dig Dis Sci. 1992;37:1141–1144. doi: 10.1007/BF01300301. [DOI] [PubMed] [Google Scholar]
- 27.Ahlawat SK. EUS-guided FNA diagnosis of pancreatic tuberculosis. Gastrointest Endosc. 2007;65:557–558. doi: 10.1016/j.gie.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Coriat R, Latournerie M, Galtier JB, Michiels C, Hillon P, Manfredi S. [The role of echoendoscopic biopsy in diagnosing tuberculosis of pancreas] Gastroenterol Clin Biol. 2008;32:98–101. doi: 10.1016/j.gcb.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Song TJ, Lee SS, Park do H, Lee TY, Lee SO, Seo DW, Lee SK, Kim MH. Yield of EUS-guided FNA on the diagnosis of pancreatic/peripancreatic tuberculosis. Gastrointest Endosc. 2009;69:484–491. doi: 10.1016/j.gie.2008.10.007. [DOI] [PubMed] [Google Scholar]


