Abstract
Direct mass spectrometry analysis of untreated samples of volumes as low as 0.2 µL were achieved using fast extraction and nanoESI (electrospray ionization) in a combined fashion. The analytes in dried samples on paper substrates were extracted by organic solvent in a nanoESI tube and ionized with a high voltage applied for generating a spray. The ionization source produced stable signals for different atmospheric pressure interfaces of triple quadrupole instruments. Analysis time more than 20 minutes were available with 10 µL solvent consumed for the entire analysis process. The performance in qualitative and quantitative analysis was characterized with a wide variety of samples. Limits of detection as low as 0.1 ng/mL (corresponding to an absolute amount of 0.05 pg) were obtained for analysis of atrazine in river water, thiabendazole in orange homogenate, and methamphetamine in blood.
Introduction
Rapid chemical analysis plays an important role in a wide range of fields, such as drug development,1–3 neonatal screening4–6 and other clinical analysis as well asfood quality control.7–9 The matrix effect due to the high complexity of the samples is one of the major challenges for achieving high sensitivity and high quantitation accuracy of the analysis.10, 11 This is particularly true for mass spectrometry analysis, where severe ionization suppression can occur due to the matrix effect. The limit of detection (LOD) or quantitation (LOQ) and linear calibration ranges obtained for analysis of pure analytes typically could not be obtained for the analysis of complex mixture samples.10–12 The development of atmospheric pressure ionization methods, including electrospray ionization (ESI)13, 14 and atmospheric pressure chemical ionization (APCI)15, 16, allowed the coupling of mass spectrometry with liquid chromatography (LC), which is a powerful tool for minimizing the matrix effect and preconcentrating the analytes from bulk samples. The LC-MS/MS has been developed as a robust and reliable method for quantitative analysis of complex samples17–20 and widely used for drug discovery,21, 22 clinical chemical analysis,23, 24 and biomedical research.25, 26 It should be noted that the raw samples, such as food stuff, blood or tissue, need to be processed and only the purified extracts can be sent to LC-MS systems for analysis.
While the standard LC-MS/MS approach has been continuously improved with the new developments in instrumentation and analytical method, alternative solutions are actively sought for fast and direct analysis of untreated samples. Many recent examples have been shown using ambient ionization methods27–29 for direct sampling ionization of untreated samples using MS. Starting with desorption electrospray ionization (DESI)30 and direct analysis in real time (DART),31 there have been more than 30 ambient ionization methods developed, all of which have been demonstrated for analysis of untreated samples with little or no sample preparation.27–29, 32 The applications of these methods have expanded to direct quantitation of untreated samples,33–35 in vivo tissue imaging,30, 35, 36 as well as study of chemical reactions.37, 38 It has been proved that the use of proper combinations of the techniques to perform real time differential extraction, desorption and ionization, high sensitivity can be achieved for direct analysis of target analytes in untreated samples with very complex matrices. Some exemplary achievements include the detection of chemical warfare agents at low-ppb levels using DART,39 0.62 pg/mL nicotine in gas phase samples using extractive electrospray ionization (EESI),40 100 fmol of peptides using Electrospray-assisted Laser Desorption Ionization (ELDI),41 and 0.2 to 40 ng drug molecules in plasma using DESI.42 These achievements suggest that the ambient MS analysis can potentially serve as a solution for performing chemical analysis at high sensitivity without complicated procedures that require special expertise or long time. This would be very important for incorporating MS technologies into the development of point-of-care and other consumer type devices for chemical analysis, which are meant to be operated by personnel untrained with analytical techniques and demand direct report of the analysis results.
In addition to the adequate LODs, many applications could potentially be well served by ambient ionization, such as those for therapeutic drug monitoring, food regulation, and biomedical diagnosis. Mandatory accuracy and reproducibility in the quantitation are typically required for these applications. Paper spray ionization43 has been investigated for quantitative ambient MS analysis.44, 45 The sample, such as whole blood, is deposited on a paper substrate cut in triangle shape to form a dried sample spot, a small amount (10 µL) of solvent is then dropped onto the paper substrate to extract the analytes from the dried sample, and a high DC voltage is applied to generate a spray ionization at the tip of the paper substrate.46 Paper spray has been investigated for therapeutic drug monitoring,47 metabolite analysis for neonatal screening,48 drug abuse screening,49 and food safety.50 With internal standards incorporated, good limits of quantitation (LOQs) have been obtained. For example, LOQs better than 5 ng/mL43 and RSDs better than 5%51 have been obtained for quantitation of therapeutic drugs in dried blood spots.
In addition to the obvious advantage of analyzing untreated samples with a fast speed and simplified procedure, use of ambient ionization also enables the analysis of samples of extremely small amounts.47 The loss of the sample associated with sequential steps in sample preparation and purification for traditional LC-MS analysis is avoided with the direct MS analysis using ambient ionization. Analysis requiring only several microliters of blood is of advantage for developing point-of-care diagnosis using less invasive method for sample taking as well for applications in preclinical studies where limited amounts of body fluid samples could be obtained. However, the efficient extraction of the analytes from the samples is critical to the sensitivity of the ambient ionization MS analysis; the matrix effects can also be significant and need to be overcome.52, 53 Quantitative analysis with small sample amounts could also be challenging, due to the large relative errors associated with measuring and handling the samples of small volumes. Various methods for adding internal standards to small amounts of samples without requiring laboratory skills have also been explored, which would be important for applications like point-of-care diagnosis.54, 55
The effectiveness of the paper spray as well as many other spray-based ambient ionization methods, such as the nanospray desorption electrospray desorption (nanoDESI) 56, 57 and liquid extraction surface analysis (LESA) methods,58, 59 revealed a fact that a simple extraction followed by a spray ionization can provide adequate performance for qualitative detection at high sensitivity and quantitative analysis at good accuracy. In this study, we demonstrate a simple version of the extraction spray ionization for quantitative analysis of untreated samples. Highly quantitative results (RSD < 5%) have been achieved for analyzing drug compounds in whole blood of only 0.2µL using this method. This extraction spray method works well with commercial instruments using different atmospheric pressure interfaces. Stable signals of analytes were obtained for more than 20 minutes with only 10 µL solvent used for the entire analysis, which is of significant advantage for quantitation at low concentration levels. The versatility of this method has been tested for analysis of illicit drugs in urine, herbicides in river water, and additives in food stuff. Although the performance of this method could be compared with many other methods developed for ambient ionization, in this manuscript we report the improved spray stability in comparison with paper spray as a step forward toward the development of the disposable sampling cartridges.
Experimental Section
Chromatography paper (grade 1) used for making sample substrates was purchased from Whatman (Whatman International Ltd., Maidstone, ENG). Borosilicate glass tubes (id 0.86 mm) for making the nanoESI tips were purchased from Sutter Instrument (Sutter Instrument Co, Novato, CA, US). All the organic solvents were purchased from Macron Chemicals (Avantor Performance Materials Inc., Phillipsburg, NJ, US), unless otherwise specified. Bovine whole blood (with EDTAK2 as anticoagulant) was purchased from Innovative Research (Novi, MI, US). Other chemicals used in the experiments were purchased from Sigma-Aldrich (Milwaukee, WI, US) including sunitinib, amitriptyline, verapamil, nicotine, methamphetamine, clenbuterol, melamine, thiabendazole, and atrazine. Stock solutions were prepared by dissolving analytes of interest in methanol:H2O 50:50 (v:v) solution, which were subsequently spiked into the raw samples. The orange homogenate was prepared with 10 g orange and 10 mL of water. Thiabendazole stock solution was then spiked into the homogenate at 1% in volume. Porcine homogenate was prepared with 2 g of pork in 15 mL of water and 1% (volume) clenbuterol stock solution was added. The river water samples were prepared with a series of dilutions from atrazine stock solution using river water. Two triple quadrupole mass spectrometers with different atmospheric pressure interfaces (APIs) were used in the study for comparison, including a TSQ Quantum Access Max (Thermo Scientific, San Jose, CA, US) with a heated capillary API and an QTRAP 4000 (Applied Biosystems, Toronto, Canada) with a pinhole type API using curtain gas. Typical instrument settings for MS/MS scans were used for the product ion scan mode, selected or multiple reaction monitoring mode.
Results and Discussion
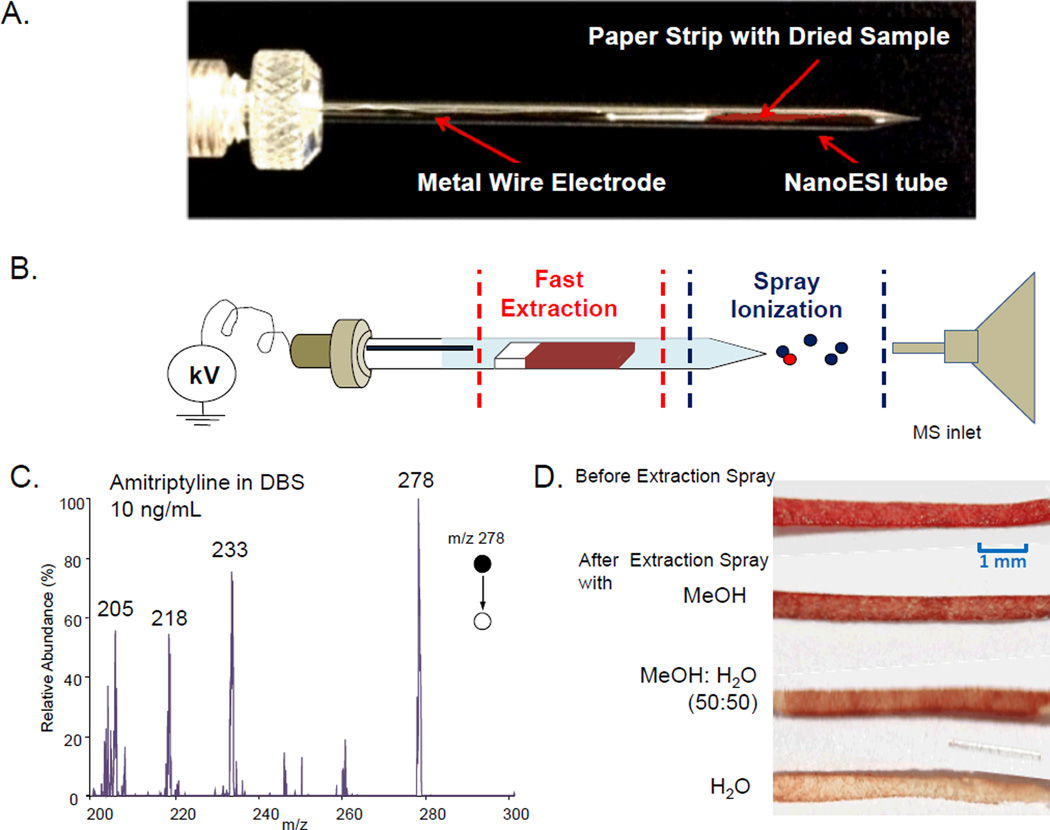
The device for the demonstration of the general concept for extraction spray ionization is shown in Figure 1a. A piece of paper strip (Whatman grade1, 1 cm length, 0.5 mm width, 0.18 mm thickness) with dried sample was inserted into a nanoESI tube, which was made from a borosilicate glass tube with the spray tip pulled using the method previously reported.60, 61 The tube was then filled with a solvent for extraction of the analytes from the sample on the paper strip; a high DC voltage (about 2 kV) was applied through a metal wire to generate nanoESI (Figure 1b). The entire procedure took about 1 minute. This method shares the same concept with paper spray, viz. a fast extraction of the analyte followed by an electrospray. The advantage of the extraction spray will be further discussed later in compassion with paper spray in this manuscript. Paper spray is a very simple version of the extraction spray, with the analytes extracted and subsequently spray-ionized from a single substrate. A great advantage of spraying from the tip of a paper substrate is that the clogging would not occur.25, 62 However, the spray could not be well regulated in terms of maintaining flow rate and the spray current constant. The spray pattern is highly dependent on how fast the solvent is consumed through the spray as well as the evaporation.52 With the extraction and spray separated in the device shown in Figure 1a, it was expected to have the advantage of the good stability and ionization efficiency associated with the nanoESI.
Figure 1.
(a) Photo and (b) schematic of an extraction spray ionization source for MS analysis. (c) MS/MS spectrum obtained by TSQ for 10 ng/mL amitriptyline in blood, dried sample prepared with 0.2 µL blood, 10 µL methanol for extraction and spray solvent. (d) Photos of paper substrates with dried blood samples before and after extraction spray process using different solvents
The initial test of this extraction nanoESI was done with an analysis of dried blood spots each prepared with about 0.2 µL whole bovine blood containing 10 ng/mL amitriptyline. In preparation of the DBS on paper, one end of the paper strip was in touch with the 0.2 µL blood (preloaded using Eppendorf 2.5 µL micropipette) on a glass slide to take the sample through the capillary effect. After the blood sample was completely dried, the paper strip was inserted into the nanoESI tube. The MeOH solvent fo 10 µL was filled into the tube for extraction and a platinum wire was inserted to apply a DC voltage of 2.0 kV for generating the spray ionization from the tip. The MS/MS spectrum recorded for protonated amitriptyline m/z 278 using TSQ is shown in Figure 1c. Good signal-to-noise (S/N) ratios were obtained for fragment ions m/z 233, 218 and 205 from amitriptyline of 10 ng/mL in blood. Each of these fragment ions could be used for quantitation in multiple reaction monitoring (MRM) mode.
Using the tube with a pulled glass tip a well-controlled spray was obtained and a good sensitivity was achieved, just like the nanoESI at relatively low flow rate but with relatively high efficiency in desolvation. However, it is well known that nanoESI generally suffers the clogging at the spray tip, which could be a major disadvantage in comparison with paper spray. A direct insert of the untreated sample into the spray tube could potentially make the situation much worse. In our previous studies of the solvent-substrate systems for the paper spray,52, 63 it was concluded that the interactions between the sample and the substrate or solvent have a significant impact on the analysis results. Using the current extraction nanoESI device, although the spray was different, the extraction process was expected to be similar to the paper spray. The effectiveness in analyzing the organic drug compounds directly from the blood is largely due to the differential extraction in favor of these compounds against others in the blood sample matrix. The blood cell, proteins and other chemicals such as salts, all bind well with cellulose in the paper substrate. Retaining the sample matrices on the paper while effectively extracting the target analytes is the key to avoid the clogging at the spray tip and to obtain a stable signal during the analysis, which is important for achieving high sensitivity and high quantitation accuracy of the analysis.
In a comparison study, pure methanol, methanol:water (1:1) solvent, and pure water were used for extraction spray analysis of the dried blood spots prepared in the same way described above. It was found that the clogging occurs with the methanol/water solvent and pure water, but not with methanol. The paper strips soaked with solvent became soft but did not swell significantly. It also stayed in the tube with a distance from the spray tip and should not have contributed to the clogging. As shown with the picture in Figure 1d for the paper strips after the analysis, more water soluble materials in the blood samples, including the blood cells that contribute to the red color of the sample, were washed off from the paper substrates with an aqueous component in the solvent composition. The coagulation of the cells and the electrochemical reactions involving salts during the nanoESI could all contribute to the clogging. Besides methanol, other pure organic solvents, including acetonitrile and isopropanol, could also be used for the extraction spray. Good sensitivity could be achieved with these solvents in analysis of amitriptyline in blood without clogging at the spray tip.
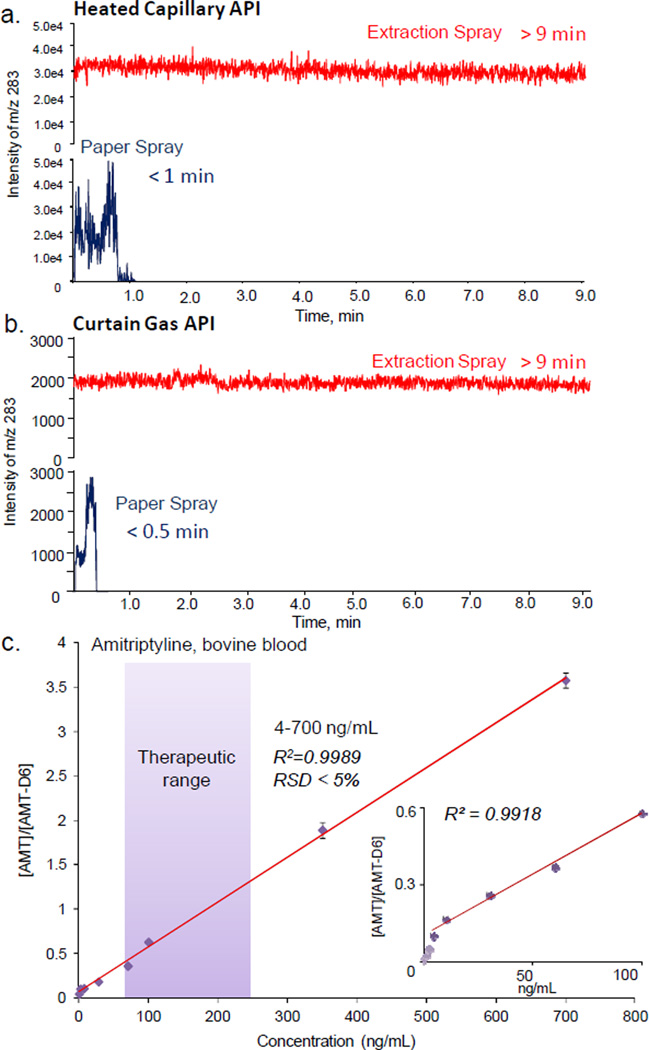
Another significant advantage gained with the extraction nanoESI is the enhanced stability and a long spray time for analysis, which is of a great significance for quantitative analysis of samples containing low concentration analytes. Comparisons between the extraction spray and paper spray were made using two triple quadrupole mass spectrometers with different atmospheric pressure interfaces, a TSQ Access with a heated capillary inlet and a QTRAP 4000 with a pinhole inlet and curtain gas. For paper spray, triangle substrates (1 cm height, 0.8 cm base width) each with a dried blood spot prepared from 1 µL blood containing 200 ng/mL sunitinib were used. For extraction spray, paper strips each with dried blood prepared from 0.2 µL of the same blood sample were used in this analysis. MeOH of 10 µL was utilized as the spray solvent in both cases. A DC voltage of 3.5 kV and 2 kV were used for paper spray and extraction nanoESI, respectively. The selected reaction monitoring (SRM) with transition of m/z 399 to m/z 283 was performed and the intensity of m/z 283 was monitored as a function of analysis time. The spray time for paper spray with TSQ was about 1 min (Figure 2a), which is consistent with the observations in previous studies.46 The spray time with the QTRAP was much shorter, less than 20s (Figure 2b). This is due to the fast drying of the spray solvent with the curtain gas flowing out of the atmospheric pressure interface. In our previous investigations using paper spray for quantitation of the therapeutic drugs in blood samples, good results could be obtained for TSQ but much poorer LOQs or RSDs were obtained with QTRAP 4000, which is due to the short analysis time and highly instable signals of the analytes.
Figure 2.
Ion chronograms recorded with SRM transition m/z 399 to m/z 283 for sunitinib by (a) TSQ and (b) QTRAP4000 using paper spray (blue) and extraction spray (red), 200 ng/mL sunitinib in blood, 1 and 0.2 µL used for preparing dried samples for paper spray and extraction spray, respectively; 10 µL methanol used as extraction/spray solvent. (c) Calibration curve for analysis of amitriptyline in blood samples using extraction spray, MRM transition m/z 277 to 223 for amitriptyline and m/z 283 to 223 for internal standard amitriptyline-d6 (200 ng/mL), 0.2 µL blood used for DBS, 10 µL methanol for extraction and spray
For paper spray using a discrete amount of solvent applied for on-paper extraction and spray, it has been observed that the pattern of the signal intensity measured for the analyte was not necessarily corresponding to that of the spray current.64 Larger droplets are generated at the beginning of the spray, while the highest signal for the analyte could be measured at a later time when the solvent flow (or exhausting) rate is relatively lower and presumably smaller droplets are generated.52 This pattern could certainly be affected by other factors, such as the accelerated evaporation by the curtain gas flow from a QTRAP 4000. With the extraction nanoESI, for both instruments the spray time could last more than 20 min and signals much more stable could be obtained (Figure 2a and b). A spray voltage of 3.5 kV was needed for paper spray while 2.0 kV was adequate for extraction nanoESI, which is comparable to normal nanoESI. The highest signal intensities obtained with paper spray were slightly higher than those for extraction spray, while the spray patterns by extraction spray were much more regular.
The enhanced stability of the signal in extraction nanoESI is certainly important for quantitation using MRM, by which the intensities of the analyte and internal standard are measured in an alternating order with a time delay. The longer spray time would also allow a longer measurement time for averaging the signals, which helps significantly to improve the quantitation accuracy at low concentration levels. As a demonstration of the effect on the quantitative analysis using the QTRAP 4000 instrument, blood samples containing amitriptyline at 0, 0.6, 2, 4, 10, 28, 60, 100, 350 and 700 ng/mL were analyzed, all with the internal standard amitriptyline-d6 at a constant concentration of 250 ng/mL. MRM transitions of m/z 277 to 223 and m/z 283 to 223 were used for amitriptyline and amitriptyline-d6, respectively. The calibration curve is plotted in Figure 2c, with an LOQ of 4 ng/mL (Figure 2c inset) and a linear range of 4–700ng/mL obtained, well covering the therapeutic range of amitriptyline (80–250 ng/mL). The RSDs (n=3) better than 5% were obtained for the entire range, which indicates a good reproducibility of the method.
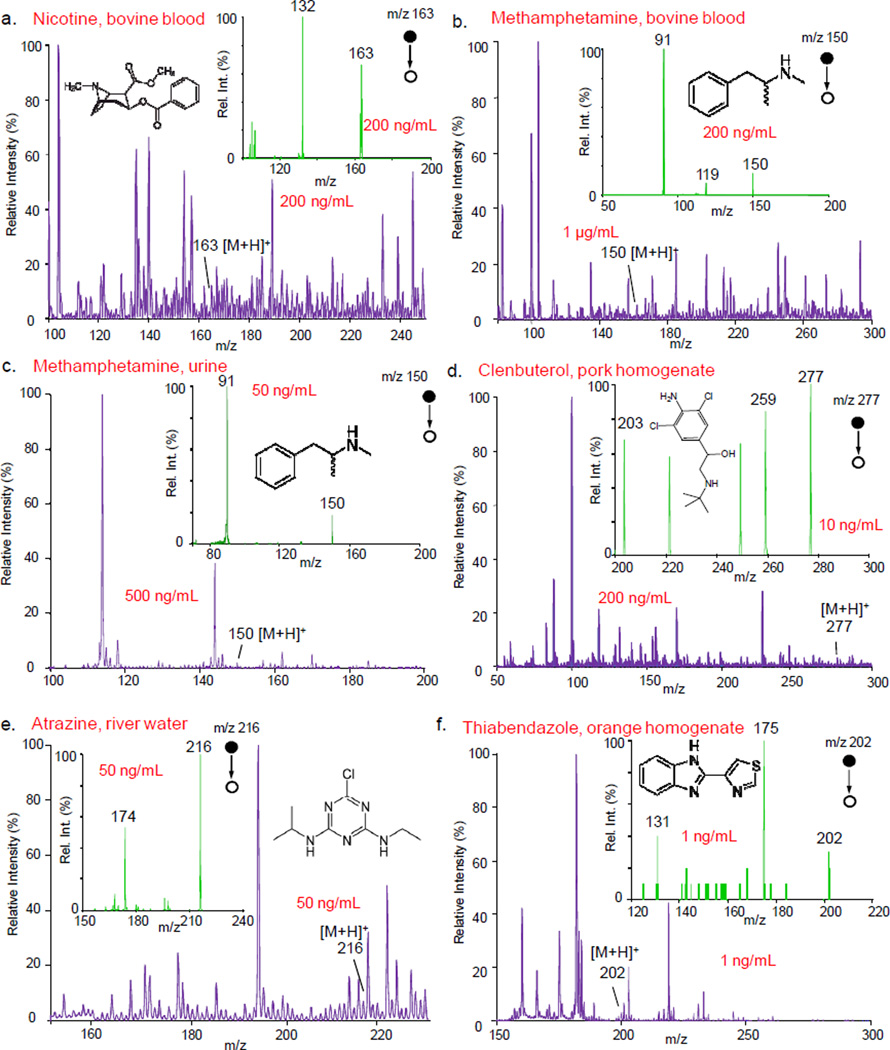
The versatility of extraction spray was characterized with analysis of a wide variety of compounds in samples with different complex matrices, including nicotine in whole blood (Figure 3a), methamphetamine in whole blood (Figure 3b) and urine (Figure 3c), clenbuterol in pork (Figure 3d), atrazine in river water (Figure 3e), and thiabendazole in orange homogenate (Figure 3f). In each test, 0.5 µL sample was used for preparing each dried sample on the paper strip and 10 µL MeOH was used for extraction and spray. The exemplary MS and MS/MS spectra are shown in Figure 3 for the samples containing the analytes at different levels in the ng/mL range. The protonated analytes could be observed in MS spectra for the analytes at the corresponding concentrations reported in Figure 3, with possible overlap with isomeric or isobaric compounds. The MS/MS spectra (Figure 3 insets) obtained with the extraction spray all show good S/N ratios for the fragment ions of the analytes.
Figure 3.
MS and MS/MS spectra (insets) obtained using QTRAP 4000 for (a) nicotine in blood, (b) methamphetamine in blood, (c) methamphetamine in urine, (d) clenbuterol in pork hommogenate, (e) atrazine in river water, and (f) thiabendazole in orange homogenate. The m/z values marked for the precursor and the fragment peaks of the analytes in the MS/MS spectra. 0.5 µL sample used to prepare each dried sample, 10 µL methanol and 2 kV used for extraction nanESI
For each type of the samples described above, a series of samples of lower analyte concentrations were prepared and analyzed using extraction nanoESI and MS/MS to evaluate the sensitivity. The LODs were identified with the MS/MS analysis and are summarized in Table 1, with 1 ng/mL as the poorest. LODs of 0.1 ng/mL were achieved for analyzing atrazine in river water, thiabendazole in orange homogenate, and methamphetamine in blood. The absolute amounts the analytes in these samples at the LOD concentrations are below 0.5 pg. The sensitivity of this method satisfies the requirements for meeting the mandatory cutoff values for these samples for regulatory or monitoring purposes (Table 1).
Table 1.
Limits of detection (LODs) of chemicals in various matrices using extraction spray method.
| hemicals | Category | Matrix | LOD | Cutoff value (ng/mL) |
|
|---|---|---|---|---|---|
| Absolute amount (pg) |
Concentration (ng/mL)/ (RSD) |
||||
| Melamine | Contaminant | Milk | 0.5 | 1 (10.4%) | 100065 |
| Clenbuterol | Contaminant | Pork homogenate | 0.25 | 0.5 (12.8%) | 566 |
| Atrazine | Herbicide | River water | 0.05 | 0.1 (5.8%) | 367 |
| Thiabendazole | Fungicide | Orange homogenate | 0.05 | 0.1 (15.5%) | 500068 |
| Methamphetamine | Psychoactive drug | Blood | 0.05 | 0.1 (19.3%) | 2049 |
| Nicotine | Psychoactive drug | Blood | 0.5 | 1 (16.1%) | NA |
| Imatinib | Therapeutic drug | Blood | 0.5 | 1 (13.7%) | 90054 |
| Verapamil | Therapeutic drug | Blood | 0.25 | 0.5 (15.2%) | 5063 |
| Sunitinib | Therapeutic drug | Blood | 0.5 | 1 (10.0%) | 20 |
Conclusions
Sensitive analysis and high accuracy quantitation can be achieved with untreated samples of small amounts using a simple combination of the fast extraction and nanoESI. Analysis time longer than 20 minutes was available with only 10 µL solvent consumed for the entire analysis. Stable signals were obtained for different types of atmospheric pressure interface with a heated capillary or curtain gas. Linear response of 4–700 ng/mL was achieved in the quantitation of selected therapeutic drugs in whole blood. The success in detecting a variety of chemicals at low concentrations in different matrices suggest a potential of this hybrid method for a broad range of applications. This method was developed with an aim for the possible development of a disposable sample cartridge for direct analysis using MS, like for paper spray. The use of the nanoESI tube in addition to the paper substrate would complicate the design of the cartridge in comparison with paper spray; however, the improved stability of the spray and quantitation performance are attractive to analysis using QTRAP type instruments and miniature mass spectrometers.
Acknowledgement
We thank Professor Yu Xia (Department of Chemistry, Purdue University) for providing access to the QTAP 4000 mass spectrometer. This work was supported by the National Science Foundation (Project 0847205-CHE), National Science Foundation Instrumentation Development for Biological Research (DBI 0852740), National Center for Research Resources (5R21RR031246-03) and the National Institute of General Medical Sciences (8 R21 GM103454) from the National Institutes of Health.
REFERENCES
- 1.Gunnar T, Ariniemi K, Lillsunde P. Journal of Mass Spectrometry. 2005;40:739–753. doi: 10.1002/jms.846. [DOI] [PubMed] [Google Scholar]
- 2.Jagerdeo E, Abdel-Rehim M. Journal of the American Society for Mass Spectrometry. 2009;20:891–899. doi: 10.1016/j.jasms.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 3.White RE. Annual Review of Pharmacology and Toxicology. 2000;40:133–157. doi: 10.1146/annurev.pharmtox.40.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Chace DH, Kalas TA, Naylor EW. Clinical Chemistry. 2003;49:1797–1817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 5.Chace DH, Kalas TA. Clinical Biochemistry. 2005;38:296–309. doi: 10.1016/j.clinbiochem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter KH, Wiley V. Clinica Chimica Acta. 2002;322:1–10. doi: 10.1016/s0009-8981(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 7.He LM, Su YJ, Fang BH, Shen XG, Zeng ZL, Liu YH. Analytica Chimica Acta. 2007;594:139–146. doi: 10.1016/j.aca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Stolker AAM, Rutgers P, Oosterink E, Lasaroms JJP, Peters RJB, van Rhijn JA, Nielen MWF. Analytical and Bioanalytical Chemistry. 2008;391:2309–2322. doi: 10.1007/s00216-008-2168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambonin CG, Quinto M, De Vietro N, Palmisano F. Food Chemistry. 2004;86:269–274. [Google Scholar]
- 10.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Analytical Chemistry. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PJ. Clinical Biochemistry. 2005;38:328–334. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Dams R, Huestis MA, Lambert WE, Murphy CM. Journal of the American Society for Mass Spectrometry. 2003;14:1290–1294. doi: 10.1016/S1044-0305(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 13.Wong SF, Meng CK, Fenn JB. Journal of Physical Chemistry. 1988;92:546–550. [Google Scholar]
- 14.Yamashita M, Fenn JB. Journal of Physical Chemistry. 1984;88:4451–4459. [Google Scholar]
- 15.Horning EC, Horning MG, Carroll DI, Dzidic I. Stillwel.Rn. Analytical Chemistry. 1973;45:936–943. [Google Scholar]
- 16.Carroll DI, Dzidic I, Stillwell RN, Haegele KD, Horning EC. Analytical Chemistry. 1975;47:2369–2373. doi: 10.1021/ac60358a077. [DOI] [PubMed] [Google Scholar]
- 17.Whitehouse CM, Dreyer RN, Yamashita M, Fenn JB. Analytical Chemistry. 1985;57:675–679. doi: 10.1021/ac00280a023. [DOI] [PubMed] [Google Scholar]
- 18.Holcapek M, Jandera P, Zderadicka P, Hruba L. Journal of Chromatography A. 2003;1010:195–215. doi: 10.1016/s0021-9673(03)01030-6. [DOI] [PubMed] [Google Scholar]
- 19.Opiteck GJ, Lewis KC, Jorgenson JW, Anderegg RJ. Analytical Chemistry. 1997;69:1518–1524. doi: 10.1021/ac961155l. [DOI] [PubMed] [Google Scholar]
- 20.Jemal M. Biomedical Chromatography. 2000;14:422–429. doi: 10.1002/1099-0801(200010)14:6<422::AID-BMC25>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Gelpi E. Journal of Chromatography A. 1995;703:59–80. doi: 10.1016/0021-9673(94)01287-o. [DOI] [PubMed] [Google Scholar]
- 22.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Rapid Communications in Mass Spectrometry. 2005;19:2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 23.Maurer HH. Journal of Chromatography B. 1998;713:3–25. doi: 10.1016/s0378-4347(97)00514-8. [DOI] [PubMed] [Google Scholar]
- 24.Maurer HH. Analytical and Bioanalytical Chemistry. 2007;388:1315–1325. doi: 10.1007/s00216-007-1248-5. [DOI] [PubMed] [Google Scholar]
- 25.Balakrishnan VK, Terry KA, Toito J. Journal of Chromatography A. 2006;1131:1–10. doi: 10.1016/j.chroma.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Kammerer B, Frickenschmidt A, Muller CE, Laufer S, Gleiter CH, Liebich H. Analytical and Bioanalytical Chemistry. 2005;382:1017–1026. doi: 10.1007/s00216-005-3232-2. [DOI] [PubMed] [Google Scholar]
- 27.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang Z, Zhang XR. Analyst. 2010;135:659–660. doi: 10.1039/c003812c. [DOI] [PubMed] [Google Scholar]
- 29.Monge ME, Harris GA, Dwivedi P, Fernández FM. Chemical Reviews. 2013;113:2269–2308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 30.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 31.Cody RB, Laramee JA, Durst HD. Analytical Chemistry. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 32.Nemes P, Vertes A. Trac-Trends in Analytical Chemistry. 2012;34:22–34. [Google Scholar]
- 33.Haddad R, Sparrapan R, Kotiaho T, Eberlin MN. Analytical Chemistry. 2008;80:898–903. doi: 10.1021/ac701960q. [DOI] [PubMed] [Google Scholar]
- 34.Ifa DR, Manicke NE, Rusine AL, Cooks RG. Rapid Communications in Mass Spectrometry. 2008;22:503–510. doi: 10.1002/rcm.3377. [DOI] [PubMed] [Google Scholar]
- 35.Nemes P, Vertes A. Analytical Chemistry. 2007;79:8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- 36.Robichaud G, Barry JA, Garrard KP, Muddiman DC. Journal of the American Society for Mass Spectrometry. 2013;24:92–100. doi: 10.1007/s13361-012-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, Lanekoff IT, Laskin J, Dewald HD, Chen H. Analytical Chemistry. 2012;84:5737–5743. doi: 10.1021/ac300916k. [DOI] [PubMed] [Google Scholar]
- 38.Lu M, Wolff C, Cui WD, Chen H. Analytical and Bioanalytical Chemistry. 2012;403:355–365. doi: 10.1007/s00216-011-5679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilles JM, Connell TR, Durst HD. Analytical Chemistry. 2009;81:6744–6749. doi: 10.1021/ac900682f. [DOI] [PubMed] [Google Scholar]
- 40.Berchtold C, Meier L, Zenobi R. International Journal of Mass Spectrometry. 2011;299:145–150. [Google Scholar]
- 41.Peng IX, Loo RRO, Margalith E, Little MW, Loo JA. Analyst. 2010;135:767–772. doi: 10.1039/b923303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy JH, Wiseman JM. Rapid Communications in Mass Spectrometry. 2010;24:309–314. doi: 10.1002/rcm.4390. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Liu JJ, Cooks RG, Ouyang Z. Angewandte Chemie-International Edition. 2010;49:877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 44.Manicke NE, Yang QA, Wang H, Oradu S, Ouyang Z, Cooks RG. International Journal of Mass Spectrometry. 2011;300:123–129. [Google Scholar]
- 45.Wang H, Manicke NE, Yang QA, Zheng LX, Shi RY, Cooks RG, Zheng OY. Analytical Chemistry. 2011;83:1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JJ, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Analytical Chemistry. 2010;82:2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 47.Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. Journal of the American Society for Mass Spectrometry. 2011;22:1501–1507. doi: 10.1007/s13361-011-0177-x. [DOI] [PubMed] [Google Scholar]
- 48.Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, Ouyang Z. Analytical and Bioanalytical Chemistry. 2012;404:1389–1397. doi: 10.1007/s00216-012-6211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su Y, Wang H, Liu JJ, Wei P, Cooks RG, Ouyang Z. Analyst. 2013;138:4443–4447. doi: 10.1039/c3an00934c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang ZP, Cooks RG, Ouyang Z. Analyst. 2012;137:2556–2558. doi: 10.1039/c2an35196j. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Cooks RG, Ouyang Z. Analytical Chemistry. 2013;85:5632–5636. doi: 10.1021/ac401056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren Y, Wang H, Liu J, Zhang Z, McLuckey M, Ouyang Z. Chromatographia. 2013:1–8. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson AU, Talaty N, Cooks RG, Van Berkel GJ. Journal of the American Society for Mass Spectrometry. 2007;18:2218–2225. doi: 10.1016/j.jasms.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Espy RD, Manicke NE, Ouyang Z, Cooks RG. Analyst. 2012;137:2344–2349. doi: 10.1039/c2an35082c. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Cooks RG, Ouyang YZ. Canada: Vancover; 2012. [Google Scholar]
- 56.Lanekoff I, Thomas M, Carson JP, Smith JN, Timchalk C, Laskin J. Analytical Chemistry. 2013;85:882–889. doi: 10.1021/ac302308p. [DOI] [PubMed] [Google Scholar]
- 57.Roach PJ, Laskin J, Laskin A. Analyst. 2010;135:2233–2236. doi: 10.1039/c0an00312c. [DOI] [PubMed] [Google Scholar]
- 58.Van Berkel GJ, Kertesz V, Koeplinger KA, Vavrek M, Kong ANT. Journal of Mass Spectrometry. 2008;43:500–508. doi: 10.1002/jms.1340. [DOI] [PubMed] [Google Scholar]
- 59.Kertesz V, Van Berkel GJ. Journal of Mass Spectrometry. 2010;45:252–260. doi: 10.1002/jms.1709. [DOI] [PubMed] [Google Scholar]
- 60.Wilm MS, Mann M. International Journal of Mass Spectrometry. 1994;136:167–180. [Google Scholar]
- 61.Wilm M, Mann M. Analytical Chemistry. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 62.Huang GM, Li GT, Cooks RG. Angewandte Chemie-International Edition. 2011;50:9907–9910. doi: 10.1002/anie.201103687. [DOI] [PubMed] [Google Scholar]
- 63.Zhang ZP, Xu W, Manicke NE, Cooks RG, Ouyang Z. Analytical Chemistry. 2012;84:931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espy RD, Muliadi AR, Ouyang Z, Cooks RG. International Journal of Mass Spectrometry. 2012;325:167–171. [Google Scholar]
- 65.Yang SP, Ding JH, Zheng J, Hu B, Li JQ, Chen HW, Zhou ZQ, Qiao XL. Analytical Chemistry. 2009;81:2426–2436. doi: 10.1021/ac900063u. [DOI] [PubMed] [Google Scholar]
- 66.Salleras L, Dominguez A, Mata E, Taberner JL, Moro I, Salva P. Public Health Reports. 1995;110:338–342. [PMC free article] [PubMed] [Google Scholar]
- 67.Agency USEP, editor. 2013 http://water.epa.gov/drink/contaminants/basicinformation/atrazine.cfm.
- 68.Kruve A, Lamos A, Kirillova J, Herodes K. Proceedings of the Estonian Academy of Sciences. 2007;56:134–141. [Google Scholar]