Abstract
Regulation of cell functions by the physical properties of the extracellular matrix (ECM) has emerged as a crucial contributor to development and disease. Two specific physical properties of the ECM, stiffness and dimensionality, each influence cell signaling and function. As these ECM physical properties are linked to other properties that also regulate cell behavior, e.g., integrin ligand density, parsing the specific contributions of ECM stiffness and dimensionality has proven difficult. Here we detail a simple protocol, which can be completed in 1–2 d, for combining three-dimensional (3D) ECM engagement with controlled underlying ECM stiffness. In these ‘sandwich gels’, cells are sandwiched between a 3D fibrillar ECM and an ECM-coupled polyacrylamide gel of defined compliance, allowing the study of the specific effects of ECM compliance on cell function in physiologically relevant 3D ECMs. This type of system enables high-resolution time-lapse imaging and is suitable for a wide range of cell types and molecular perturbations.
INTRODUCTION
Cellular responses to dimensionality and compliance
Although soluble and diffusible guidance cues have long been known to regulate cell function, how cells respond to the physical attributes of the ECM—a process termed ‘ECM mechanosensing’—has now emerged as a mechanism equally important for controlling the functions of cells1–3. ECM compliance/stiffness and topology can influence cell signaling to drive major changes in cell morphology, cell-cycle progression and differentiation. Focal adhesions connecting the ECM to the actomyosin cytoskeleton enable cells to sense the ECM stiffness in a process that has been termed ‘compliance mechanosensing’4–6. In addition to ECM compliance, cells also demonstrate different morphological and migration responses to 2D versus 3D ECM engagement7,8. This topology-dependent phenomenon is referred to as ‘ECM dimensionality sensing’. Thus, compliance and dimensionality sensing induce major changes in cell signaling that modulate cell function.
The effects of altering ECM compliance on cell behavior are wide-ranging and include not only marked changes in differentiation9, cell proliferation10,11 and cell migration12, but also less obvious effects such as DNA uptake13, transcription14 and cell-cell adhesion15. These effects also include many effects that are of direct importance to human health, such as tumor cell progression and metastatic potential, which are enhanced by local matrix reorganization into more rigid substrata16–18. Thus, much effort has gone into the development of methods to control ECM compliance in cell-culture models, some of which have been based on using compliant elastic polyacrylamide gels as substrate material (as opposed to glass or plastic) to which ECM molecules can then be directly and covalently cross-linked19. These culture systems enable not only specification of ECM stiffness in a physiologically relevant range but also allow dynamic quantification of localized force applied by the cell to the underlying matrix12,20.
Dimensionality sensing also produces marked cellular changes. For example, epithelial-cell differentiation can be induced by 3D ECM engagement21. Yarmush and colleagues have demonstrated a range of cell responses to 3D ECM engagement relative to 2D cultures, including altered hepatocyte metabolism and transcriptional regulation22,23. Migrating cell morphology and directional persistence are markedly altered in 3D culture systems compared with standard 2D cultures24–26. In direct comparison studies with 2D ECMs, engagement of the dorsal cell surface by a second planar substrate of either synthetic or collagen gels was sufficient to alter cell morphology and migration in motile cells7,27,28, and had profound effects on the cytoskeletal organization29 and dynamics30, some of which appear to approximate those observed in cells in native 3D environments31.
Given this range of responses of cells to 3D environmental cues, much effort has gone into creating 3D culture environments for both basic and applied research32,33. In fact, cell responses to 3D substrates are so widely and demonstrably altered relative to the responses in conventional 2D cultures that many in vitro small-molecule assays and drug-discovery platforms have been amended to use 3D cultures to improve their relevance to in vivo models34,35. Recent studies using microfabricated channels of defined stiffness show that tumor cells exhibit enhanced migration when they experience not only optimal substrate stiffness but also an optimally confined channel in which to migrate36. This suggests that a high degree of spatially organized contact can enhance the response to substrate compliance. Microengineered culture platforms can mimic 3D tissue properties so well that higher-order properties of differentiated cells are induced, allowing testing in ‘organ-on-a-chip’ cultures to potentially replace some testing in animals33,37. Other methods that have been used to generate 3D environments for cultured cells, such as synthetic substrates with modification to add integrin ligands or proteolytic cleavage sites38, biodegradable polymers (e.g., polylactic acid39–41) and hydrogels made with fibrin, hyaluronan or collagen42,43, as well as cell-derived matrices44, all hold promise for specific applications as well.
Although the above-mentioned 3D culture systems have some unique advantages, most have a common drawback for understanding mechanosensitive biological processes. Given that the two ECM properties, compliance and dimensionality, are both sensed by cells, and that these properties are both linked to one another by the physical structure of the ECM, it is difficult to parse which property specifically induces a given cell response or signal. Under most circumstances, if ECM stiffness is increased by increasing the density of cross-links or components in the matrix, other parameters such as pore size and/or the density or spacing of adhesion sites is concomitantly altered45. Furthermore, although 3D polymers or 3D collagen gels may be manipulated to alter their stiffness or compliance by cross-linking43,46, the dynamic range of stiffness regimes of such manipulations is small, such that it may not elicit different responses in some cell types. Nevertheless, it is important to note that all 2D and 3D culture environments have various strengths and drawbacks in terms of technical difficulty, biological relevance, accessibility for imaging and cost as well as specific amenability for a given investigation. The particular culture system chosen will generally be a compromise between these traits that best suit the biological question at hand.
Here we describe the use of simple hybrid ‘sandwich gels’ that allow high-resolution live-cell imaging with control of the ECM stiffness the cell experiences while maintaining 3D engagement with a fibrillar collagen ECM of constant concentration and density. We provide an overview of the experimental design and then cover in detail the basic components of sandwich gels that must be carefully controlled for optimal results, including the compliant polyacrylamide substrates, the 3D ECM and the live-cell imaging chambers for containing the sandwich gels during microscopic observation. We also discuss the limitations of this approach and alternative approaches for studying ECM compliance/stiffness stimuli in 3D cell environments. Finally, we provide a detailed reagent list, a protocol and the expected results. The protocol described here is based on published methods used in the authors’ laboratories, and has been used to study the relative contributions of mechanosensing and dimensionality to cytoskeletal dynamics30,47. The exact protocol may be adapted in several ways to meet other specific needs, some of which we discuss throughout the protocol.
The sandwich-gel approach
Sandwich gel cultures use a deformable planar substrate bound to a glass cover slip as a solid support, with cells attached to the deformable substrate surface and overlaid with an additional matrix such that the cells are ‘sandwiched’ to encounter deformable matrices on both their ‘ventral’ and ‘dorsal’ surfaces. Approaches using either polyacrylamide sandwiches7,27 or collagen sandwiches23,29 have been used for many years to approximate 3D cell environments. To parse the contributions of ECM compliance and ECM dimensionality to cell behavior and function, hybrid sandwich gels (from here on referred to simply as sandwich gels) have been developed more recently. These hybrid sandwich gels fuse the optimal mechanical and optical properties of polyacrylamide gels with the 3D ECM engagement induced by 3D ECMs30,47. Sandwich gels allow a wide range of defined and reproducible substrate stiffness regimes to be encountered by the cell ventrally while maintaining dorsal and lateral engagement of integrin receptors with ECM molecules, thus providing effectively 3D ECM engagement, all in proximity to a microscope cover glass to allow high-resolution live-cell imaging. In addition, one can directly determine the effects of 2D ECM versus 3D ECM engagement at a given ECM stiffness by comparing cell function on 2D ECM-coupled polyacrylamide gels with cells in sandwich gels of the same stiffness30. Sandwich gels are made with inexpensive ordinary cell biology lab supplies, are straightforward to set up, allow for exchange of small molecules and media and are compatible with other techniques such as traction-force microscopy and micromanipulation. Finally, sandwich gels are also amenable to culturing a wide range of cell types, including primary neuron and glial cocultures, primary endothelial cells (ECs) from aortic tissue slices and more robust transformed cells and fibroblasts. Notably, the stiffness of the underlying polyacrylamide gel can tuned to match the responsive or physiologically relevant range of any of these diverse cell types while maintaining 3D ECM engagement. Here we describe a detailed protocol for preparing sandwich gels for use in live-cell imaging, based on our previously published methods30,47.
Potential power of sandwich gels for current questions in cell biology
The primary advantage of the sandwich-gel approach is that it allows high-resolution imaging of cell responses to 2D and 3D ECMs with specified stiffness. Although this strength has been mostly used for live-cell imaging, sandwich gels are also excellent for fixed-cell immunostaining. Given these advantages for imaging in 3D environments, the sandwich gel technique has been used to directly investigate important questions related to the regulation of cytoskeletal dynamics and EC morphology30,47. Physical interactions between ECs and their in vivo 3D ECMs have long been known to drive modification in EC shape and behaviors, including the development of spindle-shaped morphologies, the elaboration and retraction of branched protrusion and angiogenic sprouting and migration48,49, all of which are readily recapitulated by ECs cultured in sandwich gels. The sandwich-gel approach has been validated in experiments that verified previous studies of compliance mechanosensing and expieriments have highlighted the advantages of performing high-resolution imaging in 3D ECMs while maintaining the advantages of a substratum with tunable compliance. This technique has revealed that compliance mechanosensing functions synergistically with increasing ECM dimensionality to enhance EC branching frequency, a result uniquely quantifiable using the sandwich-gel approach47. In addition to cell-morphological assays, regulation of cell signaling can also be investigated using the sandwich-gel technique. Investigations of actomyosin contractility have identified that contractility via Rho/ROCK-mediated myosin II activity and/or ECM stiffness inhibits EC branching by blocking both branch initiation and branch retraction after their successful initiation47. Investigation of microtubule dynamics has shown that increasing the frequency of EC branching by both compliance and dimensionality mechanosensing is coupled to an increase in the growth speed and an increase in the catastrophe frequency of dynamic microtubules30. Notably, inhibiting the response of either actomyosin or microtubule dynamics to ECM compliance or dimensionality prevents directional EC migration. Thus, studies using the sandwich-gel approach have answered questions that previously had not been testable using traditional 2D or 3D cell culture techniques.
Because the sandwich-gel technique enables high-resolution imaging of living cells in culture, it is now possible to examine subcellular protein dynamics and molecular mechanisms that respond to either the compliance or the dimensionality of the extracellular environment. Some key experimental applications for this methodology include investigations of molecular mechanisms responsible for stem-cell differentiation, by tuning the stiffness of the sandwich gel to direct stem-cell differentiation to a specific lineage50,51. Conversely, highly differentiated cell types such as neurons can be cultured in sandwich gels to gain insight into how axon initiation and branching, as well as growth-cone navigation, are regulated by physiological stiffness within a 3D-like environment. These experiments are important because neuronal adhesion to the ECM is known to use focal complexes on a 2D glass substrate, where they are essential for growth-cone guidance52–56. However, when the same neurons are cultured on soft substrates, both the regulatory signaling pathways and the protein content of focal adhesions are modified, as is the ability of the growth cone to migrate toward specific signaling targets57,58, raising the question of which focal adhesion components are used to mediate physiological growth cone guidance. How these mechanotransduction signals are altered by 3D integrin engagement is not yet well understood but may be approached at least in part through the use of sandwich gels.
Investigations of focal adhesion dynamics and how they relate to ECM topology have intensified in recent years, partly because of the efforts to create more physiologically relevant in vitro culture systems coupled with live-cell image analyses (e.g., see ref. 59). In standard 2D cell culture on effectively infinitely stiff substrates, focal adhesions can develop into multiple classes of size and composition (reviewed in ref. 60). In so-called ‘1D’ ECMs, focal adhesions appear to be limited to adopt the morphology or conformation of the linear matrix61. Which of these organizations is more similar to 3D ECMs remains a topic of investigation59,62,63. Given that the dynamics and organization of focal adhesions therefore likely depend on both topology of the matrix and its mechanical properties, sandwich gels may be an excellent additional tool for understanding focal adhesions in more complex physical environments. In summary, the sandwich-gel technique is a method to visually and quantitatively address experimental questions about mechanobiology with mechanistic studies using high-resolution microscopy, and to test hypotheses in physiologically relevant cell-culture systems that, to date, have not been possible to investigate using state-of-the-art in vivo microscopy techniques.
Limitations of the approach
Although the sandwich-gel approach is optimal for definition of the ECM compliance and 3D integrin engagement, there are some limitations and caveats to be considered. Cells at the polyacrylamide–collagen interface will engage collagen on their ventral surface that is covalently coupled to the polyacrylamide of a defined stiffness, but also engage dorsal collagen in the 3D ECM. Because the polyacrylamide gel is much stiffer than the collagen or other ECM on top (collagen gel is ~80–200 Pa), the cells in sandwich gels experience anisotropic stiffness28. However, it has been shown that the cell mechanosensing response is dominated by the maximum stiffness encountered by the cells12,28,64, which in the case of sandwich gels will be that of the collagen coupled directly to the polyacrylamide. In sandwich cultures in which there are substantial differences between stiffness of the upper and lower gel, myosin II contractility causes cells to adopt elongated shapes in response to the stiffer matrix, whereas in homogeneous soft matrices cells adopt a more spherical shape dominated by a cortical cytoskeleton28. For this reason, it is usually preferable to operate near the lowest stiffness range that the cells optimally respond to, so that the difference between the polyacrylamide and collagen gel stiffness is minimized. It should be made clear here that, although these sandwich gels represent an excellent intermediate between standard 2D culture and fully native 3D or in vivo environments, cell geometry will be limited by the relatively planar polyacrylamide that cells cannot modify. Although this does limit true 3D migration, it also allows a more physiologically realistic substratum that is optimal for high-resolution imaging.
Other approaches
Other culture systems can yield both 3D ECM engagement and provide tunable stiffness. These range from the simple, straightforward use of two apposing cover slip–supported, ECM-coupled polyacrylamide gels to engage both dorsal and ventral sides of the cell7,27 to more sophisticated microfabricated culture platforms that can be engineered to induce 3D ECM engagement while allowing modulation of stiffness36,37,41. The former technique is simple, requires only common lab supplies, and can provide an equal mechanical input from both dorsal and ventral sides of the cell. However, this approach does not allow for adequate gas exchange or facilitate exchange of media, making long-term culture (as may be required for some primary or explant cultures) unfeasible. Meanwhile, although microengineered surfaces can be designed to allow constant exchange of media and gas as well as small-molecule delivery, they require very specialized equipment and expertise, as well as considerable cost and time to optimize and implement.
Experimental design
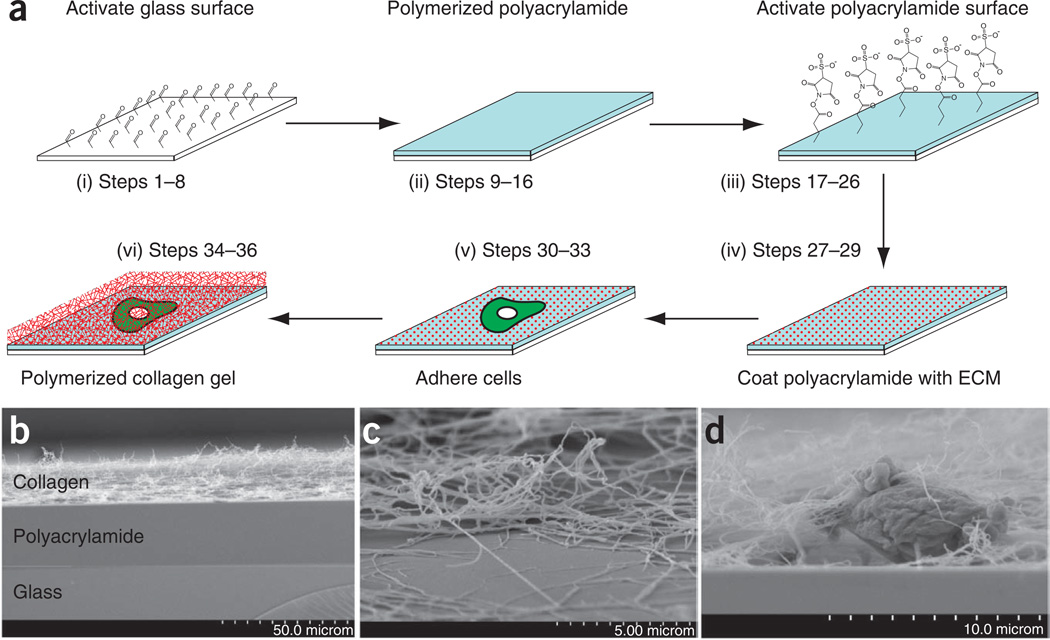
The basic design of the hybrid sandwich described here is to bind a polyacrylamide gel of defined compliance to a standard microscope cover slip and covalently cross-link to the polyacrylamide gel an ECM that is suitable to adhere the cell type in question (Fig. 1a). Cells are plated and allowed to attach to the ECM-coupled polyacrylamide gel for as long as necessary. Next, the culture medium is removed and replaced with neutralized, unpolymerized collagen, which is then allowed to polymerize on top of the cells to form a fibrillar 3D ECM. The collagen ECM can contain other ECM molecules (e.g., fibronectin, laminin) as needed for the specific cell type, or it can be another biologically relevant multicomponent ECM (e.g., Matrigel). Once this upper ECM has formed, culture medium is added and exchanged as needed. For live-cell imaging applications, the cover slip with attached ECM-coupled polyacrylamide (before addition of cells) is mounted into either a modified culture dish or another similar chamber (e.g., Rose chamber65). Thus, the cells are sandwiched within an ECM whose local compliance is determined by that of the polyacrylamide gel but will have integrin engagement defined by the ECM used. The procedure comprises four basic sections, which are separated by convenient pause points: (i) cover slip activation by glutaraldehyde; (ii) polyacrylamide sheet preparation; (iii) cross-linking of ECM molecules to the polyacrylamide sheet; and (iv) plating of cells and formation of upper collagen gel overlay (Fig. 1a). To facilitate successful execution of the following protocol, we discuss some key aspects of the methodology and highlight their advantages and potential difficulties.
Figure 1. Assembly of sandwich gels.
(a) Schematic of sandwich gels. (i) Silanized glass cover slips are activated with glutaraldehyde. (ii) A 20–25-µm-thick polyacrylamide gel (light blue) is then polymerized on top of the activated glass. (iii) The surface of the polyacrylamide gel is activated with sulfo-SANPAH, wherein the photoactivatable nitrophenyl azide group covalently bonds the polyacrylamide, whereas the NHS ester group is available to bond to free amines in the ECM proteins (red dots). Cells (green) are then allowed to adhere as for normal culture, the supernatant medium is removed and a collagen gel (red cross hatch) is polymerized on top of the cells and polyacrylamide. (b) Scanning electron microscopy (SEM) of cross-section of sandwich gels. Sandwich gels were fixed as described and critical point dried for SEM, and then the back of the cover slip was etched with a diamond pen and broken to reveal the cross-sectional area of the sandwich gel. (c) SEM of collagen fibers from the polymerized gel and attached to the polyacrylamide surface. Shown is an area where the bulk of the collagen gel has retracted during fixation, illustrating that a portion of the collagen gel remains closely associated with the polyacrylamide. (d) Cross-sectional area of a sandwich gel in SEM showing the posterior side of the cell embedded within the collagen and interacting with the polyacrylamide. Note small set of collagen fibers being condensed by dorsal surface of cell.
Polyacrylamide substrates
Polyacrylamide has been used for many years as a compliant substrate for attachment of ECM19. It is optically clear, colorless and has a similar refractive index to that of water. It is linearly elastic and can be easily and reproducibly prepared to exhibit a wide range of well-defined compliances (see Table 1 and ref. 66). Finally, polyacrylamide is biochemically inert, allowing cells to specifically engage only the ECM that is covalently coupled to its surface. Nevertheless, when using a polyacrylamide gel as the bottom layer of the sandwich gel, some aspects of polyacrylamide must be kept in mind for optimal cell health and behavior. First, as polyacrylamide is uncharged and does not react with proteins, cells will not adhere directly to polyacrylamide surfaces. Therefore, care must be taken with respect to the concentrations and identities of ECM protein(s) that are cross-linked to the polyacrylamide, such that the cells can interact with the gel. Second, unpolymerized acrylamide is highly toxic to many cell types, and therefore it is not advisable to shorten wash times once the polyacrylamide gel has been formed; overnight incubations in large volumes of buffer are optimal. Third, it has been shown that cells will ‘feel’ and respond to a stiffer substrate beneath a softer one if the soft substrate is sufficiently thin67. Thus, although it may be advantageous for some microscopy methods to make the polyacrylamide gel as thin as possible, making the polyacrylamide gel thinner than ~20 µm may allow the cells to detect the stiffness of the microscope cover slip beneath it and therefore give less reliable results with respect to mechanosensing of the compliant polyacrylamide. The protocol described here typically yields polyacrylamide gels that are ~25–50-µm thick, thus allowing the reliable compliance responses while being as thin as practical for imaging purposes.
Table 1.
Polyacrylamide shear modulus table.
| Acrylamide (%) | Bis-acrylamide (%) | Shear modulus (kPa) |
|---|---|---|
| 5 | 0.05 | 0.43 |
| 0.075 | 0.6 | |
| 0.1 | 1.431 | |
| 0.15 | 1.64 | |
| 0.2 | 2.02 | |
| 7.5 | 0.01 | 0.233 |
| 0.03 | 0.689 | |
| 0.05 | 1.535 | |
| 0.075 | 2.286 | |
| 0.1 | 2.833 | |
| 0.15 | 4.069 | |
| 0.2 | 5.356 | |
| 0.3 | 8.64 | |
| 12 | 0.145 | 16.344 |
| 0.28 | 30.067 | |
| 0.45 | 34.263 | |
| 0.55 | 42.375 | |
| 0.575 | 50.873 | |
| 0.6 | 55.293 |
Shear moduli values for polyacrylamide gels at 25 °C as determined by a cone/plate rheometer at < 10% strain; see also ref. 66. Percentages given are final dilutions of either acrylamide or bis-acrylamide to generate the desired stiffness (shear modulus).
Methods other than those described here exist to bind the polyacrylamide gel to the cover slip and to cross-link the ECM protein (collagen) to the polyacrylamide gel68. For example, in place of the (3-aminopropyl)triethoxysilane, other investigators have used allyltrichlorosilane as a one-step method to activate the glass for binding polyacrylamide67. These methods also have certain potential hazards: for example, allyltrichlorosilane may react violently with water; therefore, the appropriate references must be read carefully before making substitutions to the protocol detailed here.
Chamber dishes
For cultures that are to be used in live-cell imaging, home-made chamber dishes can be prepared by drilling (with a hole saw) or cutting (with a hot scalpel or Dremmel-type device) holes into the bottoms of 35-mm tissue-culture dishes. The perimeter of the hole on the bottom of the dish is then lined with a bead of vacuum grease, and the cover slip with ECM-coupled polyacrylamide is mounted onto the bottom of the dish with the ECMcoated gel facing up into the dish. The edges around the bottom of the cover slip are then sealed with Valap. Similar results could be achieved by replacing the existing premounted cover slip in commercial dishes (e.g., MatTek) with the polyacrylamide-bound cover slip. More sophisticated chambers such as ‘Rose chambers’ are ideal for long-term cultures65,69. Either type of chamber should be sterilized by ethanol wash and air-dried before mounting polyacrylamide- modified cover slips. Alternatively, simple slide chambers can be made at the time of imaging using nothing more than Parafilm or double-stick tape spacers and Valap70.
Collagen ECM
Fibrillar collagens such as collagen I are excellent for forming biologically active ECM as the top layer of the sandwich gel, as their mechanical and cross-linking properties are well-understood71 and they can serve as molecular scaffolds or templates for other ECM proteins including other collagens (e.g., collagen IV), fibronectin and several proteoglycans72. In choosing a source of collagen I, nonpepsinized native sources that maintain the telopeptides (e.g., rat-tail collagen I) may be preferable for many cell types. Intact telopeptides enable collagen I polymers to be stabilized via hydroxylation and cross-linking between the telopeptide and triple helical regions of adjacent molecules18,73,74.
To minimize distress to cells, collagen I ECMs are prepared with concentrated media (e.g., 10× MEM) to a final concentration of 1×. Collagen I solutions are highly acidic; as the pH and temperature of the solution is raised, polymerization is favored. Therefore, when preparing collagen ECMs, it is advisable to use buffers or media with a pH indicator dye such as phenol red to ensure optimum pH. Phenol red can be omitted from subsequent media and buffers to reduce autofluorescence during imaging. For very fragile cell types such as neurons, serum or other growth factors can be included in the ECM recipe; however, these factors should be added after neutralization of the solution. As mentioned above, in addition to collagen I, other biological ECMs such as Matrigel can be used for the upper layer of the sandwich gel, so long as it normally supports spreading and/or migration of the cell type in question. In these cases, a diluted solution of such ECM mixtures should also be used to covalently couple to the polyacrylamide gel to maintain a consistent cell environment.
Cell treatments
Sandwich gels are generally compatible with most cell treatments such as plasmid transfection and treatments with small molecules and/or drugs as well as small interfering RNA (siRNA). Expression constructs for live fluorescence imaging should be transfected at appropriate times before initial plating onto coated polyacrylamide and collagen overlay. At this time, cells that do not adhere within 1–2 h can be removed by rinsing with medium. However, for transfection techniques or expression vectors that can induce substantial cell death over a period longer than 1–2 h (e.g., some cytoskeletal protein expression constructs), preplating of transfected cells in standard culture to select for viable cells before culturing in sandwhich gels is recommended, as removal of dead cells will not be possible after collagen gel is polymerized over cells. Similar considerations should be applied to siRNA treatment of cells before plating into sandwich gels.
Drug treatments are also quite feasible in sandwich gels, but as the cells are covered with an upper ECM gel exchange or introduction of small molecules or growth factors will occur more slowly than with liquid medium alone. Many small molecules (e.g., Taxol30) can be observed to have a visible effect on the cells within 5 min of addition to the supernatant medium, depending on dosage and composition, but this should be determined empirically for any experimental condition. It should also be kept in mind that native collagen and many other ECM proteins bind to growth factors, which may alter their delivery in these cultures. Similar considerations should be made for subsequent manipulations of the cells such as fixation and immunostaining; incubation and wash times should be increased as necessary.
MATERIALS
REAGENTS
Acrylamide solution (40%; Bio-Rad, cat. no. 161-0140); this solution can be stored at 4 °C for up to 6 months ! CAUTION Acrylamide is a potent neurotoxin. Avoid contact and wear appropriate safety gear when handling solutions.
Bis-acrylamide solution (2%; e.g., Bio-Rad, cat. no. 161-0142); this solution can be stored at 4 °C for up to 6 months.
Ammonium persulfate (APS; e.g., Sigma-Aldrich, cat. no. A3678)
Tetramethylethylenediamine (TEMED; e.g., Bio-Rad, cat. no. 161-0800)
HEPES (Sigma-Aldrich, cat. no. H4034)
Phosphate-buffered saline, pH 7.4 (PBS; do not substitute with buffer containing amines) (Invitrogen, cat. no. 10010-023; http://products.invitrogen.com/ivgn/en/US/adirect/invitrogen?cmd=catProductDetail&productID=10010023)
PBS, 10× (Invitrogen, cat. no. 70011-044; http://products.invitrogen.com/ivgn/en/US/adirect/invitrogen?cmd=catProductDetail&productID=70011044)
Glutaraldehyde stock, 25% (wt/vol) (Electron Microscopy Sciences, cat. no. 16200) ▲ CRITICAL Prepare fresh each time.
Collagen I, high concentration (> 6 mg ml−1; e.g., BD Biosciences, cat. no. 354249) ▲ CRITICAL High-concentration collagen must be used; do not substitute with lower-concentration collagen stocks (> 6 mg ml−1); insufficient dilution of acids and salts, and poor gelation may result in poor cell health.
NaHCO3, 7.5% (wt/vol), cell culture–tested, sterile (Invitrogen, cat. no. 25080-094)
Cell culture media, 10× (e.g., 10× MEM; e.g., Gibco, cat. no. 11430)
(3-Aminopropyl)triethoxysilane (e.g., Sigma-Aldrich, cat. no. A3648) ! CAUTION This reagent is toxic, and high concentrations can degrade polycarbonate and polystyrene (e.g., disposable pipettes, Petri dishes and so on).
Sulfo-SANPAH (Thermo Scientific, cat. no. 22589) ▲ CRITICAL The reagent is temperature, oxidation and light sensitive. Thus, store it appropriately at all times as suggested by the supplier and use fresh aliquots of prepared stock solutions each time (see Reagent Setup).
ddH2O
NaOH pellets (Sigma-Aldrich, cat. no. S8045)
DMSO (Sigma-Aldrich, cat. no. D2650)
Ethanol
EQUIPMENT
Petri dishes (10 or 15 cm)
Parafilm
Acid-washed glass cover slips or ‘Squeaky Clean’ cover slips75; no. 1.5, 22 mm × 22 mm, stored in 100% ethanol
Glass slides (Fisher Scientific, cat. no. 12-544-1)
Disposable 15-ml conical tubes
Hydrophobic silicone spray (e.g., Rain-X original formula)
Low-lint wipes (e.g., KimWipes)
UV light chamber with light emission between 300 nm and 420 nm (e.g., Stratalinker 2400; Spectroline, cat. no. XL-1000A)
Stainless steel cover slip rack to fit cover slips of choice (e.g., Electron Microscopy Sciences, cat. no. 72239-04) Note: these racks are not absolutely necessary, but make changing solutions and manipulating the cover slips much easier.
Glass beaker or staining box (e.g., Electron Microscopy Sciences, cat. no. 70312-21) to fit the cover glass rack
Desiccator
Low-temperature oven or incubator (50 °C)
Stir plate and small magnetic stir bar
Optional for live-cell imaging
35 mm Tissue culture dishes or Rose chambers
Paraffin wax (Sigma-Aldrich, cat. no. 327204)
Vaseline (Sigma-Aldrich, cat. no. 16415)
Lanolin (Sigma-Aldrich, cat. no. L7387)
REAGENT SETUP
0.5 (wt/vol) Glutaraldehyde in PBS
The most consistent results will be obtained using premade 25% (wt/vol) stock solutions (e.g., Electron Microscopy Sciences, cat. no. 16200) and 10× PBS diluted with ddH2O to a final concentration of 1× PBS/0.5% glutaraldehyde. ! CAUTION Steps with glutaraldehyde should be performed in a fume hood. Dispose of glutaraldehyde waste in accordance with local safety and waste management guidelines. ▲ CRITICAL This solution should be prepared fresh each day of use.
HEPES buffer, 1 M
Dissolve 238.4 g of HEPES in 750 ml of ddH2O. Add NaOH pellets (~5.5 g) to adjust the pH to 7.5. Bring the solution to a final volume of 1,000 ml, and filter it through a 0.2-µm filter. Store the buffer at room temperature (22–25 °C) for up to 1 year.
HEPES, 50 mM, pH 7.5
Dilute 200 ml of 1 M HEPES solution with 80 ml of ddH2O. The solution can be stored at room temperature for several months.
Valap
Mix together equal parts (by weight) of Vaseline, lanolin and paraffin wax in a pyrex beaker; heat the mixture slowly over low heat until it melts, stirring with a heat-resistant spatula or tongue depressor (use heat setting of 2 or 3 out of 10 on most stir plates). Be careful not to overheat the mixture. When the mixture becomes homogeneous, pour aliquots into smaller pyrex beakers, and allow it to cool. For use, reheat the aliquot to melt, taking care not to overheat it. Use as a sealant around the edges of the cover slip as indicated in the text. Valap can be stored cool indefinitely.
(3-Aminopropyl)trimethoxysilane, 0.5%
(3-Aminopropyl)trimethoxysilane (0.5%) diluted in ddH2O should also be made fresh, by using stock solutions of > 97% (3-aminopropyl)trimethoxysilane. ! CAUTION It should be used in a fume hood, with appropriate safety gear. This solution can rapidly degrade polycarbonate and polystyrene, especially in concentrated stocks. Concentrated solutions are best handled with glass pipettes and/or measuring vessels. ▲ CRITICAL A 0.5% solution must be made fresh before use each time.
Sulfo-SANPAH
Sulfo-SANPAH stocks should be made with DMSO at a concentration of 25 mg ml−1. Distribute 40-µl aliquots into 1.5-ml tubes and flash-freeze them in liquid nitrogen or ethanol and dry ice bath. Store the aliquots at −80 °C for up to 1 year.
APS (10% (wt/vol))
APS (10% (wt/vol)) should be prepared fresh (Step 9), but it may be used for 72 h if stored at 4 °C, or for 1 month at –20 °C. Freeze-thawing cycles should be avoided. A volume of 1 ml of 10% APS is sufficient for several cover slips.
Polyacrylamide gel mixture (Step 11)
When choosing the amount of polyacrylamide mixture to prepare, 5 ml will be more than enough to prepare several (6–10) cover slips. It is advisable to divide this solution into two ~2.5-ml portions in separate 15-ml conical tubes before adding TEMED and APS, if more than four to six cover slips are desired. Ideally, the acrylamide– bis-acrylamide mixture should be degassed before the initiation of polymerization by the addition of redox activator (TEMED) and redox initiator (APS), as the presence of oxygen is known to create an inhibition period. The most homogeneous gels are obtained with the fastest polymerization76. Although the relatively high concentrations of TEMED and APS in this protocol make degassing unnecessary, introduction of oxygen to the solution by aeration should still be avoided. The solution must be made fresh from individual reagents each time. Keep in mind that very soft gels (shear modulus < 0.7 kPa) are fragile and should be handled with care during manipulations. Even with appropriate care, soft gels will often display some ruffling at the edge of the cover slip, which is normal (Supplementary Fig. 1).
EQUIPMENT SETUP
UV light chamber
The UV-light chamber should be set to deliver 7,500 J. Place a small support (e.g., pipette tip box or a similar small box) inside the chamber to make the distance from the lamps to the substrates ~8 cm.
PROCEDURE
Cover slip activation ● TIMING 3 h
-
1|
Dry the cover slips by carefully flaming off ethanol.
-
2|
Place the cover slips in a stainless steel rack, being careful not to crack the edges of cover slips.
-
3|
Soak the cover slips in 0.5% (3-aminopropyl)trimethoxysilane (in ddH2O) at room temperature for 30 min. The use of a stir bar and stir plate will ensure even activation, although this can also be accomplished with occasional gentle agitation of the rack in the solution.
-
4|
Wash cover slips by immersing the rack in six changes of distilled H2O. When resubmerging the rack, be careful not to disturb the cover slips in the rack.
-
5|
Remove the rack from water, blot off the bottom on a paper towel, and dry the rack in an oven (~30 min, 50 °C).
-
6|
Cool the rack to room temperature. While cooling, prepare 0.5% glutaraldehyde in 1× PBS (see Reagent Setup).
-
7|
Immerse the rack in glutaraldehyde–PBS solution for 30 min at room temperature, again stirring or agitating to prevent bubble accumulation.
-
8|
Wash the rack in three changes of distilled H2O, and then air-dry on a bench.
■ PAUSE POINT Store the activated cover slips in a rack in a desiccator for up to 2 months.
Preparing the polyacrylamide substrate ● TIMING ≥1 h
-
9|
Prepare 10% APS solution in ddH2O (see Reagent Setup).
-
10|
Polish two to four glass slides with KimWipes soaked with Rain-X solution, thus making the surface hydrophobic. It is easiest to polish both sides of the slide. Put the slides into a coplin jar or slide rack and rinse well with distilled water. Drain the slides and allow them to air-dry.
-
11|
Mix together acrylamide, bis-acrylamide, 1 M HEPES and ddH2O to produce the desired gel stiffness (see Table 1; see also Reagent Setup) in a 15-ml conical tube. Mix thoroughly by inversion; vigorous vortexing is not recommended because introducing oxygen to the gel mixture can impede polymerization.
-
12|
Place one or two of the treated glass slides into a 15-cm Petri dish.
-
13|
Working quickly, for each 2.5 ml of polyacrylamide mixture add 10 µl of TEMED, mix briefly, add 15 µl of APS and mix briefly and distribute three 12-µl drops of the mixture onto one of the treated glass slides. Space the drops about 1 inch apart. Gently place one of the activated cover slips onto each of the three drops, positioning the cover slip such that one edge is slightly overhanging the edge of the slide and ensuring that the drop spreads evenly with no bubbles. Repeat this process for another set of cover slips on another treated glass slide.
▲ CRITICAL STEP The polyacrylamide in small-volume solutions will polymerize very rapidly; therefore, the number of cover slips that can be set up with a single solution of activated polyacrylamide (containing APS and TEMED) will be limited. When you first attempt the protocol, do no more than 3 or 4 cover slips at a time.
-
14|
Allow the acrylamide mixture to polymerize at room temperature for 20 min. During this time, prepare a 50 mM HEPES, pH 7.5, solution (see Reagent Setup).
-
15|
After polymerization is complete, flood the surface of the slides and cover slips with 50 mM HEPES buffer.
-
16|
Remove the cover slips from the slides by first applying gentle lateral pressure to the overhanging edge of the cover slip with forceps; this will dislodge the cover slip with attached polyacrylamide from the slide to leave one edge free in buffer. Gently lift the cover slip up and submerge the polyacrylamide side up in fresh 50 mM HEPES buffer in another Petri dish. Repeat for all cover slips (can be placed together in fresh HEPES buffer, polyacrylamide side up). Label the dish with the polyacrylamide stiffness. Rinse well (four or five exchanges) with 50 mM HEPES.
? TROUBLESHOOTING
■ PAUSE POINT Cover slips/gels can be stored in 50 mM HEPES at 4 °C for 2–3 weeks.
Cross-linking ECM to the polyacrylamide sheet ● TIMING 30 min, followed by overnight incubation
-
17|
Thaw a 40-µl aliquot of sulfo-SANPAH at room temperature, add 960 µl of ddH2O and mix well. Place a fresh sheet of Parafilm onto a support (glass plate, Petri dish lid or similar).
-
18|
Prepare a solution containing an appropriate concentration of ECM molecule, usually 100 µg ml−1 in PBS. The pH of this solution must be 7.4–7.8 for optimal cross-linking. Keep the solution on ice.
▲ CRITICAL STEP The pH of the solutions can be verified by the addition of a small amount of phenol red–containing solution (e.g., culture medium without serum). Add just enough to be able to see color in the solution. If the color is pink, it will be in the optimal range. If not, adjust the color to pink with small additions of NaHCO3 or NaOH.
-
19|
Using forceps, take a cover slip with polyacrylamide gel and wick off excess buffer with a KimWipe. Do not allow the gel to dry. Place the cover slip on a Parafilm sheet supported by a Petri dish bottom, polyacrylamide side up. The Parafilm will help prevent the cover slip from sliding around during handling, and will prevent wicking of the sulfo-SANPAH solution away from the cover slip surface.
-
20|
Add 200 µl of sulfo-SANPAH to the polyacryladmide surface.
-
21|
Repeat Steps 19 and 20 for additional cover slips.
-
22|
Place polyacrylamide gels with sulfo-SANPAH in a UV light cross-linker. For most commercial cross-linkers, follow instructions to deliver ~7,500 J of energy. If you are using short-wavelength (300–350 nm) UV light lamps, placing the gels 8 cm away from two 15-W bulbs for 6–8 min will suffice. Sulfo-SANPAH will darken over this time.
-
23|
Dip cover slip with polyacrylamide gels one at a time in a large volume (~300 ml) of PBS to rinse, and remove excess buffer. Replace cover slips on a Petri dish–supported Parafilm.
-
24|
Repeat Steps 19–22.
-
25|
During the second UV-light activation step, place a second piece of Parafilm onto another support. Pipette 75-µl drops of ECM solution onto the Parafilm, one for each cover slip.
-
26|
Remove excess sulfo-SANPAH solution from polyacrylamide-bound cover slips, dip them into PBS to rinse and remove excess buffer. Wipe excess liquid from the back of the cover slips and invert (polyacrylamide side down) onto drops of ECM solution.
▲ CRITICAL STEP Step 26 should be performed as rapidly as possible, as the half-life of the NHS ester (N-hydroxysuccinimide) in sulfo-SANPAH is likely < 20 min at neutral pH. From this point onward, coverslips and gel assemblies should be handled and kept under sterile conditions.
-
27|
Allow ECM to covalently couple to polyacrylamide overnight at 4 °C. Incubation in a humidified chamber (Petri dish or other closed container with a damp KimWipe) will help prevent evaporation of the ECM solution.
-
28|
By using sterile forceps, remove cover slips with gels from ECM drops and place gel side up into sterile PBS in separate wells of a six-well dish. From this point, all work should be done in a sterile field, with sterile solutions.
-
29|
Rinse extensively with sterile PBS, with four to six changes of buffer in total.
■ PAUSE POINT ECM-coupled, polyacrylamide-bound cover slips can be stored in PBS at 4 °C for up to 2 weeks.
Cell plating and formation of 3D collagen ECM overlay ● TIMING ~3 h (plus 2–12 h of incubation)
-
30|
When you are ready to plate cells (or place tissue explant and so on), rinse ECM-coated polyacylamide gels with warm (37 °C), sterile PBS and then with warm cell-culture medium.
-
31|
If cells are to be imaged live, the cover slip with ECM-coated polyacrylamide should now be mounted into an appropriate imaging chamber (see ‘Chamber dishes’), taking care not to let ECM-coated polyacrylamide dry out.
-
32|
Seed cells onto coated gels (typically 30,000–50,000 cells for a 22 mm × 22 mm cover slip) in full growth medium, and allow them to adhere for 2–3 h.
-
33|
Prepare 3 ml of a 2 mg ml−1 collagen I (or other ECM). On ice in a sterile field, add the following reagents in order: X ml of sterile, cold ddH2O, 0.3 ml of 10× medium and Y ml of collagen I solution, where Y is enough to dilute stock collagen to 2 mg ml−1 final concentration and X is enough to bring the final volume to 3 ml. Mix well, and then titrate to pH 7.4 by adding small amounts of NaHCO3 (10–20 µl); solutions with a pH indicator will turn into the appropriate color. Mix the solution by pipetting and place it back on ice.
-
34|
Remove the supernatant medium from cells on polyacrylamide-coupled cover slips. Overlay enough collagen I solution to cover the cells on each cover slip (for a 22 mm × 22 mm cover slip, 50 µl of collagen solution can cover the cells completely). If you are using a live-cell imaging chamber, be sure to leave enough space for some cell culture medium to be added later. Allow collagen I to for a gel on cells at 37 °C in a humidified incubator for 2–12 h.
▲ CRITICAL STEP Shorter incubation times than 2 h at 37 °C may result in collagen that has not fully polymerized, whereas longer than 12 h without supernatant medium may be deleterious to the cells. When the collagen has sufficiently polymerized, there will be little to no movement of the gel if the dish is tilted to a 45° angle. Some excess liquid at this stage is normal.
? TROUBLESHOOTING
-
35|
Overlay sandwich gels with cell-culture medium.
▲ CRITICAL STEP For open cover slips in tissue culture dishes, this must be done slowly, as the collagen gel is fragile and loosely attached to the underlying ECM-coupled polyacrylamide gel. Vigorous additions will dislodge the collagen gel.
? TROUBLESHOOTING
-
36|
Maintain cultures in this state in a tissue culture incubator (they can be maintained for several days); medium changes should be kept to a minimum.
? TROUBLESHOOTING
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
Table 2.
Troubleshooting table.
| Step | Problem | Possible reason(s) | Solution |
|---|---|---|---|
| 16 | Gel peeling | Cover slips not clean; incomplete activation | Use ‘Squeaky Clean’ cover slips, see protocol in ref. 75 |
| Gel ruffling (Supplementary Fig. 1) | Soft gel, drying | No action necessary, take care not to allow these areas to rip | |
| 35 | Collagen gel is dislodged from polyacrylamide | Collagen polymerization time not optimal | Shorter than 2 h at 37 °C can result in incomplete collagen polymerization, increase incubation time or increase collagen concentration, if possible |
| Insufficient cross-linking of collagen; sulfo-SANPAH cross-linking suboptimal | Steps 17–26 should be done quickly; sulfo-SANPAH should be thawed quickly, diluted and kept on ice until placed onto polyacrylamide | ||
| Collagen gel edges have retracted | Gelling collagen on top of polyacrlyamide, whereas in Rose chamber or other imaging chamber will allow collagen to adhere to the sides, preventing dislodging. Pipette medium on top slowly and carefully | ||
| 34–36 | Cells do not appear healthy | Unpolymerized acrylamide and free radicals not washed out of polyacrlyamide | Longer wash times, larger volumes of wash buffer and overnight incubation in wash buffer can help to remove free radical and unpolymerized acrylamide, which are toxic to cells |
| 36 | Cells do not appear healthy | Cells are sensitive to minimal medium during collagen polymerization | Collagen gel can be made up with X volume of collagen I stock, 0.1× volume of 10× MEM, and Y volume of complete cell culture media, such that X + Y make a collagen concentration of 2 mg ml−1 |
| Contaminated culture | Aseptic technique not used during early parts of procedure | Use aseptic technique throughout procedure. ECM-coated polyacrylamide sheets can be sterilized in a sterile laminar flow hood with UV germicidal lamp exposure. Keep gel covered in thin layer of PBS to prevent drying, 30–60 min of exposure to UV light |
● TIMING
Steps 1–8, activation of cover slip: 3 h
Steps 9–16, preparation of polyacrylamide sheet: ~1 h
Steps 17–29, cross-linking of ECM: 30–40 min, followed by overnight incubation at 4 °C
Steps 30–32, plating of cells: ~30 min, followed by incubation for 2–3 h
Steps 33–36, formation of collagen gel: 20–30 min, followed by incubation for 2–12 h
ANTICIPATED RESULTS
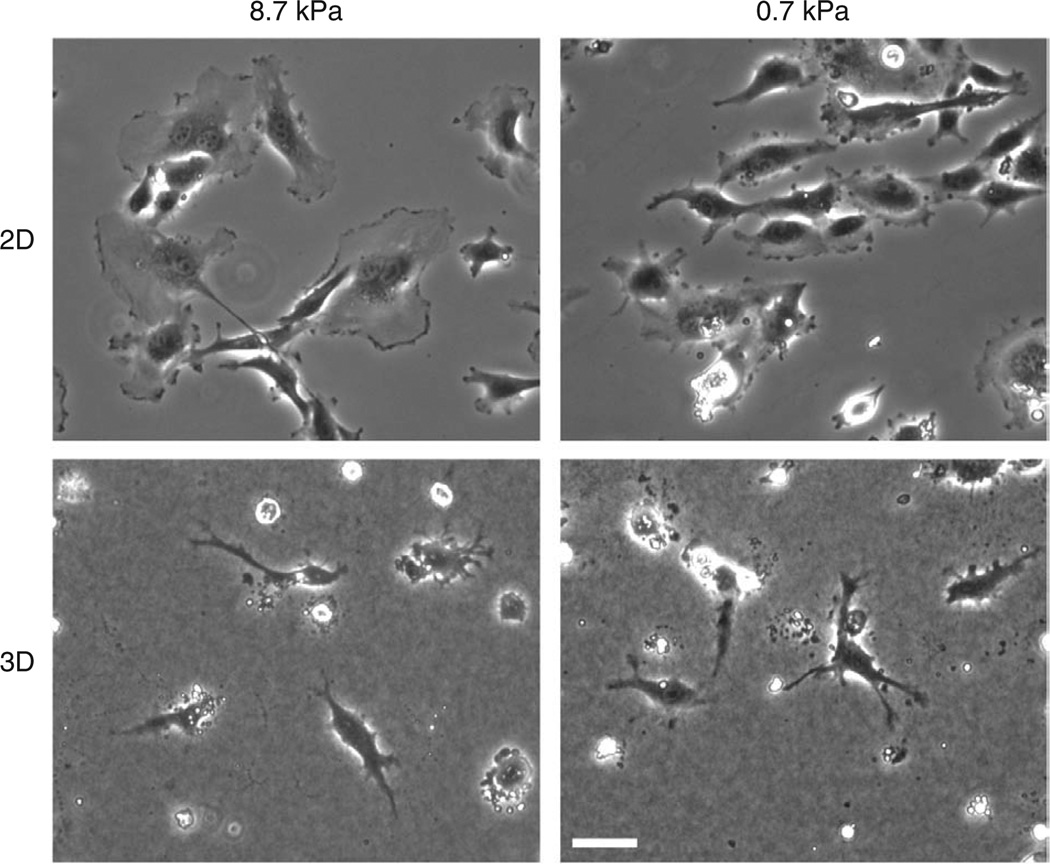
When the sandwich gel is correctly assembled, the collagen gel will form a loose interface with the coated polyacrylamide on which the cells will migrate. Cells will interact with both the collagen bound to the polyacrylamide and to the collagen surrounding the cell (Fig. 1b–d), which can be observed by a differing morphology or behavior (Fig. 2). Cell imaging can be performed as soon as the supernatant medium can be applied, and most cells will be maximally migratory within the first 24 h of plating, as observed for standard 2D cultures. In general, most molecular and morphological phenotypes will be observed during this period, and differences between 2D compliant substrates and their corresponding 3D-like sandwich gels will be similarly evident almost immediately upon cell spreading. For example, HUVECs plated on compliant (0.7 or 8.7 kPa) 2D substrates spread differently based on the stiffness of the polyacrylamide, where the stiffer substrates induce typical flattened lamellar morphology, whereas softer substrates allow spreading but to a lesser degree (Fig. 2). However, when the same substrates are combined in a sandwich gel culture, the HUVECs create a spindle-shaped morphology, with increased branching on softer substrates (Fig. 2). Accompanying these changes are other molecular changes in both cytoskeleton dynamics and signaling pathways30,47.
Figure 2. Anticipated results with sandwich gel culture setup.
Phase contrast images of HUVECs on 0.7-kPa or 8.7-kPa polyacrylamide gels without (2D) and with (3D) collagen gel on top. Note that when adhered to 2D collagen–coated polyacrylamide gels, cells spread to a larger area on stiffer ECM. In contrast, in 3D sandwich gels, cells are less spread and more branched, and softer ECM (0.7 kPa) induces more cell branches. This illustrates the different effects of stiffness in 2D and 3D ECMs. Scale bar, 50 µm.
Supplementary Material
ACKNOWLEDGMENTS
R.S.F., K.A.M. and C.M.W. are supported by the NHLBI Intramural Research Program. M.L.G. is supported by the NIH Director’s Pioneer Award (DP10D00354). We thank Sergey Plotnikov for insightful discussion. We also acknowledge members of the NIH Electron Microscopy Core Facility and are grateful for the particularly excellent technical skills of M. Daniels and P. Connelly.
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS R.S.F. and K.A.M. conducted experimental work, M.L.G. performed rheometry measurements, and R.S.F., K.A.M. and C.M.W. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, et al. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. USA. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 3.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 5.Pelham RJ, Jr, Wang YL. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol. Bull. 1998;194:348–349. doi: 10.2307/1543109. [DOI] [PubMed] [Google Scholar]
- 6.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc. Natl. Acad. Sci. USA. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, et al. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007;9:299–399. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 9.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo CM, Wang HB, Dembo M, Wang YL, et al. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, et al. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat. Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 14.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker EL, Lu J, Yu D, Bonnecaze RT, Zaman MH, et al. Cancer cell stiffness: integrated roles of three-dimensional matrix stiffness and transforming potential. Biophys. J. 2010;99:2048–2057. doi: 10.1016/j.bpj.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker R, Rogers KD, Shepherd N, Stone N. New relationships between breast microcalcifications and cancer. Br. J. Cancer. 2010;103:1034–1039. doi: 10.1038/sj.bjc.6605873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YL, Pelham RJ., Jr Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 20.Frey MT, Engler A, Discher DE, Lee J, Wang YL. Microscopic methods for measuring the elasticity of gel substrates for cell culture: microspheres, microindenters, and atomic force microscopy. Methods Cell Biol. 2007;83:47–65. doi: 10.1016/S0091-679X(07)83003-2. [DOI] [PubMed] [Google Scholar]
- 21.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res. Treat. 2009;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J. Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 24.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin. Exp. Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, et al. A Rac switch regulates random versus directionally persistent cell migration. J. Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beningo KA, Wang YL. Double-hydrogel substrate as a model system for three-dimensional cell culture. Methods Mol. Biol. 2007;370:203–212. doi: 10.1007/978-1-59745-353-0_14. [DOI] [PubMed] [Google Scholar]
- 28.Rehfeldt F, Brown AE, Raab M, Cai S, Zajac AL, et al. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr. Biol. (Camb.) 2012;4:422–430. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 29.Ezzell RM, Toner M, Hendricks K, Dunn JC, Tompkins RG, et al. Effect of collagen gel configuration on the cytoskeleton in cultured rat hepatocytes. Exp. Cell Res. 1993;208:442–452. doi: 10.1006/excr.1993.1266. [DOI] [PubMed] [Google Scholar]
- 30.Myers KA, Applegate KT, Danuser G, Fischer RS, Waterman CM, et al. Distinct ECM mechanosensing pathways regulate microtubule dynamics to control endothelial cell branching morphogenesis. J. Cell Biol. 2011;192:321–334. doi: 10.1083/jcb.201006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–369. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shakesheff KM, Rose FR. Tissue engineering in the development of replacement technologies. Adv. Exp. Med. Biol. 2012;745:47. doi: 10.1007/978-1-4614-3055-1_4. [DOI] [PubMed] [Google Scholar]
- 33.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutmacher DW, Horch RE, Loessner D, Rizzi S, Sieh S, et al. Translating tissue engineering technology platforms into cancer research. J. Cell Mol. Med. 2009;13:1417–1427. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maltman DJ, Przyborski SA. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitroanalyses. Biochem. Soc. Trans. 2010;38:1072–1075. doi: 10.1042/BST0381072. [DOI] [PubMed] [Google Scholar]
- 36.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, et al. Reconstituting organ-level lung functions on a chip. Science. 2011;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, et al. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J. Biomed. Mater. Res. 1993;27:183–189. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 40.Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, et al. Prevascularization of porous biodegradable polymers. Biotechnol. Bioeng. 1993;42:716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 41.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;9:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin. Cancer Biol. 2010;20:139–145. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu. Rev. Cell Dev. Biol. 2009;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 44.Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng. Part A. 2010;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–6435. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr. Biol. 2009;19:260–265. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat. Rev. Clin. Oncol. 2009;6:315–326. doi: 10.1038/nrclinonc.2009.64. [DOI] [PubMed] [Google Scholar]
- 49.Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Curr. Opin. Hematol. 2008;15:228–234. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- 50.Buxboim A, Discher DE. Stem cells feel the difference. Nat. Methods. 2010;7:695–697. doi: 10.1038/nmeth0910-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 52.Myers JP, Gomez TM. Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. J. Neurosci. 2011;31:13585–13595. doi: 10.1523/JNEUROSCI.2381-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev. Neurobiol. 2011;71:901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J. Neurosci. Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 55.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat. Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 56.Santiago-Medina M, Myers JP, Gomez TM. Imaging adhesion and signaling dynamics in Xenopus laevis growth cones. Dev. Neurobiol. 2012;72:585–599. doi: 10.1002/dneu.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. Effect of dynamic stiffness of the substrates on neurite outgrowth by using a DNA-crosslinked hydrogel. Tissue Eng. Part A. 2010;16:1873–1889. doi: 10.1089/ten.TEA.2009.0574. [DOI] [PubMed] [Google Scholar]
- 58.Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 59.Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat. Cell Biol. 2011;13:3–5. doi: 10.1038/ncb0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 61.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 2010;12:598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng. Part A. 2011;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 65.Wadsworth P. Using cultured mammalian cells to study mitosis. Cold Spring Harb. Protoc. 2012;2012:205–212. doi: 10.1101/pdb.ip067850. [DOI] [PubMed] [Google Scholar]
- 66.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 67.Buxboim A, Rajagopal K, Brown AE, Discher DE. How deeply cells feel: methods for thin gels. J. Phys. Condens. Matter. 2010;22:194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo WH, Wang YL. In: Live Cell Imaging: A Laboratory Manual. Goldman RD, Swedlow JR, Spector DL, editors. Cold Spring Harbor; 2010. p. 43. [Google Scholar]
- 69.Rieder CL, Hard R. Newt lung epithelial cells: cultivation, use, and advantages for biomedical research. Int. Rev. Cytol. 1990;122:153–220. doi: 10.1016/s0074-7696(08)61208-5. [DOI] [PubMed] [Google Scholar]
- 70.Lutz DA, Hamaguchi Y, Inoue S. Micromanipulation studies of the asymmetric positioning of the maturation spindle in Chaetopterus sp. oocytes: I. Anchorage of the spindle to the cortex and migration of a displaced spindle. Cell Motil. Cytoskeleton. 1988;11:83–96. doi: 10.1002/cm.970110202. [DOI] [PubMed] [Google Scholar]
- 71.Phillips JB, Bunting SC, Hall SM, Brown RA. Neural tissue engineering: a self-organizing collagen guidance conduit. Tissue Eng. 2005;11:1611–1617. doi: 10.1089/ten.2005.11.1611. [DOI] [PubMed] [Google Scholar]
- 72.Olsen BR. Life without perlecan has its problems. J. Cell Biol. 1999;147:909–912. doi: 10.1083/jcb.147.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avery NC, Sims TJ, Bailey AJ. Quantitative determination of collagen cross-links. Methods Mol. Biol. 2009;522:103–121. doi: 10.1007/978-1-59745-413-1_6. [DOI] [PubMed] [Google Scholar]
- 74.Slatter DA, Avery NC, Bailey AJ. Collagen in its fibrillar state is protected from glycation. Int. J. Biochem. Cell Biol. 2008;40:2253–2263. doi: 10.1016/j.biocel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Waterman-Storer CM. Microtubule/organelle motility assays. Curr. Protoc. Cell Biol. 2001;13:13.1.1–13.1.21. doi: 10.1002/0471143030.cb1301s00. [DOI] [PubMed] [Google Scholar]
- 76.Omidian H, Rocca JG, Park K. Advances in superporous hydrogels. J. Control Release. 2005;102:3–12. doi: 10.1016/j.jconrel.2004.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.