Abstract
Objective
The pathologic indices of Alzheimer's disease, cerebrovascular disease, and Lewy body disease accumulate in the brains of older persons with and without dementia, but the extent to which they account for late life cognitive decline remains unknown. We tested the hypothesis that these pathologic indices account for the majority of late life cognitive decline.
Methods
856 deceased participants from two longitudinal clinical-pathologic studies, Rush Memory and Aging Project and Religious Orders Study, completed a mean of 7.5 annual evaluations including 17 cognitive tests. Neuropathologic examinations provided quantitative measures of global Alzheimer's pathology, amyloid load, tangle density, macroscopic infarcts, microinfarcts, and neocortical Lewy bodies. Random coefficient models were used to examine the linear relation of pathologic indices with global cognitive decline. In subsequent analyses, random change point models were used to examine the relation of the pathologic indices with the onset of terminal decline and rates of preterminal and terminal decline (i.e., non-linear decline).
Results
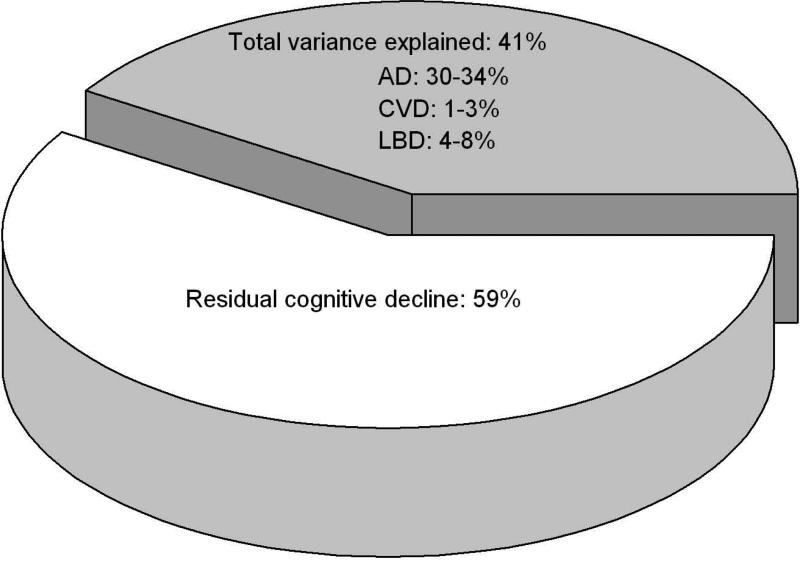
Cognition declined a mean of about 0.11 unit per year (estimate=−0.109, SE=0.004, p<0.001) with significant individual differences in rates of decline; the variance estimate for the individual slopes was 0.013 (SE=0.112, p<0.001). In separate analyses, global Alzheimer's pathology, amyloid, tangles, macroscopic infarcts, and neocortical Lewy bodies were associated with faster rates of decline and explained 22%, 6%, 34%, 2% and 8% of the variation in decline, respectively. When analyzed simultaneously, the pathologic indices accounted for a total of 41% of the variation in decline and the majority remained unexplained. Further, in random change point models examining the influence of the pathologic indices on the onset of terminal decline and the preterminal and terminal components of the cognitive trajectory, the common pathologic indices accounted for a less than a third of the variation in the onset of terminal decline and rates of preterminal and terminal decline.
Interpretation
The pathologic indices of the common causes of dementia are important determinants of cognitive decline in old age and account for a large proportion of the variation in late life cognitive decline. Surprisingly, however, much of the variation in cognitive decline remains unexplained, suggesting that other important determinants of cognitive decline remain to be identified. Identification of the mechanisms that contribute to the large unexplained proportion of cognitive decline is urgently needed to prevent late life cognitive decline.
INTRODUCTION
Aging is characterized by progressive cognitive decline and the prevention of cognitive impairment and dementia rank among the top public health challenges of the 21st century. Cognitive decline in old age is a complex phenomenon, however, and there is considerable heterogeneity in individual trajectories; whereas some persons decline rapidly, others exhibit slower decline, and still others remain stable or even improve as a result of practice and learning1-4. The three most common causes of dementia in old age, Alzheimer's disease (AD), cerebrovascular disease (CVD), and Lewy body disease (LBD), account for the vast majority of dementia cases and most cases of mild cognitive impairment (MCI)5,6. In addition, pathologic indices of AD, CVD, and LBD accumulate in varying degrees in persons without dementia or without any overt cognitive impairment (i.e., no dementia or even MCI) and are associated with level of and rate of change in cognition7-13. Although we previously examined the relation of these pathologic indices with cognitive decline in a smaller sample than that used here12, we are not aware of a prior study that quantified the extent to which these indices account for individual differences in rates of cognitive decline. This gap in knowledge likely is due to the difficulty of obtaining the large sample sizes and detailed longitudinal cognitive and neuropathologic data required to do so. Knowledge of the degree to which the common causes of dementia explain individual differences in cognitive decline has direct implications for the establishment of research priorities and interventions to combat the large and rapidly increasing public health problem posed by cognitive impairment and dementia.
We examined the relation of the pathologic indices of the three most common causes of dementia, AD, CVD, and LBD, with cognitive decline (i.e., the longitudinal rate of change in cognition over time) and tested the hypothesis that these pathologies account for the majority of individual differences in rates of cognitive decline. Participants were more than 850 deceased persons from the Rush Memory and Aging Project and the Religious Orders Study with detailed annual cognitive function data available for up to 18 years and quantitative measures of multiple pathologic indices determined at autopsy (i.e., global AD pathology, amyloid load, tangle density, macroscopic infarcts, microinfarcts, and neocortical Lewy bodies)14,15. Random coefficient models were used to characterize linear rates of cognitive decline, examine the relation of the pathologic indices with rates of cognitive decline, and quantify the extent to which variation in cognitive decline is explained by common, age-related pathologic indices. In subsequent analyses, random change point models were used to examine the relation of the pathologic indices with the onset of terminal decline and rates of preterminal and terminal decline (i.e., non-linear decline). Finally, in a supporting analysis, we investigated the relationship of measures of neural reserve (i.e., presynaptic proteins) to cognitive decline after accounting for the pathologic indices.
METHOD
Participants
Participants came from two clinical-pathologic cohort studies of aging and dementia: the Religious Orders Study and the Memory and Aging Project14,15. The Religious Orders Study began in 1994 and involves older Catholic nuns, priests, and monks recruited from more than 40 groups across the United States. The Rush Memory and Aging Project began in 1997 and involves older lay persons recruited from retirement communities, subsidized housing facilities, and social service agencies in the Chicago metropolitan area. Persons in both studies agreed to annual clinical evaluations and brain autopsy at death. Written informed consent was obtained in each study after procedures were fully explained, and both studies were approved by the Institutional Review Board of Rush University Medical Center. The follow up participation rates for both studies exceed 95% of survivors and autopsy rates exceed 80%. At the time of these analyses, data were available from 856 deceased persons with at least 2 cognitive evaluations (mean number of annual evaluations=7.5, SD=3.8, range: 2-18 years); notably, more than 80% of the persons included in these analyses had 4 or more cognitive assessments, about 60% had 5 or more, and about 25% had more than 9 assessments.
Clinical and cognitive evaluation procedures
Participants in both studies underwent uniform, structured, annual clinical evaluations that included detailed annual cognitive testing and neurologic examinations, as previously reported14,15. The two studies have 19 cognitive performance tests in common. Scores from 17 of those tests were converted to z scores using the mean and standard deviation from the baseline evaluation of participants included in these analyses, and z-scores were averaged to yield a summary measure of global cognition13,16. Z-scores were used in order to place scores from diverse cognitive tests on a common scale so that they could be combined with approximately equal weighting; further, the use of a composite score has the advantage of minimizing floor and ceiling effects and other sources of random variability and is preferable for studies examining change in cognition over many years.
Quantification of the pathologic indices
Multiple pathologic indices were examined, including three measures of AD, (i.e., global AD pathology, tangles, and amyloid), two measures of CVD (macroscopic infarcts and microinfarcts), and a measure of neocortical Lewy body pathology13,16,17,18. Details of the brain autopsy procedures and the quantification of the pathologic indices are provided in Supplemental Methods.
Quantification of presynaptic proteins
In addition, for a supporting analysis, we examined putative structural elements of reserve, presynaptic proteins24. Details regarding the procedure used to quantify these are provided in Supplemental Methods.
Statistical Analyses
We first describe the demographic, clinical and pathologic characteristics of the participants. Next, we used random coefficient models to characterize linear rates of cognitive decline, examine the relation of pathology with cognitive decline, and determine the contribution of the pathologic indices to the reduction of between-subject variation in cognitive decline19. Important advantages of the random coefficient model are that it considers the starting level of cognition as well as the variable intervals used to calculate slope. Specifically, the model estimates individual slopes using all datapoints available for each individual, including the starting level of cognition, and accounts for the correlation of intercept and slope by modeling the variance-covariance structure of the random intercept and slope. Each individual path of change is assumed to follow the mean path of change in cognitive function except for random effects that cause the starting level of function to be higher or lower and the rate of change to be faster or slower, and the estimation accounts for the variable number of cognitive assessments across participants. We began with an unadjusted model with only the term for time; time is defined as the time (in years) prior to death. The estimate for time (the slope) corresponds to the mean rate of decline in cognition, and the estimate for the variance in random slope captures how individual slopes deviate from the mean. We then added in terms for the pathologic indices and their interactions with time in a series of models. Each significant time-by-pathology term characterizes the difference from the mean slopes of cognitive decline due to the particular pathologic index. Consequently, the time-by-pathology term accounts for a proportion of the total random slope variance, and the reduction of the random slope variance reflects the between-subject variation in cognitive decline explained by the corresponding pathology. The core (fully adjusted) model included terms for time, each of the pathologic indices, and the interactions of each of the pathologic indices with time. In subsequent analyses, we varied the order of entry of the pathologic variables into the core model and added additional terms for demographics and their interactions with time. We also conducted a series of supporting analyses in which we repeated the core model with the following modifications: first, we used only a single indicator of AD pathology (i.e., tangles); second, we modeled time as time from baseline (forward) instead of time prior to death (backward); third, we excluded persons with dementia at baseline; and fourth, we used level of cognition proximate to death as the outcome (instead of cognitive slope). The core finding was essentially unchanged in all supporting analyses (see Supplementary Results). Analyses were performed in SAS®20.
In addition, because cognitive decline can accelerate in the years just prior to death, we conducted random change point models21 to first determine when the rate of cognitive decline increased prior to death (indicating the onset of terminal decline) and characterize rates of cognitive decline before and after its onset; then, we examined the relation of the pathologic indices with the onset of terminal decline and rates of change before and after its onset. The random change point models included four basic components: the change point, preterminal slope, terminal slope, and intercept proximate to death. The change point indicates when the onset of terminal decline began, and the preterminal and terminal slopes indicate the rate of change before and after its onset; the intercept reflects the level of cognition proximate to death. Each component was parameterized as a linear function of variables of interest, including the pathologic indices. The term for each pathologic index on the change point indicates the modifying effect of that pathologic index on the onset of terminal decline, the term for each pathologic index in the preterminal slope indicates the modifying effect of that pathologic index on the rate of preterminal decline, and the term for each pathologic index in the terminal slope indicates the modifying effect of that pathologic index on the rate of decline during the terminal period. The pathologic indices, if significant, account for a proportion of the total variances in random change point, preterminal and terminal slopes, and the reduction of the random slope variance reflects the between-subject variation in cognitive decline explained by pathologic indices. Model estimation was done with a Bayesian Monte Carlo Markov Chain approach implemented in OpenBugs software22,23.
RESULTS
Descriptives
Participants (n=856) in these analyses completed a mean of 7.5 (SD=3.8, range= 2-18) years of annual cognitive function testing and were an average of 88.2 (SD=6.5, range=66-108) years of age at the time of death; additional descriptive data are provided in Table 1. The distribution of the global cognitive measure at baseline was approximately normal (mean=-0.01, SD=0.62). At autopsy, 850 (99%) of persons had evidence of plaques or tangles, 311 (36%) had one or more gross infarcts, 242 (28%) had one or more microinfarcts, and 90 (11%) had neocortical Lewy bodies. Table 2 shows the intercorrelations among pathology variables and demographics.
Table 1.
Descriptive characteristics of the cohort
| Variable | Mean, SD, range (or # and percent) |
|---|---|
| Age at death | 88.2, 6.5, 66-108 |
| Education | 16.5, 3.5, 3-30 |
| % Female | 548, 64% |
| % white, non-Hispanic | 817, 95% |
| Baseline MMSE | 27.2, 3.2, 4-30 |
| Proximate to death MMSE | 21, 9.1, 0-30 |
| Baseline global cognition | −0.01, 0.62, −2.89-1.56 |
| Proximate to death global cognition | −0.71, 1.14, −3.93-1.44 |
| Global AD pathology | 0.70, 0.61, 0-3.1 |
| Amyloid | 3.76, 4.02, 0-22.9 |
| Tangles | 6.30, 7.88, 0-78.52 |
| Gross infarcts (1+ present) | 311, 36% |
| Microinfarcts (1+ present) | 242, 28% |
| Neocortical Lewy bodies (present) | 90, 10% |
Table 2.
Intercorrelations among the pathologic indices and demographics
| Variable | Amyloid | Tangles | Gross infarcts | Microinfarcts | Neo-neocortical Lewy bodies | Age | Sex | Educ |
|---|---|---|---|---|---|---|---|---|
| Global AD pathology | 0.78** | 0.67** | 0.05 | −0.02 | 0.12** | 0.16** | −0.12** | −0.004 |
| Amyloid | 0.48** | 0.06T | −0.003 | 0.08* | 0.19** | −0.08* | −0.06T | |
| Tangles | 0.06T | 0.023 | 0.12** | 0.28** | −0.16** | −0.05 | ||
| Gross infarcts | 0.23** | −0.05 | 0.13** | 0.05 | −0.04 | |||
| Microinfarcts | −0.05 | 0.05 | 0.03 | −0.03 | ||||
| Neocortical Lewy bodies | 0.02 | 0.02 | 0.01 |
*Based on Spearman correlations, T=trend, p<0.10
p<0.001
p<0.05
Heterogeneity of age-related cognitive decline
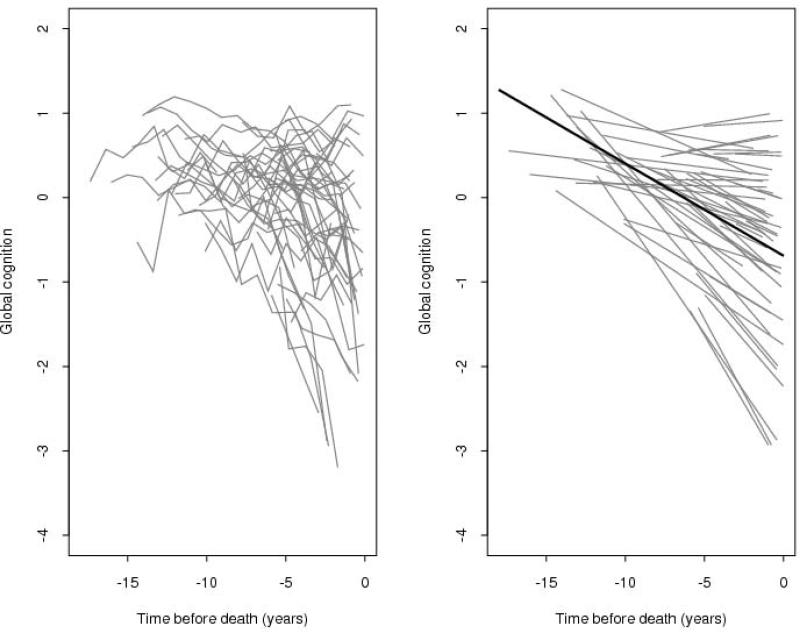
We constructed random coefficient models to first characterize the annual rate of decline (slope) in cognition and estimate the variability of such decline across individuals; the outcome measure was the longitudinal rate of change in global cognition (i.e. slope). In the initial unadjusted model, the global cognitive measure declined a mean of about 0.11 unit per year (estimate=−0.109, SE=0.004, p<0.001). Notably, although the majority of persons exhibited moderate to severe decline, there was significant between-subject variability; the variance estimate for the individual slopes was 0.013 (SE=0.112, p<0.001, Table 3). Figure 1 shows a spaghetti plot of raw data points for a random sample (N=50) of participants (left panel) as well as the model-derived mean linear slope of cognitive decline (right panel, dark line) superimposed on the estimated individual slopes from the 50 individuals whose raw data points are shown; this figure illustrates considerable heterogeneity across individuals.
Table 3.
Percent of between-subjects variance in cognitive decline explained by each of the pathologic indices
| Global cognitive decline | |||
|---|---|---|---|
| Predictor | Total Variance | Reduction | % of total variance explained by predictor variable in separate models |
| Reference model | 0.0125 | ||
| Global AD pathology | 0.0097 | 0.0028 | 22% |
| Tangles | 0.0083 | 0.0042 | 34% |
| Amyloid | 0.0117 | 0.0008 | 6% |
| Gross infarcts | 0.0122 | 0.0003 | 2% |
| Microinfarcts | 0.0124 | 0.0001 | 1% |
| Neocortical Lewy bodies | 0.0115 | 0.0010 | 8% |
Figure 1.
Spaghetti plot of individual trajectories from a random sample of persons (N=50, Left panel) and mean slope of cognitive decline superimposed on their estimated individual slopes (model derived slopes, Right panel).
Relation of common, age-related pathologic indices to cognitive decline
In separate analyses, we first examined the relation of each pathologic index with the rate of cognitive decline and determined its contribution to the reduction of between-subject variation. In the initial analysis, global AD pathology was associated with a faster rate of cognitive decline; more specifically, for a one unit increase in global AD pathology, the rate of cognitive decline was about 0.1 unit faster (estimate −0.084, SE=0.007, p<0.001), and the measure of global AD pathology explained 22% of the between-subject variability in cognitive decline (Table 3). Amyloid (estimate −0.007, SE=0.001, p<0.001) and tangles (estimate −0.008, SE=0.001, p<0.001) also were associated with a faster rate of decline and explained 6 and 34% of the variance in cognitive decline, respectively (Table 3). Similarly, gross infarcts were associated with a faster rate of decline (estimate −0.034, SE=0.009, p<0.001) and explained 2% of the between-subject variability (Table 3), but microinfarcts were not significantly associated with decline (estimate −0.014, SE=0.010, p=0.161). Neocortical Lewy bodies were associated with a faster rate of cognitive decline (estimate −0,102, SE=0.014, p<0.001) and accounted for 8% of the variance in decline (Table 3).
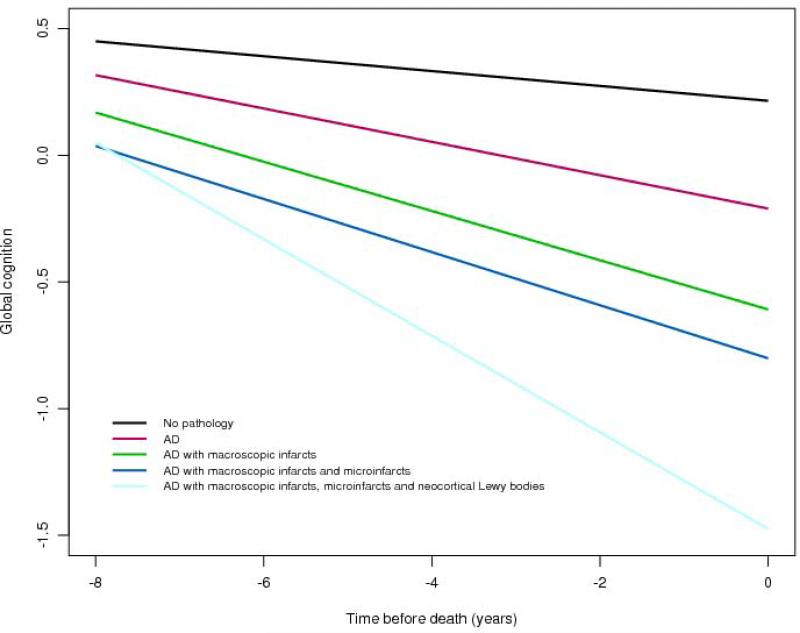
Next, to test the hypothesis that the common pathologic indices known to cause dementia account for the majority of late life cognitive decline, we examined the relation of all of the pathologic indices simultaneously with the slope of cognitive decline; in this analysis, the pathologic indices were entered in order of their prevalence (i.e., global AD pathology, amyloid, tangles, macroscopic infarcts, microinfarcts, and neocortical Lewy bodies). When considered together, tangles, macroscopic infarcts, and neocortical Lewy bodies were associated with substantially faster rates of decline (Table 4), but the pathologic indices combined only accounted for 41% of the variation in cognitive decline. Figure 2 is based on the fully adjusted model and illustrates the additive effect of each pathologic index on the rate of cognitive decline. This figure shows that AD pathology (i.e., the mean of global AD pathology, amyloid and tangles) is associated with an increased rate of decline compared to the minimal decline associated with no pathology, but co-prevalent pathologic indices are associated with an even more rapid rate of decline, and the rate of decline was greatest for persons with all three pathologic indices (i.e., AD, CVD, and LBD).
Table 4.
Association of each pathologic index with rate of cognitive decline
| Estimate | SE | p-value | |
|---|---|---|---|
| Reference slope | −0.0294 | 0.0070 | <0.001 |
| Global AD pathology | −0.0163 | 0.0100 | 0.01143 |
| Amyloid | −0.0003 | 0.0012 | 0.8121 |
| Tangles | −0.0072 | 0.0007 | <.0001 |
| Gross infarcts | −0.0313 | 0.0079 | <.0001 |
| Microinfarcts | −0.0076 | 0.0084 | 0.3628 |
| Neocortical Lewy bodies | −0.0857 | 0.0122 | <.0001 |
Figure 2.
Contributions of combinations of the pathologic indices to cognitive decline (model derived slopes).
Next, because the order of entry of the pathologic indices into the model (done above in order of their prevalence) likely affected the degree to which each pathologic index contributed to between-subject variation in decline, we conducted a series of analyses in which we varied the order of entry of the pathologic variables and exhausted all possible permutations. Then, we determined the range of variance accounted for by each of the pathologies. In these analyses, the AD pathologic indices explained 30-34% of the between-subjects variation, infarcts explained 1-3%, and neocortical Lewy bodies explained 4-8%, depending on the order of entry (data not shown). Figure 3 summarizes the cumulative influence of the pathologic indices on the between-subject variation in cognitive decline, as well as the residual variation (i.e., the decline not explained by the common pathologic indices).
Figure 3.
Variation in cognitive decline explained by the pathologic indices (grey) and the residual, unexplained variation in cognitive decline (white) derived from fully adjusted models.
Finally, we examined the role of demographics by adding age at the time of death, sex, and education and their interactions with time to the core model examining all pathologic indices together. Findings were essentially unchanged in this analysis (data not shown).
Relation of pathologic indices with preterminal and terminal cognitive decline
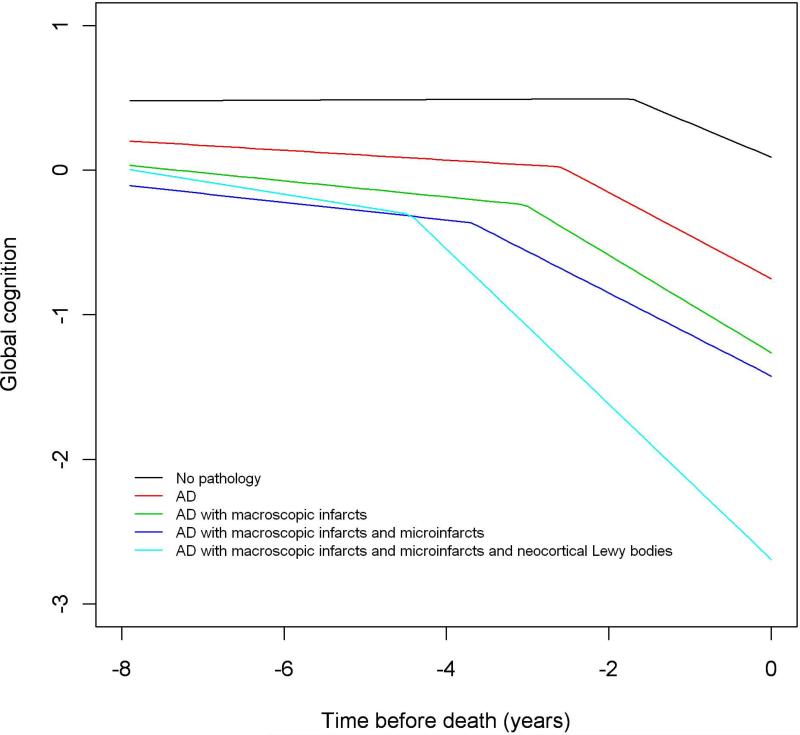
Next, because prior work has shown that the slope of cognitive decline can become steeper in the years just prior to death, a phenomenon referred to as terminal decline, we used random change point models to characterize the onset of terminal decline and rates of preterminal and terminal decline and then to examine where in the cognitive trajectory the pathologic indices exerted their deleterious effects. These analyses were done in a subset of persons (N=573) with at least 5 cognitive assessments (as needed to estimate terminal decline); the mean number of assessments was 8.56 (SD=2.95, range: 5-18). In the initial analysis, the onset of terminal cognitive decline (i.e., the change point) began 3 years prior to death (95% CI: −3.227, −2.774). The rate of decline in the preterminal period was −0.045 unit per year (95% CI: −0.052, −0.038) and this increased more than 7-fold in the terminal period to -0.319 (95% CI: −0.353, −0.287). In a model examining all of the pathologic indices together, measures of AD were associated with the onset of terminal decline (i.e., tangles), the rate of preterminal decline (i.e., global AD pathology and tangles), and the rate of terminal decline (i.e., tangles) (Table 5). Gross infarcts were associated with the onset of terminal decline and the rate of preterminal decline, and microinfarcts were associated with the onset of terminal decline. Neocortical Lewy bodies were associated with the onset of terminal decline as well as rates of preterminal and terminal decline. However, together, the pathologic indices accounted for only 25% of the variation in the onset of terminal decline, 32% of the variation in preterminal decline, and 21% of the variation in terminal decline. Figure 4 is based on this analysis and shows the additive effect of the pathologic indices on rates of preterminal and terminal cognitive decline; again, the figure illustrates co-prevalent pathologic indices were associated with faster rates of decline.
Table 5.
Association of the pathologic indices with the onset of accelerated decline as well as rates of preterminal and terminal cognitive decline.
| Model Term | Estimate | SD | 95% C.I. | ||
|---|---|---|---|---|---|
| Lower 2.5% | Upper 2.5% | ||||
| Intercept | −0.752 | 0.067 | −0.889 | −0.621 | |
| Preterminal slope | −0.034 | 0.0046 | −0.042 | −0.025 | |
| Terminal slope | −0.299 | 0.0213 | −0.342 | −0.258 | |
| Change point | −2.592 | 0.142 | −2.872 | −2.319 | |
| Intercept | Global AD pathology | −0.413 | 0.0802 | −0.569 | −0.257 |
| Preterminal slope | Global AD pathology | −0.013 | 0.006 | −0.024 | −0.001 |
| Terminal slope | Global AD pathology | −0.030 | 0.020 | −0.069 | 0.011 |
| Change point | Global AD pathology | −0.477 | 0.142 | −0.748 | −0.193 |
| Intercept | Tangles | −0.450 | 0.069 | −0.588 | −0.319 |
| Preterminal slope | Tangles | −0.025 | 0.006 | −0.037 | −0.013 |
| Terminal slope | Tangles | −0.036 | 0.0166 | −0.068 | −0.003 |
| Change point | Tangles | −0.242 | 0.120 | −0.473 | −0.006 |
| Intercept | Amyloid | 0.020 | 0.061 | −0.100 | 0.141 |
| Preterminal slope | Amyloid | 4.15E-04 | 0.004 | −0.008 | 0.009 |
| Terminal slope | Amyloid | 6.14E-04 | 0.0152 | −0.029 | 0.030 |
| Change point | Amyloid | −0.116 | 0.107 | −0.327 | 0.094 |
| Intercept | Gross infarcts | −0.511 | 0.105 | −0.718 | −0.303 |
| Preterminal slope | Gross infarcts | −0.022 | 0.007 | −0.036 | −0.008 |
| Terminal slope | Gross infarcts | −0.038 | 0.027 | −0.093 | 0.015 |
| Change point | Gross infarcts | −0.444 | 0.198 | −0.832 | −0.048 |
| Intercept | Microinfarcts | −0.161 | 0.110 | −0.374 | 0.060 |
| Preterminal slope | Microinfarcts | −0.005 | 0.007 | −0.020 | 0.009 |
| Terminal slope | Microinfarcts | 0.050 | 0.030 | −0.008 | 0.106 |
| Change point | Microinfarcts | −0.661 | 0.217 | −1.096 | −0.228 |
| Intercept | Neocortical Lewy bodies | −1.268 | 0.172 | −1.603 | −0.931 |
| Preterminal slope | Neocortical Lewy bodies | −0.029 | 0.011 | −0.051 | −0.007 |
| Terminal slope | Neocortical Lewy bodies | −0.249 | 0.044 | −0.339 | −0.167 |
| Change point | Neocortical Lewy bodies | −0.752 | 0.269 | −1.268 | −0.220 |
Figure 4.
Contributions of combinations of the pathologic indices to rates of preterminal and terminal cognitive decline, respectively (model derived slopes).
Do factors other than the common neurodegenerative pathologic indices affect the rate of cognitive decline?
Taken together, the above findings suggest that much of the variation in late-life cognitive decline is not accounted for by the pathologic indices of the three most common causes of dementia. Because in prior work we showed that neuronal density in the locus coeruleus, a putative structural element of neural reserve, was related to cognitive decline after controlling for the common pathologic indices35, we conducted a final analysis examining the relation of other possible indicators of reserve, the presynaptic protein complexin-1 and a presynaptic protein-protein interaction critical for neurotransmission (SNAP-25-syntaxin), with cognitive decline and determined the contribution of these indices to the variation in decline after accounting for the pathologic indices of AD, CVD, and LBD. To do so, we used data from a subsample of 265 persons (mean number of cognitive assessments=4.93, SD=2.25, range=2-12) from the Memory and Aging Project in whom these presynaptic protein measures were available and repeated the core model used to test our primary hypothesis but with additional terms for complexin-1, the SNAP-25-syntaxin protein-protein interaction, and their interactions with time. In this analysis, both complexin-1 and the SNAP-25-syntaxin protein-protein interaction were associated with a reduced rate of cognitive decline (complexin-1 estimate = 0.029, SE = 0.012, p=0.018 and SNAP-25-syntaxin estimate = 0.025, SE = 0.011, p=0.025). Further, complexin-1 and the SNAP-25-syntaxin protein-protein interaction explained an additional 6% of between-subjects variation in cognitive decline after accounting for the common pathologic indices. Figure 5 is based on this model and shows the rates of decline for persons with different levels of presynaptic proteins.
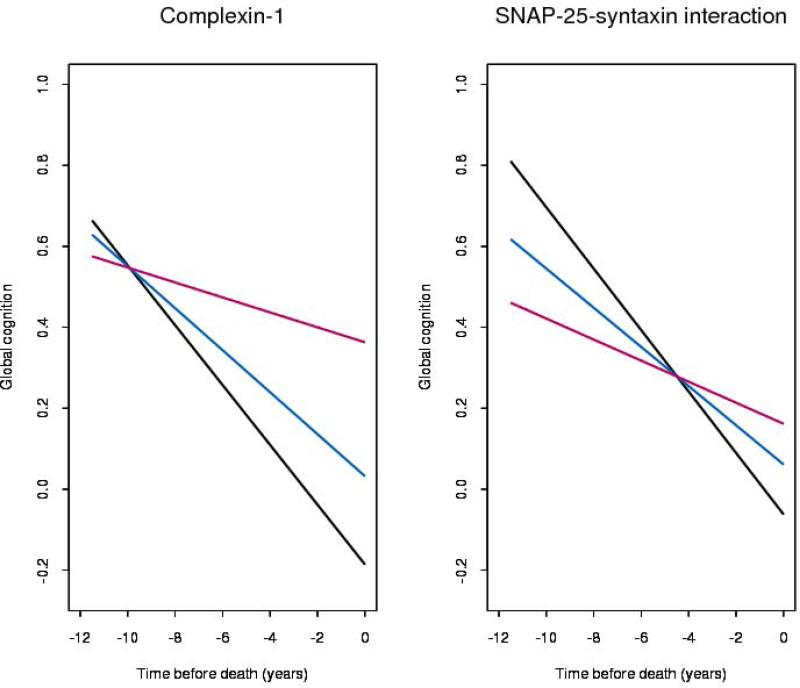
Figure 5.
Rate of cognitive decline associated with high (red, 90th percentile), medium (blue, 50th percentile) and low (black, 10th percentiles) levels of presynaptic proteins (model derived).
DISCUSSION
We examined the relation of the standard pathologic indices of the three most common causes dementia in old age, AD, CVD and LBD, with cognitive decline and determined the extent to which these pathologic indices account for individual differences in rates of cognitive decline in a cohort of more than 850 deceased persons with detailed annual cognitive data for up to 18 years. Results showed that the pathologic indices of the common causes of dementia are important determinants of cognitive decline in old age. To our surprise, however, even when multiple pathologic indices of AD, CVD, and LBD were examined simultaneously, they only explained a total of 41% of the variation in cognitive decline. Thus, the majority of the variation in cognitive decline remained unexplained. This suggests that a large proportion of late life cognitive decline is driven by factors other than the pathologic indices of AD, CVD, and LBD that are the primary focus of scientific efforts to prevent cognitive impairment and dementia. New research seeking the neurobiologic basis of this large residual (i.e., unexplained) cognitive decline is urgently needed to effectively combat the looming public health challenge posed by cognitive decline in old age.
Many prior studies have shown that there is a gap between the severity of pathologic burden and the degree of cognitive impairment in old age16,17,25,26. That is, whereas a large proportion of persons diagnosed with clinical dementia meet criteria for pathologic AD, a sizeable proportion do not; conversely, upwards of 30% of persons who die without a diagnosis of clinical dementia meet pathologic criteria for AD7,8,11,25,26. Numerous reports also have documented a mismatch between the degree of pathologic burden and the level of cognitive function at a point before death, and these findings stimulated an important body of work aimed to understand the basis of this pathology-cognition gap7,8,16,26,27. However, relatively few published reports have examined the relation of the pathologic indices known to cause dementia with longitudinal changes in cognition, and these studies typically focused on a single pathologic index (e.g., AD, often using Braak or other staging methods rather than continuous measures as used here), despite evidence that cognitive impairment is most commonly due to mixed pathologic indices9-11,28,29. Thus, there was a gap in knowledge regarding the extent to which variation in cognitive decline was due to common pathologic indices. This lack of knowledge is not surprising when one considers the data needed derive these estimates: large numbers of non-demented persons at study entry who agree to brain donation, numerous assessments of cognition over time and proximate to death, and high rates of brain autopsy with multiple pathologic indices.
We recently examined the relation of the common pathologic indices with rates of preterminal and terminal cognitive decline in a smaller group of persons than studied here (N=354) and reported that the pathologic indices were associated with a substantially increased rate of cognitive decline, particularly in the preterminal phase12. The present study builds on those findings in two important ways. First, whereas the prior study used a fixed change point for each individual, in this study we used a random change point model that allowed for individual variation in the onset of terminal decline. Thus, the random change point model more closely approximates the true effect of the disease processes studied here. Second, in this study we calculated the amount of variance explained by the pathologic indices first in a linear model and then in a fully articulated random change point model using data from a large cohort of persons with the full spectrum of cognitive function. Given that there is considerable heterogeneity in individual trajectories, with many persons declining, others remaining stable, and some improving, a direct estimate of the degree to which common pathologic indices explain individual differences in the trajectory of cognitive decline is needed to inform efforts to prevent or delay the onset of dementia and even milder forms of cognitive dysfunction in old age. In linear models, we found that the pathologic indices of AD, CVD, and LBD were associated with an increased rate of cognitive decline; however, to our surprise, they accounted for only 41% of the variation in cognitive decline. Further, in random change point models examining the effect of the pathologic indices on the onset of terminal decline and rates of decline before (i.e., preterminal) and after its onset (i.e., terminal decline), we found that the pathologic indices were important determinants of decline, particularly in the preterminal phase that typically precedes dementia; this finding is highly consistent with our prior work12. However, the pathologic indices accounted for even less of the variation in rates of decline when cognition was modeled non-linearly (i.e., the pathologic indices combined explained less than a third of the variation in the onset of terminal decline and rates of preterminal and terminal decline). Taken together, these findings suggest that pathologic indices of AD, CVD, and LBD are important determinants of cognitive decline but they only account for a minority of the variation in rates of cognitive decline. Other factors that explain the residual variation remain to be identified.
Several factors likely contribute to our unexpected finding. First, because the pathologic indices of AD, CVD, and LBD all represent markers of diseases that involve complex cascades of events that over time result in cognitive decline and/or dementia, we are certainly underestimating the true burden of these diseases. In other words, the pathologic indices commonly identified using post-mortem tissue are markers of disease processes that damage the brain in a myriad of ways that are as yet poorly understood and quantified. Second, some of the pathologic indices may lack specificity; for example, as it is currently measured, amyloid includes some contribution by vascular amyloid, and thus this measure may be an imprecise indicator of AD-related changes. Third, our measures of CVD are incomplete and we were unable to examine the contribution of small vessel disease, an important determinant of cognitive impairment in old age30,31. Fourth, we did not account for hippocampal sclerosis, which is relatively common and impairs cognition, or one other recently described pathology that impairs cognition and may be more common than originally thought, tar binding protein-43 (TDP-43)32,33. We also did not measure other pathologic indices such as inflammatory markers, although we are not aware of any study that has demonstrated their relation to cognition after controlling for the common pathologic indices. Finally, there are individual differences in the ability to tolerate or respond to pathology7,8, a concept sometimes called neural or cognitive reserve27, and the brain may actively compensate such that there are functional alterations that affect the degree to which pathologic processes are expressed. Similarly, there are structural elements of reserve that can influence late life cognitive decline. For example, in a prior study, we found that neuronal density in the locus coeruleus was associated with a reduced rate of cognitive decline after controlling for the common pathologic indices35. Further, whereas some have reported that synapses and neurons are decreased in MCI and dementia and loss of these elements may be a consequence of disease pathology36, in prior work we found that presynaptic proteins were associated with better cognition proximate to death after controlling for the pathologic indices of AD and CVD24. Here, we extended prior work to show that presynaptic proteins were associated with a slower rate of decline and accounted for an additional 6% of the between-subjects variation in cognitive decline even after accounting for the pathologic indices of the three most common causes of dementia. Together, the present findings and emerging data on resilience markers suggest that, in addition to AD, CVD, and LBD, less well studied disease processes and resilience markers influence cognitive decline in old age and are important determinants of individual differences in rates of cognitive change. Some of these (e.g., resilience markers) require further investigation and others await discovery.
Modeling longitudinal trajectories of cognitive decline and parameterizing the explained versus unexplained variance are approaches that have broad implications for studies of cognitive decline in old age. Rates of cognitive decline are the phenotype of cognitive aging that we need to understand in order to develop preventions for dementia as well as even milder forms of cognitive decline, which have important functional consequences; for example, we have previously shown in one of these cohorts that cognitive decline fully within the normal range (i.e., among persons without dementia or even mild cognitive impairment) is associated with impaired health and financial decision making40. Thus, efforts to prevent even very subtle cognitive decline are needed. In addition, the ability to separate out the explained versus unexplained variance will allow for more focused efforts to identify risk factors for cognitive decline due to common, age-related pathologic indices versus risk factors associated with cognitive decline due to as yet unknown processes. For example, it is well appreciated that some risk factors for cognitive decline and dementia work through AD and/or CVD pathology37; however, others are not related to any of the three common pathologic indices and likely work through other mechanisms38,39. Our finding that about 60% of late life cognitive decline was not explained by the standard pathologic indices of the common causes of dementia suggests that additional measures of these diseases are needed and furthers the idea that other determinants of late life cognitive decline exist. This has important implications for setting research priorities related to the prevention of cognitive decline in old age. Specifically, efforts to identify the determinants of the residual cognitive decline are urgently needed and will have timely and translational implications for the development of therapeutic targets.
The study has strengths and limitations. Strengths are that all subjects were recruited from the community without known dementia and underwent detailed annual cognitive evaluations for up to 18 years, and autopsy rates were very high. In addition, all post-mortem evaluations were performed by experienced and trained examiners shielded to all clinical data and multiple biologically specific measures of pathology were quantified. The study also has limitations. Most notably, our measures of the disease processes, particularly CVD are incomplete, and there were additional pathologic indices we did not quantify. In addition, the findings are from a selected cohort and their generalizability remains to be demonstrated. Future work is needed to better understand the impact of the common causes of dementia in population-based studies and to identify as yet unknown processes that contribute to cognitive decline in old age.
Supplementary Material
Acknowledgments
We thank the participants in the Religious Orders Study and the Memory and Aging Project and the staff of the Rush Alzheimer's Disease Center.
Funding: The study was supported by NIH/NIA grants P30AG10161, R01AG15819, R01AG17917, R01AG34374, R01AG33678, Canadian Institutes of Health Research grant CBG-101827, the Illinois Department of Public Health, and the Scottish Rite Charitable Foundation of Canada.
Study concept and design: Boyle, Bennett, Schneider, Wilson
Acquisition of data: Bennett, Schneider, Barr, Honer
Analysis and interpretation of data: Boyle, Bennett, Wilson, Yu
Drafting of the manuscript: Boyle
Critical revision of the manuscript for important intellectual content: Wilson, Yu, Bennett, Schneider, Barr, Honer
Statistical analysis: Yu
Obtained funding: Bennett, Boyle
Study supervision: Bennett, Schneider
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data and preparation, review, and approval of the manuscript.
Footnotes
Author contributions: Dr. Boyle had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: The authors have no relevant disclosures to report.
REFERENCES
- 1.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Aging. 2011;40:684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodge HH, Wang CN, Chang CC, Ganguli M. Terminal decline and practice effects in older adults without dementia: The MoVIES project. Neurology. 2011;77:722–730. doi: 10.1212/WNL.0b013e31822b0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psych Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 4.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: Separating practice effects from the effects of growing older. Psych Aging. 2006;21:774–789. doi: 10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- 5.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologic indices account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 7.Crystal D, Dickson P, Fuld D, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38:682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 8.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll I, Resnick SM, Troncoso JC, et al. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanian AB, Kawas CH, Peltz CB, et al. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79:915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Leurgans SE, Boyle PA, et al. Neurodegenerative basis of cognitive decline in old age. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider JA, Arvanitakis Z, Yu L, et al. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135:3005–3914. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Cur Alzheimer Res. 2012;9:630–647. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the Rush Memory and Aging Project. Cur Alzheimer Res. 2012;9:648–665. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Wilson RS, Boyle PA, et al. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012 doi: 10.1002/ana.23654. 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 18.Arvanitakis Z, Leurgans SE, Barnes LL, et al. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird NM, Ware JH. “Random-effects models for longitudinal data”. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 20.SAS 9.2. Cary, NC: [Google Scholar]
- 21.Hall CB, Lipton R, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis. Chapman & Hall; London, UK: 1995. [Google Scholar]
- 23.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – A Bayesian modeling framework: Concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 24.Honer WG, Barr AM, Sawada K, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurology. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 28.Green MS, Kaye JA, Ball MJ. The Oregon Brain Aging Study: neuropathology accompanying healthy aging in the oldesto ld. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologic indices account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 30.Inaba M, White L, Bell C, et al. White matter lesions on brain magnetic resonance imaging scan and 5-year cognitive decline: the Honolulu-Asia aging study. J Am Geriatr Soc. 2011;59(8):1484–9. doi: 10.1111/j.1532-5415.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, Mack WJ, Decarli C, Weiner MW, Mungas DM, Chui HC, Jagust WJ. The aging brain and cognition: contribution of vascular injury and aβ to mild cognitive dysfunction. JAMA Neurol. 2013 Apr 1;70(4):488–95. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, Thal LJ. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48(1):154–60. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay C, St-Amour I, Schneider J, et al. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol. 2011;70(9):788–98. doi: 10.1097/NEN.0b013e31822c62cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Nag S, Boyle PA, et al. Neural reserve, neuronal density in the locus coeruleus, and cognitive decline. Neurology. doi: 10.1212/WNL.0b013e3182897103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheff SW, Price DA, Schmitt FA, et al. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA, Schneider JA, Wilson RS, et al. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76(9):1194–9. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyle PA, Buchman AS, Wilson RS, et al. Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry. 2012;69(5):499–505. doi: 10.1001/archgenpsychiatry.2011.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 40.Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, Bennett DA. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer's disease or mild cognitive impairment. PLoS One. 2012;7(8):e43647. doi: 10.1371/journal.pone.0043647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.