Abstract
Introduction: Hyssopus officinalis (L) (Hyssop, Family: Lamiaceae), one of the endemic Iranian perennial herb with a long history of medicinal use, was studied to detect some biologically active chemical constituents of the plant. Methods: The flavonoids of the hydromethanolic extract of the aerial parts of Hyssopus officinalis (L.) were studied by VLC and crystalisation of the major compound in subsequent fractions. Furthermore, the composition of its essential oil, total phenolic content and antioxidant activities were studied by GC-MS, Folin–Ciocalteau and DPPH reagents respectively. Results: Apigenin 7-O-β-D-glucuronide was isolated as the major flavonoid. All structural elucidation was performed by spectral means. A total of 20 compounds representing 99.97% of the oil have been identified. Myrtenylacetate, Camphor, Germacrene, Spathulenol were the main compounds The total phenol content of the n-butanol and ethylacetate extracts were determined spectrophotometrically according to the Folin–Ciocalteau procedure to be 246 mgGAE g-1 and 51 mgGAE g-1 in the aerial parts of Hyssopus officinalis . The antioxidant activities of apigenin 7-O-β-D-glucuronide, ethylacetate and n-butanol extracts were also determined by DPPH radical scavenging assay with IC50 values of 116×10-3, 103×10-3, 25×10-3 mg mL-1 respectively. The purified flavonoid showed weak radical scavenging activity (IC50 = 116×10-3mg mL-1). N-butanol extract, because of the highest content of total phenolic compounds (246 mgGAE100-1g) had the best antioxidant activity (IC50 = 25mg mL-1). Conclusion: On the whole, the findings of the study revealed that Hyssop possesses valuable antioxidant properties for culinary and possible medicinal use.
Keywords: Hyssopus officinalisf, Total phenols, Radical scavenging, Essential oil, Antioxidant, Culinary herb
Introduction
In recent years, using traditional medicinal knowledge in drug discovery seems so promising that even large pharmaceutical companies have begun to show interest in this field.1,2 The genus Hyssopus L. comprises about 10-12 species distributed mainly in the east Mediterranean to central Asia. Hyssopus officinalis (L) (Hyssop, Family: Lamiaceae), a perennial herb with a long history of medicinal use, is one of the endemic Iranian species of the genus Hyssopus.3,4 Traditionally, H. officinalis named Zufa in Iran, have been used as a carminative, tonic, antiseptic, expectorant and cough reliever.4 Despite having a slightly bitter taste, H. officinalis is often used as a minty flavor and condiment in food industries. The merit of the traditional use of H. officinalis has been supported by some prior studies from the genus Hyssopus, providing several biologically active constituents especially main compounds from essential oils.5-7 Studies dealing with the antioxidant, anti-platelet, and anti-fungal activities of essential oil from H. officinalis have been previously reported.5,7-9 Surveys such as that conducted by Miyazaki et al. have shown that H. officinalis extracts revealed alpha-glucosidase inhibitory effects on intestinal carbohydrate absorption, indicating significant activity against hyperglycemia.10 Numerous studies refer to the composition of H. officinalis oil from different parts of the world4,11,12 However, a few studies have been conducted on flavonoid structures present in H. officinalis.13-15 It is well known that phenolics and flavonoids from plants act as free radical scavenger. There is interest in knowing the phenolic content of fruits and vegetables in order to increase their potential use as nutraceuticals or functional foods. However, scientific information on antioxidant properties of various plants, particularly those that are used in culinary and medicine is still rather scarce.16 Hence, the present study was carried out to detect some biologically active chemical constituents of the plant. This survey is an ongoing point in our study on the plants of Iranian flora.12,17-19Herein, we describe the analysis of essential oil, isolation and structural elucidation of a main flavonoid isolated from aerial parts of H. officinalis. The free radical scavenging activity of the flavonoid, essential oil and methanolic extract were also determined using (2,2-diphenyl-1-picrylhydrazyl) DPPH.
Materials and Methods
General
All solvents and the Folin-Ciocalteu reagent used for the present work were purchased from Merck (Germany), DPPH (1,1-diphenyl-2- picryl-hydrazyl, 90%) was from Sigma (Germany).
UV spectra (λ max) were recorded on a Shimadzu 2100 spectrophotometer. 1H-NMR and 13C-NMR spectra were taken on a Bruker instrument 200 MHz (1H-NMR) and 50 MHz (13C-NMR) in MeOH-d4.
Plant material
The aerial parts of Hyssopus officinalis were collected during flowering stage on June from north of Iran and authenticated by Dr. Mazandarani. Voucher specimens have been deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Extraction and isolation of flavonoids
The dried aerial parts (200g) were ground and defatted with 1L n-hexane. The defatted powdered aerial parts were extracted with 2L 70% methanol in water by maceration at room temperature. The methanol of resultant hydroalcoholic extract was evaporated at 40°C under reduced pressure and affording the aqueous extract. Subsequently, the aqueous extract was partitioned with EtOAc (ETE) and n- butanol respectively. According to TLC analysis, the n-butanol flavonoid rich extract (NBE) was subjected to VLC on silica gel as the stationary phase and eluted with step-gradient EtOAc-MeOH mixtures (8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, each 200 ml) and MeOH (300 ml) providing nine fractions (A-I). All farctions were controlled by TLC on analytical Silica gel GF254 plate using a mixture of EtOAc-MeOH-H2O (6:4:1) as eluent and 5% AlCl3 reagent for detection. On separation, the fraction B and F, each containing one major flavonoid, were dried and dissolved in small volume of methanol and kept in refrigerator at +2°C. The crystals formed in fraction B and F, was separated by filteration and dried to provide Apigenin 7-O-glucuronide (compound 1, 33.6 mg) and compound 2 (14.3 mg).
The structure of compound 1 was elucidated by interpretation and comparison of its spectral data (UV, 1H-NMR and 13C-NMR) with those published references. Due to the lack of sufficient and complete spectroscopic data, Compound 2 has not been identified.
Essential oil isolation and GC- MS analysis
Air-dried plant material was subjected to hydro distillation using a Clevenger-type apparatus for 3 h (1. 3% yield). The obtained essential oil was dried over anhydrous sodium sulphate and stored at +4 °C until tested and analyzed.
Analysis of the essential oil was performed using a Shimadzu, QP 5050A, (E.I Quadrapole) equipped with a FID detector and a DB-1 capillary column (60 m × 0.25 mm i.d.).
For GC–MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium was the carrier gas, at a flow rate of 1 ml/min and split injection with split ratio 1:11. Injector, detector and MS transfer line temperatures were set at 250 °C, 280 °C and 290 °C, respectively. The program used was 60–200 °C at a rate of 1.5 °C/min, held isothermal for 10 min and finally raised to 250 °C at 5 °C/min. Diluted samples (1/100 v/v in methylene chloride) of 1.0 μl were injected manually. The components were identified based on the comparison of their relative retention times and mass spectra with those of standards, Nist 21, Nist 107 and Wiley 229 library data of the GC–MS system and literature data. 20
Determination of Total Phenolic Content of Plant Extracts
Total phenolic content of ETE and NBE were measured according to the Folin-Ciocalteu assay.21 Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dry weight. Briefly, 100 ml of crude extracts and the standard, previously dissolved in methanol, was diluted with water to 8 mL, 0.5 mL of Folin- Ciocalteu phenol reagent was added, and the flasks were shaken vigorously. After 8 min, 1.5 mL of 20% sodium carbonate solution was added, and the mixtures were mixed thoroughly again. The mixtures were allowed to stand for 1 h protected from light. The absorbance of the blue color produced was measured with a spectrophotometer (Shimadzu 2100) at 750 nm. The concentration of total phenolic compounds for each extract was calculated on the basis of a standard curve obtained using gallic acid.
DPPH radical-scavenging assay
DPPH was used to determine free radical-scavenging potential of copmpound1, ETE and NBE. DPPH solution in MeOH (80 g mL-1) was used. IC50 (50% inhibitory concentrations) of compound 1 and extracts were calculated versus MeOH as a negative control and Quercetin was used as a positive control.22
Briefly, stock solutions of compound 1, ETE and NBE were prepared in MeOH to achieve the concentration of 1mg mL-1. Dilutions were made to obtain concentrations of 5×10-2, 5×10-3,5×10-4,5×10-5,5×10-6, 5×10-7mg mL-1. 2 ml of each solution was added to 2 ml of DPPH solution. The absorbance was measured at 517 nm after 30 min of reaction at 25ºC. The experiments were performed in duplicate and the average absorption was noted for each concentration.
Results and discussion
The n-butanol extract (NBE) of H. officinalis was fractionated by using VLC on silica gel. Further purification was achieved by crystallization in methanol to give flavonoid compounds 1-2. The structures of compound 1 was determined by 1H NMR and 13C NMR experiments and UV data. Compound 1 was obtained as yellow crystals. The UV spectra of compound I obtained in this study was as follows: UV, λmax (nm) (MeOH) 268.4, 332.4; (MeONa) 271.0, 300 sh, 386; (AlCl3) 273.6, 297.4, 345,381.8; (AlCl3/HCl) 275.2, 297.4, 340, 379.2, 403; (NaOAc) 265.8, 389.4, 407; (NaOAc/H3BO3) 266.8, 341.8. These UV data were compared with published data (Mabry et al., 1970 and Markham, 1982). The UV spectrum in methanol and its changes after the addition of the customary shift reagents suggested that compound 1 is a 7- substituted flavone with free hydroxyl groups at positions C-5and C-4′.
The 1H NMR spectrum of 1 (Table 1) displayed signals corresponding to a momosubstituted B-ring of the flavonoid nucleus at δ 7.92 (d, J = 8.0) and 6.92 (d, J = 8.0 Hz) each integrating for two protons, which were assigned to H-2′ and H-6′, and H-3′ and H-5′, respectively. A typical flavone H-3 signal at δ 6.82 (1H, s) was observed. The A ring of flavonoid 1 showed two doublets (J =2.1 Hz, meta-coupling) at δH 6.43 and 6.77 attributed to hydrogen atoms H-6 and H-8, respectively.
Table 1. 1H NMR and 13C NMR data of compounds 1 in MeOH-d4.
| Position | dH (J in Hz)(ppm) | dC(ppm) |
| 2 | - | 165.2 |
| 3 | 6.82 | 103.82 |
| 4 | - | 182.78 |
| 5 | - | 162.44 |
| 6 | 6.43(d, J=2.1) | 100.35 |
| 7 | - | 163.80 |
| 8 | 6.77(d, J=2.1) | 95.5 |
| 9 | - | 157.79 |
| 10 | - | 106 |
| 1' | - | 121.60 |
| 2' | 7.92(d, J=8.15) | 129.35 |
| 3' | 6.92(d, J=8.15) | 116.86 |
| 4' | - | 162.4 |
| 5' | 6.92(d, J=8.15) | 116.86 |
| 6' | 7.92(d, J=8.15) | 129.35 |
| ß-D-glucuronide (at C-7) | ||
| 1'' | 5.15 | 100.35 |
| 2'' | m* | 73.77 |
| 3'' | m | 74.98 |
| 4'' | m | 72.78 |
| 5'' | 4.25(d, J=9) | 77.2 |
| 6'' | - | 173.49 |
*multiple
The 1H NMR spectrum suggested that compound 1 is a monosaccharide of apigenin on the basis of a signal in the sugar region at 5.15 (d, J = 7.1 Hz), corresponding to the anomeric protons of β- linked glycosidic bond. The remaining sugar protons appeared in the range δ 3.2- 4.3 ppm (Table 1).
The 13C NMR spectrum of compound 1 (Table 1) revealed 21 carbon signals, 15 of which were assigned to an apigenin unit. The anomeric carbon absorbed at δ 100.35. Two signals at 182.78 ppm and 173.49 ppm attributed to C-4 of flavones skeleton and C-6′′ of sugar moiety, respectively.23-25
The sugar moiety was determined to be β-D- glucuronide from analyses of 1H and 13C-NMR spectroscopic data.23,24 The structure of compound 1 is therefore established as apigenin 7-O-β-D-glucuronide (figure 1).
Figure 1.
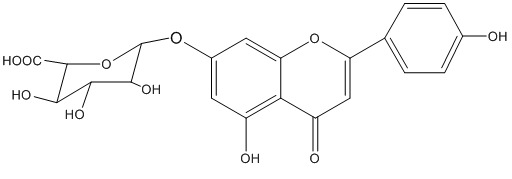
Compounds 1 isolated from H. officinalis aerial parts n-butanol extract.
This flavonoid is determined to be the main flavonoid of the aerial parts of H. officinalis. Apigenin 7-O-β-D-glucuronide was previously isolated from the aerial parts from the study of Wang et al.13
The composition of the oil of H. officinalis is given in Table 2. Constituents were listed in order of their elution from the DB-1 capillary column. Nineteen components were identified, accounting for 99.97% of the sample collected in Iran. The main constituents of the oil were Myrtenyl acetate (74.08%), Camphor (6.76%), Germacrene (3.39%), Spathulenol (2.14%), Caryophyllen Oxide (2.13%) and β-Caryophyllene (2.10%) with lesser amts. of Cis- sabinol (1.75%), β- Bourbonene (1.47%), Bornyl acetate (1.42%).
Table 2. Composition of Hyssopus officinalis L. essential oil.
| Peak No. | Components | Composition (%) |
| 1 | 1,8- Cineole | 0.31 |
| 2 | L- linalool | 0.56 |
| 3 | Cis- sabinol | 1.75 |
| 4 | Camphor | 6.76 |
| 5 | Terpineol-4 | 0.28 |
| 6 | Myrtenal | 0.43 |
| 7 | Bornyl acetate | 1.42 |
| 8 | Myrtenyl acetate | 74.08 |
| 9 | a- Cubebene | 0.53 |
| 10 | ß- Bourbonene | 1.47 |
| 11 | ß-Caryophyllene | 2.10 |
| 12 | a- Caryophyllene | 0.64 |
| 13 | Germacrene | 3.36 |
| 14 | Cubenol | 0.33 |
| 15 | d- Cadinene | 0.47 |
| 16 | Nerodiol Z and E | 0.75 |
| 17 | Spathulenol | 2.14 |
| 18 | Caryophyllene Oxide | 2.13 |
| 19 | Elemol | 0.46 |
| Total | 99.97 | |
We could not find an explanation for the exceptionally large proportion of Myrtenylacetatein the H. officinalis from north of Iran. A review of the published literature indicates that the oil composition of H. officinalis may vary considerably within a single species from one growth season to another affected by climatic parameters and agrotechnical factors, such as fertilization, water supply, and harvesting, especially the phase of plant development at the time of harvest.26
Phenolic content of NBE extract was 246 mgGAE g-1, while in ETE 51 mg GAEg-1. This fact is in correlation with polarity of the solvents used for extraction and solubility of phenolic compounds in them.
The antioxidant activities of apigenin 7-glucuronide, ETE and NBE were examined by comparing them with the known antioxidant, quercetin, by employing the DPPH radical scavenging. The IC50 values for apigenin 7-glucuronide, ETE, NBE and quercetin were found to be 116×10-3, 103×10-3, 25×10-3 and 5.22×10-5mg mL-1.
Our results showed a direct linear correlation between total phenolic content and antioxidant activities. This correlation suggests that the phenolic compounds contribute directly to the antioxidative action, but probably other compounds, to some extent, are also responsible for these activities.
Therefore, H. officinalis L. as a culinary herb and medicinal plant may be considered as natural food ingredients to replace synthetic antioxidants.
Acknowledgement
Authors are thankful to the staff of the Medicinal plants laboratory at the Drug Applied Research Center for their help in conducting GC-MS analysis.
Conflict of interest
The authors report no conflicts of interest in this work.
References
- 1.Do QT, Bernard P. Pharmacognosy and reverse pharmacognosy: a new concept for accelerating natural drug discovery. IDrugs. 2004;7:1017–1027. [PubMed] [Google Scholar]
- 2.Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, Vuorela H. Natural products in the process of finding new drug candidates. Curr Med Chem. 2004;11:1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- 3.Kazazi H, Rezaei K, Ghotb-sharif SJ, Emam-djomeh Z, Yamini Y. Supercriticial fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chemistry. 2007;105:805–811. [Google Scholar]
- 4.Khazaie HR, Nadjafi F, Bannayan M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis) Industrial Crops and Products. 2008;27:315–321. [Google Scholar]
- 5.Tognolini M, Barocelli E, Ballabeni V, Bruni R, Bianchi A, Chiavarini M, Impicciatore M. Comparative screening of plant essential oils: phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006;78:1419–1432. doi: 10.1016/j.lfs.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 6.De almeida LF, Frei F, Mancini E, De martino L, De feo V. Phytotoxic activities of mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghfir B, Fonvieille JL, Koulali Y, Ecalle R, Dargent R. Effect of essential oil of Hyssopus officinalis on the lipid composition of Aspergillus fumigatus. Mycopathologia. 1994;126:163–167. doi: 10.1007/BF01103770. [DOI] [PubMed] [Google Scholar]
- 8.Babovic N, Djilas S, Jadranin M, Vajs V, Ivanovic J, Petrovic S, Zizovic I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innovative Food Science & Emerging Technologies. 2010;11:98–107. [Google Scholar]
- 9.Ghfir B, Fonvieille JL, Dargent R. Influence of essential oil of Hyssopus officinalis on the chemical composition of the walls of Aspergillus fumigatus (Fresenius) Mycopathologia. 1997;138:7–12. doi: 10.1023/A:1006876018261. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki H, Matsuura H, Yanagiya C, Mizutani J, Tsuji M, Ishihara C. Inhibitory effects of hyssop (Hyssopus officinalis) extracts on intestinal alpha-glucosidase activity and postprandial hyperglycemia. J Nutr Sci Vitaminol (Tokyo) 2003;49:346–349. doi: 10.3177/jnsv.49.346. [DOI] [PubMed] [Google Scholar]
- 11.Langa E, Cacho J, Palavra AMF, Burillo J, Mainar AM, Urieta JS. The evolution of hyssop oil composition in the supercritical extraction curve:Modelling of the oil extraction process. The Journal of Supercritical Fluids. 2009;49:37–44. [Google Scholar]
- 12.Varga E, Hajdu Z, Veres K, Mathe I, Nemeth E, Pluhar Z, Bernath J. Investigation of variation of the production of biological and chemical compounds of Hyssopus officinalis L. Acta Pharm Hung. 1998;68:183–188. [PubMed] [Google Scholar]
- 13.Wang N, Yang XW. Two new flavonoid glycosides from the whole herbs of hyssopus officinalis. J Asian Nat Prod Res. 2010;12:1044–1050. doi: 10.1080/10286020.2010.533120. [DOI] [PubMed] [Google Scholar]
- 14.Marin FR, Ortuno A, Benavente-garcia O, Del rio JA. Distribution of flavone glycoside diosmin in Hyssopus officinalis plants: changes during growth. Planta Med. 1998;64:181–182. doi: 10.1055/s-2006-957401. [DOI] [PubMed] [Google Scholar]
- 15.Hilal S, El-sherei M, El-alfy T. Study of the flavonoid content of Hyssopus officinalis L. Egyptian Journal of Pharmaceutical Sciences. 1979;20:271–278. [Google Scholar]
- 16.Miliauskas G, Venskutonis PR, Van beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 2004;85:231–237. [Google Scholar]
- 17.Modaressi M, Delazar A, Nazemiyeh H, Fathi-azad F, Smith E, Rahman MM, Gibbons S, Nahar L, Sarker SD. Antibacterial iridoid glucosides from Eremostachys laciniata. Phytother Res. 2009;23:99–103. doi: 10.1002/ptr.2568. [DOI] [PubMed] [Google Scholar]
- 18.Fathiazad F, Khosropanah MK, Movafeghi A. Cycloartane-type glycosides from the roots of Astragalus caspicus Bieb. Nat Prod Res. 2010;24:1069–1078. doi: 10.1080/14786410902975582. [DOI] [PubMed] [Google Scholar]
- 19.Movafeghi A, Abedini M, Fathiazad F, Aliasgharpour M, Omidi Y. Floral nectar composition of Peganum harmala L. Nat Prod Res. 2009;23:301–308. doi: 10.1080/14786410802076291. [DOI] [PubMed] [Google Scholar]
- 20.Adams R. Identification of Essential Oils Components by GasChromatography Quadrupole Mass Spectroscopy. USA: Allured Publishing Corporation; 2001. [Google Scholar]
- 21.Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci. 2009;22:102–106. [PubMed] [Google Scholar]
- 22.Nahar L, Russell WR, Middleton M, Shoeb M, Sarker SD. Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm. 2005;55:187–193. [PubMed] [Google Scholar]
- 23.Agrawal PK. Carbon-13 NMR of Flavonoids. The Netherlands: Elsevier Science Publishers; 1989. [Google Scholar]
- 24.Harborne JB. The Flavonoids: Advances in research since 1986. London: Chapman & Hall; 1994. [Google Scholar]
- 25.Mabry T J, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York: Springer; 1970. [Google Scholar]
- 26.Kerrola K, Galambosi B, Kallio H. Volatile Components and Odor Intensity of Four Phenotypes of Hyssop (Hyssopus officinalis L.) J Agric Food Chem. 1994;42:776–781. [Google Scholar]


