Abstract
Purpose: This study is aimed at determining chemical constituents and antimicrobial activities of a common brand of black tea (Lipton®) in Nigeria. Method: Standard methods were employed for testing carbohydrates, tannins, saponins, flavonoids, alkaloids, steroids and terpenes in the tea. Antimicrobial activities of methanolic and aqueous extracts of the tea on four standard strains of organisms: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis were also determined by standard methods. Result: Results showed that the tea contains tannin and reducing sugar. Concentrations of 1%, 2%, 4%, 6%, 8% and 10% aqueous and methanolic extract of black tea were prepared and their zones of inhibition determined against the four test organisms using the cup plate method. This was compared with zones for standard disc Gentamicin (10 µg) and Erythromycin (15 µg). Pseudomonas aeruginosa was sensitive to 2% to 10% aqueous extracts and intermediate to 6%, 8% and 10% methanolic extracts. E. coli was intermediately sensitive to 6%, 8% and 10% aqueous extract and 2% to 10% methanolic extracts. B. subtilis was intermediately sensitive to 4%, 6% and 8% aqueous extract and 4% to 10% methanolic extract but sensitive to 10% aqueous extract. Staph.aureus was intermediately sensitive to 4% to 10% aqueous extracts and 2% to 10% methanolic extracts. B. subtilis had the lowest MIC values of both aqueous and methanolic extracts. In conclusion, this study has shown that Lipton® has antimicrobial properties on E.coli, Staph.aureus, B.subtilis and Ps.aeruginosa and contains tannin and reducing sugar.
Keywords: Phytochemical screening, Antimicrobial properties, Black tea, Nigeria
Introduction
Tea is the second most commonly drunk liquid after water and the most common beverage drunk.1 Tea, the drink, is an infusion of variously processed leaves and flowers of one of the varieties of an evergreen shrub botanically called Camellia sinensis. Black tea is one of a varieties of preparations made from Camellia sinensis. Others include green teas, white tea and oolong tea. The processes of obtaining the various types of tea are different.
The chemical compositions of C. sinensis is not completely known but is understood to be quite complex. Black tea is more complex than green tea partly because of the oxidation process that occurs during fermentation.2 Chemical reactions take place when dried and finished tea is extracted into water increasing the complexity of the chemical mix in a cup of tea. When left to stand, further chemical reactions still takes place in the tea. C. sinensis has been used for different purposes which could be classified as edible use, medicinal use (Internal and external uses) and other uses which include dye, essential oil, food flavouring and perfumery.3
C. sinensis has been reported to have physiological and pharmacological effects,4,5 strengthening capillary, slows down catabolism of catecholamine, exert anti inflammatory effects by enhancing effect of Vitamin C. It acts as an anti oxidant, also as hypocholesterolemic action. It inhibits angiotensin converting enzyme and the growth of implanted malignant cells.6 One of the earliest reports of C. sinensis regarding its anti microbial activity is its recommendation by an army surgeon in soldiers’ water bottles as a prophylactic against typhoid.7
There are several reports on the antibacterial effects of tea in vitro and in vivo mainly on intestinal pathogen.8-10 The Japanese and others in a series of well conducted systemic studies showed that tea extract inhibit and kill various species of Staphylococcus, Salmonella, Shigella and Vibrio.11
The aim of this study is to determine the chemical constituents of a common brand of black tea (Lipton®) found in the Nigerian environment and to compare its antimicrobial properties with standard antimicrobial agents using Gentamicin and Erythromycin disc.
Materials and Methods
Extraction of Black tea
Aqueous extraction was done as follows: sterile distilled water was allowed to boil, 100ml of the water was measured into a conical flask with five (5) Lipton® bags and allowed to extract (infusion) for about two minutes.
Alcoholic extraction was done using 90% Methanol. About 100ml of Methanol was measured with a measuring cylinder into a conical flask containing five Lipton® tea bags and allowed to extract for 12 hours.
The Aqueous and Methanolic extracts were poured into separate crucibles and evaporated to dryness on a water bath (Aqueous at less than 100oC and Methanolic at 60oC). Two grams (2g) of powdered dry extract was dissolved in 20ml volume each of distilled water of both Aqueous and Methanolic extract to produce 10% solutions. Serial dilution was done to obtain 8%, 6%, 4%, 2% and 1% concentrations. The different concentrations were made inside labelled, sterile bottles.
Preliminary phytochemical screening of the extracts
About 2g of the dried extract was weighed and dissolved in distilled water. The solution obtained was then subjected to various phytochemical screening to identify the chemical components present in the extract using qualitative chemical tests with the use of chemical reagents. Standard methods were employed for testing carbohydrates, tannins, saponins, flavonoids, alkaloids, steroids and terpenes.12,13
Antimicrobial screening of the extract
Culture of the test organisms (Escherichia coli ATCC 25722, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923 and Bacillus subtilis NCTC8325B76) were prepared by an inoculating surface scrapes on solid media into 5ml of sterile nutrient broth which was then incubated at 37oC overnight. The overnight cultures were diluted to obtain approximately 106 colony forming units according to standard practice (1 in 1000 dilutions for Gram positive and 1 in 5000 dilutions for Gram negative organisms). Already prepared 20ml sterile nutrient agar were melted and allowed to cool to body temperature and poured into sterile petri dish. The melted agar were allowed to solidify and then flooded with the required final dilution of the organism, the excess was discarded and the petri dishes labelled. This was allowed to diffuse into the agar for 30minutes. Wells were made on the already flooded agar using cup borer (10mm) and the wells were sealed with a drop of the melted agar. Then 0.1ml of the prepared extract concentrates to be tested was then filled into the hole and labelled. This was allowed to diffuse for at least an hour and then incubated for 24 hours at 37oC. The diameter of the zones where growth was inhibited was measured in millimetres (mm) and recorded. For the sensitivity test of standard antibiotics instead of drilling holes, the sensitivity disc (Gentamicin and Erythromycin) were placed on the agar in the petri disc and labelled. This was allowed to stand for one hour to enable antibiotics to diffuse. The plates were incubated at 37oC for 24 hours. The zones where growth was inhibited was measured (in mm) and recorded.
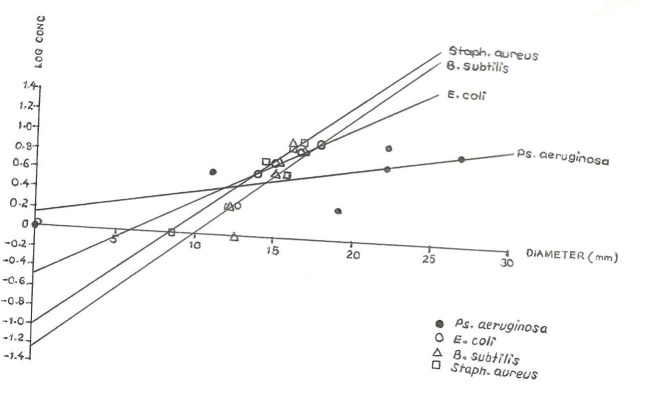
The Minimum Inhibitory Concentration (MIC) was determined by plotting the graph of log concentration against the diameter in mm. The MIC from the graph was the antilog of the intercept value obtained at the Y-axis (log conc.) axis.
Results
As shown on Table 1, the phytochemical screening carried out on the Lipton brand of Camellia sinensis extract was found to contain reducing sugar and tannins.
Table 1. Phytochemical screening of lipton® Camellia sinensis) extract.
| Phytochemical compound | Result |
| Carbohydrate | Absent |
| Reducing sugar | Present |
| Tannin | Present |
| Saponin | Absent |
| Flavonoid | Absent |
| Alkaloid | Absent |
| Steroid and triterpenes | Absent |
Generally, the observed mean zone of inhibition on Table 2 shows an increase in zone with increase in the C. sinensis concentrate. However, there were instances of repeated zone of inhibition like 4% and 6% aqueous concentration on B. subtilis. There were instances of low zone of inhibition between two high zones like 4% aqueous extract on Ps. aeruginosa and 2% and 4% Methanolic concentrates of Ps. aeruginosa and E.coli respectively. High zone of inhibition between two low zones were observed at 4% aqueous and 8% aqueous concentrate of Staph.aureus and Ps.aeruginosa respectively.
Table 2. Mean zone of inhibitions of various concentrations of aqueous extract, methanolic extract and standard disc (gentamicin 10µg, erythromycin 15µg) on Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus.
| Mean zone of inhibition | ||||||||||||||
| - | Aqueous extract (mm) | Methanolic extract (mm) | Standard disc (mm) | |||||||||||
| Organisms | 1% | 2% | 4% | 6% | 8% | 10% | 1% | 2% | 4% | 6% | 8% | 10% | G | E |
| Ps. Aeruginosa | - | 19 | 11.5 | 22 | 26.5 | 22 | 11 | 10.5 | 12.5 | 14 | 16 | 15.5 | 27 | 16 |
| E. coli | - | 12.5 | 13 | 15 | 16.5 | 17.5 | - | 14 | 13.5 | 15.5 | 16 | 16.5 | 24.5 | 6.5 |
| B. subtilis | 12.5 | 12 | 15 | 15 | 16.5 | 18 | - | 13 | 14.5 | 15 | 16 | 17 | 25.5 | 14 |
| Staph aureus | 8.5 | 12 | 15.5 | 14 | 16 | 16.5 | - | 15 | 9 | 15.5 | 14.5 | 15 | 28 | 12 |
| Cup borer size: 1cm (10mm; G: Gentamicin; E: Erythromycin | ||||||||||||||
| Clinical and laboratory standard institute (CLSI) values: | ||||||||||||||
| Gentamicin (10µg): sensitivity (>18mm), intermediate (-), resistance (>13mm) | ||||||||||||||
| Erythromycin (15µg): sensitivity (>18mm), intermediate (14-17mm), resistance (>13mm). | ||||||||||||||
With the exception of 4% methanolic concentrate the zone of inhibition on Staph.aureus is almost the same ranging between 14.5 to 15.5mm using CLSI standard values; all the organisms were sensitive to Gentamicin while only Ps.aeruginosa and B.subtilis showed moderate or intermediate sensitivity to Erythromycin. Gentamicin gave a higher zone of inhibition as compared to erythromycin showing that it is more effective.
Figures 1 and 2 show the graphs of log concentration against diameter for methanolic and aqueous extracts on various organisms respectively. Table 3 above shows the MIC values of aqueous and methanolic extract of C.sinensis which indicates the minimum concentration required to inhibit the growth of an organism calculated from figures 1 and 2.
Figure 1.
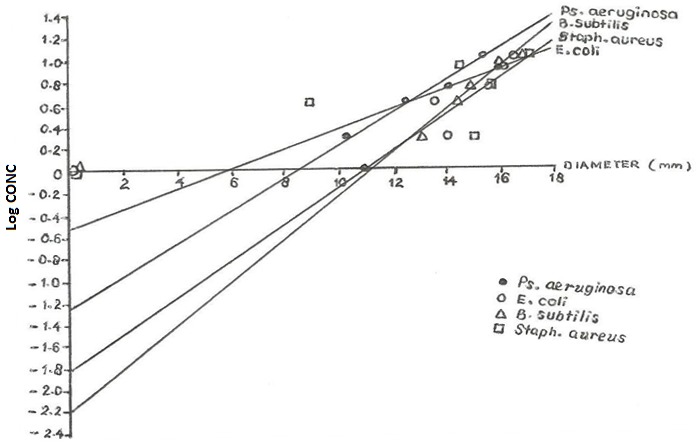
Graph of log concentration against diameter for methanolic extract on various organisms
Figure 2.
Graph of log concentration against diameter for aqueous extract on various organisms
Table 3. MIC value of aqueous and methanolic extract of Camellia sinensis.
| - | MIC value (x 10 µg/ml) | |
| Organisms | Aqueous | Methanolic |
| Ps.aeruginosa | 1.413 | 0.050 |
| Staph.aureus | 0.089 | 0.013 |
| E.coli | 0.280 | 0.250 |
| B.subtilis | 0.056 | 0.005 |
Discussion
From the agar diffusion experiment carried out to determine the susceptibility of Ps.aeruginosa, Staph.aureus, B.subtilis and E.coli to different concentrations of black tea extract, almost all the organisms were sensitive at all concentration with the exception of 1% aqueous extract on Ps.aeruginosa and E.coli and 1% methanolic extract on E.coli, B.subtilis and Staph.aureus. The antimicrobial properties could be as a result of the presence of tannin which is known generally for antimicrobial activity.
The inconsistency in the zone of inhibition aside from other factors such as batch work that could affect the result could also be as a result of unavoidable variation in the time of preparation of the concentrate and its use. A cup of black tea when left to stand for a while changes colour from black to milky black colour especially when left to cool. This physical change could indicate chemical changes or reaction taking place in the black tea.
Using the Clinical and Laboratory Standard Institute(CLSI) standard value for Gentamicin and Erythromycin, Ps.aeruginosa gave a similar zone of inhibition to the sensitivity value of Erythromycin and Gentamicin at 4% aqueous extract and above and B.subtilis at 10% aqueous extract. Using the 14 to 17mm CLSI intermediate value for Erythromycin, Ps.aeruginosa, E.coli, B.subtilis and Staph.aureus gave similar zone of inhibition to the intermediate sensitivity value of Erythromycin at 6%, 2%, 4%, 6% methanolic extract and above respectively. For the aqueous extract, E.coli, B.subtilis and Staph.aureus similar zones were at 6%, 4% and 4% concentrations and above respectively. Organisms that gave zone of inhibition from 13 and below matches with the resistance value of both Gentamicin and Erythromycin at the various concentrations.
The MIC value of an agent is the lowest concentration that prevents growth after overnight incubation. For quantitative estimates of antibiotics activities, dilutions of the antibiotic may be incorporated into broth or agar medium and incubated with the test organism. The MIC value obtained graphically showed that the aqueous extract has increasing MIC values on the organism in the following order: B.subtilis, Staph.aureus, E.coli and Ps.aeruginosa. The methanolic extract on the other hand has increasing MIC value on the organism in the order B.subtilis, Staph.aureus, Ps.aeruginosa and E.coli. The smaller the MIC value the more effective the extract, that is, the smaller the concentration required to inhibit the growth of the organism hence methanolic extract on B.subtilis is the most effective and aqueous extract on Ps.aeruginosa is the least effective.
Generally, the methanolic extract gave smaller MIC values on each organism than the aqueous extract. This could be as a result of the inherent antimicrobial property of the methanol itself. Considering the zone of inhibition however, once the aqueous extract starts eliciting its effect, it gives greater zone of inhibition than the methanolic extract. This can be seen in B.subtilis though it has a 0.05µg/ml methanolic MIC value did not inhibit at 10% concentration while Ps.aeruginosa with 14.13µg/ml of aqueous extract MIC value was inhibited at 2%. These differences might be as a result of the mechanism of action of the tea and also because of difference in their cell walls.
Conclusion
Within the limits of experimental errors, it was found out that Lipton brand of Camellia sinensis contains tannin and reducing sugar. It has also been shown to have antimicrobial properties on E.coli, Staph.aureus, B.subtilis and Ps.aeruginosa. From the MIC values obtained, B.subtilis requires the least concentration of either methanolic or aqueous extract to inhibit its growth.
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Okubo S, Ikigai H, Toda M, Shimamura T. The anti-haemolysis activity of tea and coffee. Lett Appl Microbiol. 1989;9(2):65–6. [Google Scholar]
- 2.Hamilton-miller JMT. Antimicrobial properties of tea (camellia sinensis l.) Antimicrob Agents Chemother. 1995;39(11):2375–7. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samman S, Sandstrom B, Toft MB, Bukhaye K, Jensen M, Sorensen S, Et AL. Green tea extracts to foods reduces non home-iron absorption. Am J Clinc Nutri. 2001;73(3):607–12. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 4.Min Z, Paige X. Quantitative analysis of the active constituents in green tea. Pyrometer Res. 1991;5:239–40. [Google Scholar]
- 5.Stagg GV, Millin DJ. The nutritional and therapeutic value of tea-a review. J Sci Food Agric. 1975;26:1439–59. [Google Scholar]
- 6.Hattori M, Kusumoto IT, Namba T, Ishigami T, Hara Y. Effect of tea polyphenols on glucan synthesis by glucosyltransferase from streptococcus mutans. Chem Pharm Bull. 1990;38(3):717–20. doi: 10.1248/cpb.38.717. [DOI] [PubMed] [Google Scholar]
- 7.Using tea to fight typhoid. Tea and Coffee Journal 1923;129. [Google Scholar]
- 8.Das DN. Studies on the antibiotic activity of tea. J Ind Chem Soc. 1962;39:849–54. [Google Scholar]
- 9.Ryu E. Prophylactic effect of tea on pathogenic micro-organism infection to human and animals (1) Int J Zoonoses. 1980;7(2):164–70. [PubMed] [Google Scholar]
- 10.Scalbert A. Antimicrobial properties tannins. Phytochemistry. 1991;30:3875–83. [Google Scholar]
- 11.Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lelt Appli Microbial. 1989;8:123–5. [Google Scholar]
- 12.Trease GE, Evans WC. Introduction and general methods in pharmacognosy. 14th ed. London: Alden press; 2002. [Google Scholar]
- 13.Sofowora EA. Traditional medicine and medicinal plant of africa. Nigeria: Spectrum books publishers; 1982. [Google Scholar]