Abstract
Purpose: The present study was designed to explore the effect of intraperitoneal administration of cisplatin in germinal epithelium of mice. There are few reports on the side effect of cisplatin on spermatogenesis when are used as anticancer drug. Therefore, in the present study the effect of cisplatin on spermatogenesis was evaluated by electron microscopy. Methods: Twenty balb/c mice aging 6-8 weeks was used in this study. The mice were divided into two groups, control and cysplatin treated. cysplatin was injected for five days as 2.5 mg /kg. The mice were sacrificed after 5 weeks and testicular specimens were removed, fixed in boueins, formaldeyd fixative and 2.5% Glutaraldehide then prepared for light and electron microscopic study. Results: Observation with optic microscope in treated group thickness of germinal epithelium was reduced a lot and increased the number of apoptotic cells. In some seminiferous tubules only sertoli cells were observed and nucleus of spermatogony cells was hetrochromatin. The electron microscopic observations showed some irregularity waviness and thickening in basal layer. Also myoid cells of this group were thick and contracted. In this group many apoptotic cells and damaged organelles were seen. Conclusion: It was indicated that cisplatin affected testicular germinal epithelium by both cytotoxic effect and induction of apoptosis.
Keywords: Cisplatin, Electron microscope, Apoptosis, Germinal epithelium
Introduction
Infertility is one of the major problems following chemotherapy in cancer treatment.1In young male patients, chemotherapy regimes affect fertility by influence on testicular function. Although the cancer controlled by chemotherapy, the ability to have a normal child as a factor of life quality becomes a major issue. Testicular dysfunction is the most common long-term side effects of chemotherapy in men.
Spermatogenesis is influenced by radiotherapy and cancer cytotoxic drugs.2-5 Recently, the Cisplatin is used for treatment of various malignancies including cancer of testis, ovary, lung, bladder and Hodgkin and non-Hodgkin lymphoma6-10 The previous studies on the side effects of chemotherapeutic drugs such as procarbazine, cyclophosphamide and chlorambucil showed long-term (up to years) azoospermia after treatment.3,11 There are a few reports on the side effect of Cisplatin on the spermatogenesis when they are used as anticancer drugs, in the literature.12 Tumors are characterized by division, which is no longer controlled as it is in normal tissue. Cancer cells no longer have the normal checks and balances in place that control and limit cell division. The process of cell division, normal or cancerous cell, is through the cell cycle. The cell cycle goes from the resting phase, through active growing phases and then to mitosis (division). The ability of chemotherapy to kill cancer cells depends on its ability to halt cell division. So chemotherapy is most effective at killing cells that are rapidly dividing. Unfortunately, chemotherapeutic agents can not differentiate between the cancerous and the normal cells. It has been shown that one of the mechanisms in cell destruction following chemotherapy is apoptosis.12-16 Some agents including genes factors, Testicular ischemia, heat stress, exposure to irradiation and toxic substances, could increase the rate of germ cell apoptosis in testis.5,12-17Cisplatin is not a classical DNA directed alkylation agent but it involve in transcription, translation and repairing of DNA (like alkylation agents) and currently used in high dose therapy approaches to the treatment of various malignancies including, testis, ovary, lung, bladder cancers and Hodgkin and non-Hodgkin lymphoma.6-10 These studies have not provided enough information about ultra-structural changes in germinal epithelium of testis and spermatogenesis. On the other hand, the results of electron microscope in evaluating morphological pattern of nucleus, organelles, membrane and cell adhesion is more valuable in comparison to light microscope. In the meantime, the knowledge about the ultra structural changes of germinal stem cells and spermatogenic cells following the administration of Cisplatin can help to understand the mechanisms of action of Cisplatin.
Materials and Methods
Animals: Twenty balb/c mice aging 6-8 weeks was used in this study. The mice were divided into two groups, control and Cisplatin treated. Cisplatin was injected intraperitoneally for five days as 2.5 mg /kg. Since spermatogenic cycle in mice is 35 days, mice in both groups were sacrificed 35 days after last injection. The testes were removed from the abdominal cavity and separated from the epididymis with care by using a surgical blade and then half of testicular specimen from right testis was fixed in bouein̕s fixative for 48 h for quantitative studies. For quantitative study, we used Motic Image plus 20 software. That is, in 20 microscopic fields in the cross sectioned seminiferous tubule the number of spermatogonia and sertoli cells, thickness of germinal epithelium and spermatogenesis index (SI) were determined. The obtained data were analysed with T-test and using spss.13 softwere. Another half of the testis fixed in formaldehyde for histochemical study by using apoptosis Kit for detection of apoptotic cells. Then both groups processed for light microscopy.
The specimens from the left testis were fixed in 2.5% Glutaraldehyde (Pro. Sci. Tech. Au) for 12 h and washed with 0.2 M phosphate buffer and post fixed with 2% osmium tetroxide (TAAB, UK) for 2 h. After Dehydration, Clearing and Infiltration they embedded in resin (Pro. Sci. Tech. Au) and sectioned using ultramicrotome (Richert-Jung, Au). Semithin stained with toluidine blue and studied with light microscope. The thin sections (80 nm thickness) were stained with uranyl acetate and lead citrate and were studied with Leo 906 transmission electron microscope (Leo, Germany).
Results
Light microscopy
The result from light microscopy revealed that in control group the germinal epithelium of seminiferous tubules composed of multilayer cells and showed features of active spermatogenesis. So the 12 stages of spermatogenesis could easily be realized according to morphological characteristics of germinal epithelium in the seminiferous tubules (Figure 1.A).
Figure 1.

Photomicrography of seminiferous tubules of mice: A) in control group, Note different spermatogenenic cells in tubules. PAS staining .660 X. B) treated with cisplatin. Note the destroyed germinal epithelium and hyper chromic nuclei, PAS staining. 330 X. C) treated with cisplatin, sertoli cells are only observed inside tubules, PAS staining, 1650 X. D) which shows apoptotic cells in cisplatin receiving group Tunnel staining.660X.
In experimental group, the thickness of germinal epithelium was reduced and / in many cases it was completely depleted and only sertoli cells were left. Therefore, the stages of spermatogenesis were not detectable (Figure 1.B,C).
Morphometric evaluation
Morphometric study showed that the mean number of spermatogonia/tubule in control group was 43.22±1.55 and in treated group was 21.42±2.44. Statistical analysis showed that the mean number of spermatogonia in treated group in comparison to control group is significantly (P<0.05) reduced (table 1). Spematogenic Index (SI) in control group was 24.43±9.22, in treated group was 1.52±1.39. Statistical analysis showed that the SI values in control and treated group is significantly (p=0.000) different (Table 1). The mean number of sertoli/tubule cells in control group was 5.50±0.52, in treated group was 8.61±0.80. Statistical analysis showed that the difference between controle and treated group is significant (P<0.05) (Table 1). Thickness of germinal epithelium in control group was 57.33±4.1 µm but it reduced to 20.90±3.84 µm in treated group. Statistical analysis showed that the difference between control and treated group is significant (P<0.05) (Table 1).
Table 1. Comparison of different parameters in testis from control and cisplatin receiving group.
| Groups |
No. of Spermatogonia cells |
No. of Sertoli cells |
Thickness of germinal Epi (µm) |
No. of Apoptotic cells |
SI value |
| Control | 43.22±1.55 | 5.50±0.52 | 57.33±4.1 | 1.27±0.20 | 24.43±9.22 |
| Cisplatin | 21.42±2.44* | 8.61±0.80* | 20.90±3.84* | 7.21±0.90* | 1.52±1.39* |
*: Significantly different from control group
Histochemistery evaluation
The 3 micron samples was provided from fixed samples in formaldehyde and after deparafination, incubated in sodium citrate 0.1M buffer for 5 min, and then DNA fragmentation evaluated by Tunnel Kit based on instruction of company in order to show apoptotic cells. Histochemical study showed that the mean number of apoptotic cells/tubule in control group was 1.27±0.20 and in treated group was 7.21±0.90 which is significantly (P<0.05) different (table 1) and (Figure 1.D).
Electron microscopy
Ultrastructural studies revealed that in control group, the spermatogonia were regularly arranged and rested on basal lamina. The cells had a spherical or oval nucleus, with higher magnification they contained few mitochondria and poorly developed endoplasmic reticulum (Figure 2.A,B). Sertoli cells were also rested on basal lamina and had large nuclei with prominent nucleolus (Figure 2.A). The Junction between two adjacent sertoli cells was obvious (Figure 3.A). This structure has important role in normal spermatogenesis by forming Blood Testes Barrier (BTB) and is composed of three elements (Figure 3.A).
Figure 2.
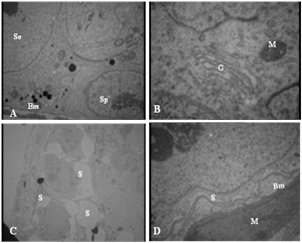
Electronmicrograph of seminiferous tubule in mice: control group (A, B) and experimental group (D, C). A) Spermatogonia (Sp) and sertoli cells (Se) on basement membrane (Bm), 3097 X. B) mitochondria (M) and Golgi (G), 12930 X. C) Note separation of cells from each other (s). D) Sertoli cell is separated from basement membrane (S). The basement membrane is wavey (Bm). A contracted myoid cell is shown in the figure (M). 3097 X.
Figure 3.
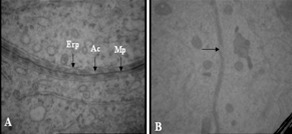
Electronmicrograph of Blood-Testes-Barrier: A) Control group, Mp: merged plasma membrane, Ac: actin filaments, Er: endoplasmic reticulum, 46460 X. B) experimental group, Note the rupture areas in BTB (arrow), 21560 X.
Merged plasmalemma of adjacent sertoli cells in the center.
Dilated endoplasmic reticulum in both sides.
Bundles of filaments that are sandwiched between plasmalemma and endoplasmic reticulum.
Study of testis in the treated group showed that spermatogonia and sertoli cells were separated from basal lamina and neighboring cells by large spaces (Figure 2.C). Basement membrane was morphologically irregular and wavey. Myoid cells in the boundary tissue were thickened and condensed (Figure 2.D). BTB in this group was ruptured and appeared thinner than in the control group (Figure 3.B).
In this group also a lot of apoptotic cells in germinal epithelium were observed that they were recognized based on DNA fragmentation (Figure 4.A). In this group also interacellular granules in interstitial cells were dramatically increased (Figure 4.B).
Figure 4.

Electronmicrograph of: A) An apoptotic cell with fragmentation of DNA 10000 X, B) Interstitial cell of mice from experimental group. Note numerous secretary granules. 6000 X, D) Part of spermatogonial cell in the seminiferous tubule in of mice in experimental group. Note ruptured mitochondria (arrow). 3097 X.
Spermatogenic cells had also several damaged organelles such as vacuolated mitochondria (Figure 4.C). Due to destructive effect of Cisplatin different spermatogenic cells and stage of spermatogenesis was rarely observed.
Discussion
The aim of this study was to evaluate the adverse effect of cysplatin, as an anticancer drug, on germinal epithelium. The study revealed that in treated group which was received cysplatin the thickness of germinal epithelium, number of spermatogonial cells and SI were decreased. In addition, the nuclei of spermatogonial cells were dense in comparison to control group. This finding is similar to those obtained with other chemotherapeutic agents.2,18
Other findings of the present study were that cellular proliferation in the seminiferous tubules was reduced and nuclear hetrochromatin was increased. Both of which is indicating that decreased thickness of germinal epithelium in our study, is partly due to decreased cell division. In support of our finding other researcher has also been reported a reduction in mitosis and DNA changes following treatment with chemotherapeutic drugs.19,20 On the other hands, it shown that the agents that are used in chemotherapy tend to act on activety dividing cells such as spermatogonia and cease their division.3,21 Our results also showed that apoptotic cells were increased in cysplatin receiving group. In support of this finding there are some reports showing that anticancer drugs could induced apoptosis in germinal epithelium.12-16 On the other hand, apoptosis has a critical role on the removal of damaged spermatogonial cells to prevent the formation of abnormal sperms.22 It is also shown that spermatocytes that fail to complete their mitotic division are removed by apoptosis.23 It appears that cysplatin as a chemotherapic agent induces apoptosis on spermatogenic cells. Other changes, such as separation of spermatogonial cells from each other and basal lamina could also be considered as preapoptotic sign. Other finding such as nuclear condensation, chromatin margination and mitochondrial vacuolization are additional evidence for appearance of apoptotic cells. There were also several separations between cells of germinal epithelium and between them and basal lamina. In this regard, it has been shown that cell adhesion enhances the survival of germ cells and cadherins mediated adhesion between sertoli and germ cells in vitro.24Cadherins as an important molecular system controls interaction between sertoli and germ cells and promotes the survival of germ cells. It has thought that cadherin based adhesion generates intracellular signaling cascades that control cell survival, migration and maturation.25 In our TEM study both BM and myoid cells were altered structurally in experimental group. BM in the treated group was irregular and had some swirls. Similar findings were observed in irradiated rats26 and after efferent ligation.27 The swirls may be due to contraction of myoid cells or reduction of tubular diameter. In this study the BM was continuous even in the absence of germ cells. It means that germ cells are probably not involved in BM production. It has been shown that during tubular injury, for survival and regeneration of stem cells, the BM is produced.26 Testicular BM is synthesized by both sertoli and myoid cells.28 In our TEM study it appeared that the thickness of BM is increased in cysplatin treated group. Increasing of BM thickness similarly occurs in: aging,29 efferent ligation27 and after irradiation.26 This alteration either is due to increasing of production by sertoli or myoid cells or reduction of proteolysis rate in ECM. In efferent ligation the gene expression of laminin is changed in sertoli cells and their protein synthesis is enhanced. According to our results intracellular granules in interstitial cells were increased in treated group. In this regard is appears that, interstitial cells are the only cells that have LH receptor and in response to LH secrets estrogen and testosterone. On the other hand it is shown that following chemotherapy, level of FSH, LH and testosterone increases.3,21,30 Therefore it can be concluded that the increased granules in interstitial cells are a response to increased LH which results from cysplatin treatment. The nucleus of myoid cells in cysplatin treated groups was appeared shorter and had a contractile form in comparison to control group. Myoid cells contain abundant actin filaments which are distributed in the cells in a species specific manner. Myoid cells also have myosin, desmin and actinin.31 In other words they are kind of smooth muscle cells.32 Testosterone and several substances such as prostoglandins, oxytocin, TGF-β have been suggested to affect the contraction of these cells.31 These finding suggest that probably after treatment with cysplatin, secretion of testosterone increased or damaging of epithelium stimulates secretion of some factors such as oxytocin or prostaglandins and cause myoid cells contraction.
Acknowledgment
The authors are thankful to Drug Applied Research Center, Tabriz University of Medical Sciences, for their financial support of the project.
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.Nejad MD, Rad SJ, Roshankar L, Karimpor M, Ghanbari AA, Azami A, Valilou MR. Effect of Thiotepa on mice Spermatogenesis using light and electronic microscope. Pak J Biol Sci. 2008;11:1929–34. doi: 10.3923/pjbs.2008.1929.1934. [DOI] [PubMed] [Google Scholar]
- 2.Codrington AM, Hales BF, Robaire B. Exposure of male rats to cyclophosphamide alters the chromatin structure and basic proteome in spermatozoa. Hum Reprod. 2007;22:1431–42. doi: 10.1093/humrep/dem002. [DOI] [PubMed] [Google Scholar]
- 3.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;(34):12–7. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 4.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005;(34):6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 5.Liu GP, Gong P, Bernstein LR, Bi Y, Gong S, Cai L. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit Rev Toxicol. 2007;37:587–605. doi: 10.1080/10408440701493061. [DOI] [PubMed] [Google Scholar]
- 6.Duale N, Lindeman B, Komada M, Olsen A, Andreassen A, Soderlund EJ. et al. Molecular portrait of cisplatin induced response in human testis cancer cell lines based on gene expression profiles. Mol Cancer. 2007;6:53. doi: 10.1186/1476-4598-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment – could arsenic have a role. J Ovarian Res. 2009;2:2. doi: 10.1186/1757-2215-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 9.Shipley WU, Prout GR JR, Einstein AB, Coombs LJ, Wajsman Z, Soloway MS. et al. Treatment of invasive bladder cancer by cisplatin and radiation in patients unsuited for surgery. JAMA. 1987;258:931–5. [PubMed] [Google Scholar]
- 10.Velasquez WS, Cabanillas F, Salvador P, Mclaughlin P, Fridrik M, Tucker S. et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–22. [PubMed] [Google Scholar]
- 11.Sieniawski M, Reineke T, Nogova L, Josting A, Pfistner B, Diehl V, Engert A. Fertility in male patients with advanced hodgkin lymphoma treated with beacopp: a report of the German Hodgkin Study Group (GHSG) Blood. 2008;111:71–6. doi: 10.1182/blood-2007-02-073544. [DOI] [PubMed] [Google Scholar]
- 12.Sawhney P, Giammona CJ, Meistrich ML, Richburg JH. Cisplatin-induced long-term failure of spermatogenesis in adult c57/bl/6j mice. J Androl. 2005;26:136–45. [PubMed] [Google Scholar]
- 13.Andriana BB, Tay TW, Maki A, Awal MA, Kanai Y, Kurohmaru M, Hayashi Y. An ultrastructural study on cytotoxic effects of mono (2-ethylhexyl) phthalate (MEHP) on testes in shiba goat in vitro. J Vet Sci. 2004;5:235–40. [PubMed] [Google Scholar]
- 14.Bakalska M, Atanassova N, Koeva Y, Nikolov B, Davidoff M. Induction of male germ cell apoptosis by testosterone withdrawal after ethane dimethanesulfonate treatment in adult rats. Endocr Regul. 2004;38:103–10. [PubMed] [Google Scholar]
- 15.Habermehl D, Kammerer B, Handrick R, Eldh T, Gruber C, Cordes N. et al. Proapoptotic activity of ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer. 2006;6:14. doi: 10.1186/1471-2407-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou M, Chrysis D, Nurmio M, Parvinen M, Eksborg S, Soder O. et al. Doxorubicin induces apoptosis in germ line stem cells in the immature rat testis and amifostine cannot protect against this cytotoxicity. Cancer Res. 2005;65:9999–10005. doi: 10.1158/0008-5472.CAN-05-2004. [DOI] [PubMed] [Google Scholar]
- 17.Sakallioglu AE, Ozdemir BH, Basaran O, Nacar A, Suren D, Haberal MA. Ultrastructural study of severe testicular damage following acute scrotal thermal injury. Burns. 2007;33:328–33. doi: 10.1016/j.burns.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrisons Principles of Internal Medicine. 16th ed. New York: McGraw Hill Medical Publishing Division; 2005. [Google Scholar]
- 19.Khilkevich LV, Kurilo LF. The gametatoxic effect of antenatal exposure to thiotepa in mice. Ontogenez. 1992;23:401–6. [PubMed] [Google Scholar]
- 20.Evenson DP, Baer RK, Jost LK, Gesch RW. Toxicity of thiotepa on mouse spermatogenesis as determined by dual-parameter flow cytometry. Toxicol Appl Pharmacol. 1986;82:151–63. doi: 10.1016/0041-008x(86)90447-3. [DOI] [PubMed] [Google Scholar]
- 21.Howell SJ, Shatel SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7:363–9. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 22.Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56:1490–7. doi: 10.1095/biolreprod56.6.1490. [DOI] [PubMed] [Google Scholar]
- 23.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–30. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Newton SC, Blaschuk OW, Millette CF. N-cadherin mediates Sertoli cell-spermatogenic cell adhesion. Dev Dyn. 1993;197:1–13. doi: 10.1002/aja.1001970102. [DOI] [PubMed] [Google Scholar]
- 25.Honecker F, Kersemaekers AM, Molier M, Van weeren PC, Stoop H, De krijger RR. et al. Involvement of E-cadherin and beta-catenin in germ cell tumours and in normal male fetal germ cell development. J Pathol. 2004;204:167–74. doi: 10.1002/path.1614. [DOI] [PubMed] [Google Scholar]
- 26.Sawada H, Esaki M. Electron microscopic observation of 137Cs-irradiated rat testis: production of basal laminae for germ cells, despite their absence. J Electron Microsc (Tokyo) 2003;52:391–7. doi: 10.1093/jmicro/52.4.391. [DOI] [PubMed] [Google Scholar]
- 27.Richardson LL, Kleinman HK, Dym M. Altered basement membrane synthesis in the testis after tissue injury. J Androl. 1998;19:145–55. [PubMed] [Google Scholar]
- 28.Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–7. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siu MK, Cheng CY. Extracellular matrix: Recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004;71:375–91. doi: 10.1095/biolreprod.104.028225. [DOI] [PubMed] [Google Scholar]
- 30.Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, Meistrich ML. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology. 2000;141:1735–45. doi: 10.1210/endo.141.5.7446. [DOI] [PubMed] [Google Scholar]
- 31.Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- 32.Virtanen I, Kallajoki M, Narvanen O, Paranko J, Thornell LE, Miettinen M. et al. Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec. 1986;215:10–20. doi: 10.1002/ar.1092150103. [DOI] [PubMed] [Google Scholar]


