Abstract
Purpose: An efficient method has been described for synthesis of 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1, 6-dihydropyrimidine-2-thiol, as a beneficial antimicrobial, anticonvulsant and anticancer agents. Methods: The clalcones of title compounds were synthesized in three steps and subsequently these chalcones were further reacted with thiourea in the presence of KOH in ethanol, which led to the formation of dihydropyrimidine derivatives (4a-j). Compounds 4a-j were screened for their in vitro antimicrobial activity by agar well method and their anticonvulsant activity by the MES model. Anticancer activity of two newly synthesized heterocycles were evaluated at National Cancer Institute (NCI) Maryland, USA against 60 cell lines of different human tumor at a single dose of 10-5 M. Results: Compound 4b, 4c, 4d, 4i and 4j were exhibited significant antimicrobial potential against tested strains at 50μg/ml and 100μg/ml concentrations. Out of the ten compounds studied 4a, 4b, 4c, 4h and 4j showed comparable MES activity to Phenytoin and Carbamazepine after 0.5h. Tested compounds did not showed to be more potent than standard drugs after 4h. Compound 4a and 4d were found active on Non-Small Cell Lung Cancer (HOP-92). Conclusion: Ten noveldihydropyrimidine analogues has been synthesized, characterized and found to bepromising antibacterial, anticonvulsant and antitumor agents.
Keywords: Chalcones, Condensation, Dihydropyrimidine, Antimicrobial activity, MES activity, Antitumor agent
Introduction
Heterocycles bearing a symmetrical triazole moiety were reported to show a broad spectrum of pharmacological properties like anticancer,1,2 antimicrobial,3-6 anticonvulsant,7 antiinflammatory, analgesic8,9 antidepressant,10 antitubercular,11,12 antimalarial13 and hypoglycemic14 activities. The pyrimidine ring system is a six membered heterocyclic ring structure composed of two nitrogen atoms and used in the synthesis of pharmaceuticals. The pyrimidine moiety is a versatile lead molecule in pharmaceutical development and has a wide range of biological activities. In the past few years, the therapeutic interest of pyrimidine derivatives in pharmaceutical and medicinal field has been given a great attention to the medicinal chemist. Literature survey reveals that pyrimidine derivatives are well known to have antimicrobial,15-17 antimalarial,18 anticonvulsant,19 anticancer,20 antiinflammatory, analgesic,21,22 antitubercular23 activities. In recent years, the extensive studies have been focused on pyrimidine derivatives because of their diverse chemical reactivity, accessibility and wide range of biological activities. We have recently reported the in vitro antimicrobial potential of 1-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-3-(substituted aryl) prop-2-en-1-one (chalcones) and MIC values of different derivatives were determined by liquid broth method.24
The widespread properties of 1,2,4-triazoles and pyrimidines have prompted us to synthesize them in single molecular framework in order to study their pharmacological activity. Hence, the present investigation was undertaken to study the antimicrobial, anticonvulsant and antitumorpotential ofpyrimidine derivatives containing 1,2,4-triazole moiety. In this dissertation, we achieved the successful synthesis and significant antimicrobial, anticonvulsant and anticancer potential of a series of 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4a-j).
Materials and Methods
The chemicals and solvents used for the experimental work were commercially procured from E. Merck India and Qualigens India. The melting points of all synthesized compounds were determined by open tube capillary using Thermonik precision apparatus in Celsius scale and uncorrected. IR spectra were recorded using KBr pellets on PERKIN ELMER 8201 PC IR spectrophotometer, 1H-NMR spectra of the final compounds 4a-j were recorded on BRUKER DRX NMR spectrometer (400 MHz). All spectra were obtained in DMSO. Mass spectra (FAB-MS) of compounds 4a-j were recorded on 70V on JEOL D-300 spectrophotometer (Jeol Ltd., Tokyo, Japan). Elemental analysis for C, H and N were performed on a PERKIN ELMER 240 elemental analyzer.
Synthesis protocol
Compounds 1, 2, and 3a-j were synthesized according to the reported method.24
Synthesis of 3,5-diphenyl-1H-1,2,4-triazole (1)
Benzohydrazide (0.1 mole) was dissolved in methanol, to this solution benzamide (0.1 mole) was added and stirred to get clear solution, then the resulting reaction mixture was refluxed for two hours on water bath. Therafter the reaction mixture was cooled at room temperature and poured in ice cold water to get precipitated 3,5-diphenyl-1H-1,2,4-triazole. Then obtained product was recrystallized by dioxane:ethanol mixture with an yield 83 %, m.p. 196-198°C.
Synthesis of 1-(3,5-diphenyl-1H-1, 2, 4-triazol-1-yl) ethanone (2)
To a solution of compound 1 (0.05 mole) dissolved in methanol, acetic anhydride (0.05 mole) and 2-3 drops of concentrated sulfuric acid were added, then the resulting reaction mixture was warmed on a water bath for 20 min. The reaction mixture was cooled at room temperature and poured in to ice cold water to get precipitate of compound 2. The precipitate of compound 2 was purified by dioxane:ethanol mixture with an yield 76 %, m.p. 176-178°C.
General procedure for synthesis of 1-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-3-(substituted aryl) prop-2-en-1-one (Chalcones) 3a-j
Compound 2 (0.05 mole) in methanol was treated with substituted aromatic aldehydes (0.05 mole) and 20% 10 ml NaOH, afterward stirred the reaction mixture for 7-8 hours at room temperature. The mixture was poured in ice cold water to get precipitate of compounds 3a-j, subsequently recrystallized by dioxane:ethanol mixture.
General procedure for synthesis of 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1, 6-dihydropyrimidine-2-thiol (4a-j)
A mixture of 0.01 mole of the required chalcones (3a-j), 0.01 mole thiourea and KOH (1gm) in 20 ml ethanol was heated under reflux for 6 hours, then cooled and poured on to crushed ice. The solid product obtained was filtered and recrystallized from dioxane:ethanol mixture.
Physical and spectral data of the compounds 4a-j are mentioned bellow.
Physical and spectral data of compounds 4a-j.
6-(4-chlorophenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4a).
Yield 67 %, m.p. 120-122°C. Elemental analysis Found C 64.89; H 4.02; N 15.70, Calculated for (C24H18ClN5S), C 64.93; H 4.09; N 15.78. IR(KBr, cm–1): 2940, 3075, 3151 (Ar-CH), 1629 (C=N, triazole), 1415 (C=C, pyrimidine), 787 (-Cl), 2626 (-SH). MS m/z: 443(M+). 1H NMR (400 MHZ, DMSO) δ: 7.69-8.40 (14H, m, Ar-H), 4.46 (d, 1H, NH-CH), 5.92 (d, 1H, CH), 13.39 (S, 1H, Ar-SH), 5.27 (s, 1H, NH).
6-(2-chlorophenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4b).
Yield 71 %, m.p. 125-127°C. Elemental analysisFound C 64.84; H 4.12; N 15.72, Calculated for (C24H18ClN5S), C 64.93; H 4.09; N 15.78.IR(KBr, cm–1): 2939, 3073, 3175 (Ar-CH), 1624 (C=N, triazole), 1419 (C=C, pyrimidine), 779 (-Cl), 2616 (-SH). MS m/z: 443(M+). 1H NMR (400 MHZ, DMSO) δ: 7.65-8.47(14H, m, Ar-H), 4.48 (d, 1H, NH-CH), 5.94 (d, 1H, CH), 13.41 (S, 1H, Ar-SH), 5.25 (s, 1H, NH).
6-(3-nitrophenyl)-4-(3, 5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4c).
Yield 65 %, m.p. 107-109°C. Elemental analysisFound C 63.44; H 4.06; N 18.40, Calculated for (C24H18N6O2S), C 63.42; H 3.99; N 18.49.IR(KBr, cm–1): 2942, 3078, 3180 (Ar-CH), 1628 (C=N, triazole), 1417 (C=C, pyrimidine), 1551 (-NO2), 2619 (-SH). MS m/z: 454(M+). 1H NMR (400 MHZ, DMSO) δ: 7.64-8.38 (14H, m, Ar-H), 4.45 (d, 1H, NH-CH), 5.96 (d, 1H, CH), 13.44 (S, 1H, Ar-SH), 5.26 (s, 1H, NH).
6-(4-methoxyphenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4d).
Yield 68 %, m.p. 130-132°C. Elemental analysisFound C 68.33; H 4.82; N 15.83, Calculated for (C25H21N5OS), C 68.32; H 4.82; N 15.93.IR(KBr, cm–1): 2947, 3075, 3181 (Ar-CH), 1625 (C=N, triazole), 1415 (C=C, pyrimidine), 1159 (-OCH3), 2618 (-SH). MS m/z: 439(M+). 1H NMR (400 MHZ, DMSO) δ: 7.64-8.38 (14H, m, Ar-H), 4.44(d, 1H, NH-CH), 5.96 (d, 1H, CH), 13.55 (S, 1H, Ar-SH), 3.82 (3H, s, OCH3),5.27 (s, 1H, NH).
6-(4-dimethylaminophenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4e).
Yield 74 %, m.p. 140-142°C. Elemental analysisFound C 69.06; H 5.33; N 18.57, Calculated for (C26H24N6S), C 69.00; H 5.35; N 18.57.IR(KBr, cm–1): 2939, 3076, 3174 (Ar-CH), 1623 (C=N, triazole), 1412 (C=C, pyrimidine), 3159, 3146 (-NCH3), 2606 (-SH). MS m/z: 452(M+). 1H NMR (400 MHZ, DMSO) δ: 7.61-8. 56 (14H, m, Ar-H), 4.47(d, 1H, NH-CH), 5.90 (d, 1H, CH), 13.49 (S, 1H, Ar-SH), 3.19 (6H, s, -N(CH3)2), 5.23 (s, 1H, NH).
6-(phenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4f).
Yield 72 %, m.p. 115-117°C. Elemental analysisFound C 70.36; H 4.60; N 17.19, Calculated for (C24H19N5S), C 70.39; H 4.68; N 17.10.IR(KBr, cm–1): 2946, 3072, 3182 (Ar-CH), 1621 (C=N, triazole), 1411 (C=C, pyrimidine), 2609 (-SH). MS m/z: 409(M+). 1H NMR (400 MHZ, DMSO) δ: 7.56-8.39 (15H, m, Ar-H), 4.45 (d, 1H, NH-CH), 5.93 (d, 1H, CH), 13.61 (S, 1H, Ar-SH), 5.29 (s, 1H, NH).
6-(2-furul)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4g).
Yield 68 %, m.p. 111-123°C. Elemental analysisFound C 66.12; H 4.34; N 17.44, Calculated for (C22H17N5OS), C 66.15; H 4.29; N 17.53.IR(KBr, cm–1): 2942, 3079, 3178 (Ar-CH), 1628 (C=N, triazole), 1416 (C=C, pyrimidine), 1224 (C-O-C), 2611 (-SH). MS m/z: 399(M+). 1H NMR (400 MHZ, DMSO) δ: 7.06-8. 37 (13H, m, Ar-H), 4.46 (d, 1H, NH-CH), 5.97(d, 1H, CH), 13.47 (S, 1H, Ar-SH), 5.28 (s, 1H, NH).
6-(4-bromophenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4h).
Yield 58 %, m.p. 117-119°C. Elemental analysisFound C 59.04; H 3.67; N 14.39, Calculated for (C24H18BrN5S), C 59.02; H 3.71; N 14.34.IR(KBr, cm–1): 2936, 3066, 3173 (Ar-CH), 1625 (C=N, triazole), 1413 (C=C, pyrimidine), 698 (-Br), 2615 (-SH). MS m/z: 488(M+). 1H NMR (400 MHZ, DMSO) δ: 7.55-8.48 (14H, m, Ar-H), 4.47 (d, 1H, NH-CH), 5.94 (d, 1H, CH), 13.38 (S, 1H, Ar-SH), 5.27 (s, 1H, NH).
6-(4-hydroxyphenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4i).
Yield 72 %, m.p. 127-129°C. Elemental analysisFound C 67.79; H 4.49; N 16.42, Calculated for (C24H19N5OS), C 67.74; H 4.50; N 16.46.IR(KBr, cm–1): 2946, 3073, 3175 (Ar-CH), 1622 (C=N, triazole), 1412 (C=C, pyrimidine), 3357 (-OH), 2616 (-SH). MS m/z: 425(M+). 1H NMR (400 MHZ, DMSO) δ: 7.48-8.59 (14H, m, Ar-H), 4.49 (d, 1H, NH-CH), 5.96 (d, 1H, CH), 13.41 (S, 1H, Ar-SH), 10.16 (s, 1H, Ar-OH), 5.26 (s, 1H, NH).
6-(2,4-dimethoxyphenyl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol (4j).
Yield 65 %, m.p. 133-135°C. Elemental analysisFoundC 67.79; H 4.49; N 16.42, Calculated for (C24H19N5OS), C 67.74; H 4.50; N 16.46.IR(KBr, cm–1): 2946, 3073, 3175 (Ar-CH), 1622 (C=N, triazole), 1412 (C=C, pyrimidine), 3357 (-OH), 2616 (-SH). MS m/z: 425(M+). 1H NMR (400 MHZ, DMSO) δ: 7.48-8.59 (14H, m, Ar-H), 4.49 (d, 1H, NH-CH), 5.96 (d, 1H, CH), 13.41 (S, 1H, Ar-SH), 10.16 (s, 1H, Ar-OH), 5.26 (s, 1H, NH).
Pharmacological assessment
Antimicrobial Activity
The in vitro antimicrobial activity of the target compounds 4a-j was performed by agar well method (diffusion technique) against S. aureus NCIM 2079, E. coli NCIM 2065, C. albicans NCIM 3471 and A. niger NCIM 1196. The antibiotic Ampicillin and the antifungal agent Fluconazole were used as standard drugs for the study. The fresh bacterial culture was obtained by inoculating bacteria into peptone water liquid media and incubating at 37 ± 2 °C for 18-24 hours. This culture was mixed with nutrient agar media and poured in to Petri dishes. After culture media solidification four wells were made at equal distance by using a sterile steel cork borer (8mm diameter). In to these wells different concentrations of standard drug and synthesized compounds were introduced. Dimethyl Formamide (DMF) was used as a control. After introduction of standard drug and synthesized compounds, the plates were placed in a refrigerator at 8-10 °C for proper diffusion of drugs into the media. After two hours of cold incubation, the Petri plates were transferred to incubator and maintained at 37±2 °C for 18-24 hours. After the incubation period, the petri plates were observed for growth inhibition zone by using vernier scale. The results were evaluated by comparing the growth inhibition zone shown by the synthesized compounds with standard drugs. The results were presented as the mean value of growth inhibition zone measured in millimeters of three sets (Table 1). The standard drugs and synthesized compounds were dissolved in a minimum quantity of Dimethyl Formamide (DMF) and adjusted, to made up the volume with distilled water to get 50μg/ml and 100μg/ml concentrations.
Table 1. Antimicrobial activity of compounds 4a-j.
|
Compound |
Zone of inhibition (in mm) | |||||||
| S. aureus | E. coli | C. albicans | A. niger | |||||
| 50µg | 100 µg | 50µg | 100 µg | 50 µg | 100 µg | 50 µg | 100 µg | |
| 4a | 19 | 21 | 18 | 23 | 20 | 23 | 18 | 21 |
| 4b | 18 | 21 | 19 | 23 | 22 | 25 | 23 | 22 |
| 4c | 21 | 24 | 24 | 24 | 19 | 22 | 18 | 23 |
| 4d | 19 | 22 | 19 | 22 | 23 | 22 | 22 | 21 |
| 4e | 18 | 19 | 17 | 20 | 20 | 21 | 19 | 20 |
| 4f | 15 | 17 | 14 | 17 | 18 | 19 | 16 | 18 |
| 4g | 16 | 19 | 17 | 20 | 15 | 19 | 17 | 21 |
| 4h | 19 | 21 | 21 | 19 | 19 | 22 | 21 | 19 |
| 4i | 21 | 19 | 22 | 20 | 21 | 18 | 22 | 22 |
| 4j | 22 | 21 | 20 | 20 | 19 | 18 | 16 | 19 |
| Ampicillin | 21 | 25 | 24 | 26 | - | - | - | - |
| Fluconazole | - | - | - | - | 24 | 26 | 23 | 25 |
| DMF | - | - | - | - | - | - | - | - |
Anticonvulsant Activity: Maximal Electroshock Seizure Test (MES)
The compounds 4a-j were screened for their anticonvulsant activity by Maximal electroshock seizure method. Study protocol was approved by the Institutional Animal Ethics Committee for the purpose of control and supervision on experiments on animals (IAEC, Approval No.1211/ac/08/CPCSEA) before experiment. Initial anticonvulsant evaluations of the test compounds were undertaken by following the anticonvulsant drug development (ADD) program protocol.25 The animals Albino mice (20-25 g) of either sex were stimulated through corneal electrodes to 50 mA current at a pulse of 60 Hz applied for 0.2 s. Animals were previously given the test drug intraperitoneally. Abolition of the hind limb tonic extension spasm was recorded as the anticonvulsant activity. The test compounds were suspended in a 0.5% methyl cellulose-water mixture. In preliminary screening, each compound was administered through an intraperitoneally injection at three dose levels (30, 100 and 300 mg/kg) and the anticonvulsant activity was assessed after 0.5 h and 4 h intervals of administration. The anticonvulsant efficacy was evaluated by the maximal electroshock-induced seizure (MES) and screening data of compounds 4a-j are presented in Table 2.
Table 2. Anticonvulsant and neurotoxicity screening of compounds 4a-j.
| Sr. No. | Treatment | Ar | MES screena | Neurotoxicity screena | ||
| 0.5h | 4h | 0.5h | 4h | |||
| 1 | 4a | 4-Cl | 30 | 300 | 300 | - |
| 2 | 4b | 2-Cl | 30 | 300 | - | 300 |
| 3 | 4c | 3-NO2 | 30 | 300 | - | - |
| 4 | 4d | 4-OCH3 | 100 | 300 | 300 | 300 |
| 5 | 4e | 4-N(CH3)2 | 100 | 300 | - | 300 |
| 6 | 4f | phenyl | 100 | 300 | 300 | 300 |
| 7 | 4g | 2-Furyl | 100 | 300 | - | - |
| 8 | 4h | 4-Br | 30 | 300 | 300 | 300 |
| 9 | 4i | 4-OH | 100 | 300 | - | 300 |
| 10 | 4j | 2,4-OCH3 | 30 | 300 | - | - |
| 11 | Phenytoin | - | 30 | 30 | 100 | 100 |
| 12 | Carbamazepine | - | 30 | 100 | 300 | 300 |
“a” Doses of 30, 100 and 300 mg/kg were administered. The figures in the Table indicate the minimum dose whereby bioactivity was demonstrated in half or more of mice. The animals were examined 0.5 and 4 h after injections were given. The dash “-“ indicates an absence of activity at maximum dose administered (300 mg/kg).
Neurotoxicity screening (NT)
The minimal motor impairment was measured in mice by the rotorod test.26 The Albino mice (20-25 g) were trained to stay on an accelerating rotorod that rotated at 10 rotations/min and its diameter was 3.2 cm. Only those mice were taken for the test which could stay on the revolving rod for at least one minute. Trained animals were injected intraperitoneally with the test compounds at doses of 300 mg/kg. Neurotoxicity was indicated by the inability of the animal to maintain equilibration on the rod for at least for one minute.
Anticancer activity
Anticancer activity of newly synthesized heterocycles were evaluated. Primarily compounds submitted at National Cancer Institute (NCI) Maryland, USA and selected compounds were screened for anticancer activity against 60 cell lines of different human tumor, representing leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney for one dose against the 60 cell lines at a single dose of 10-5M. The mean growth %, range of growth % and growth % relative to most sensitive cell line is depicted in Table 3.
Table 3. Anticancer screening data of selected compounds.
| Compound No. | 60 Cell lines in assay in 1-dose 10-5 M concentration | |||
| (NSC code) | Mean growth (%) |
Range growth (%) |
Most sensitive cell line |
Growth of most sensitive cell line (%) |
| 4a (764753/1) |
106.85 | -27.71-55.23 | Non-Small Cell Lung Cancer (HOP 92) |
- 27.71 |
| 4d (764754/1) |
103.26 | -19.05-50.25 | Non-Small Cell Lung Cancer (HOP 92) |
-19.05 |
Results and discussion
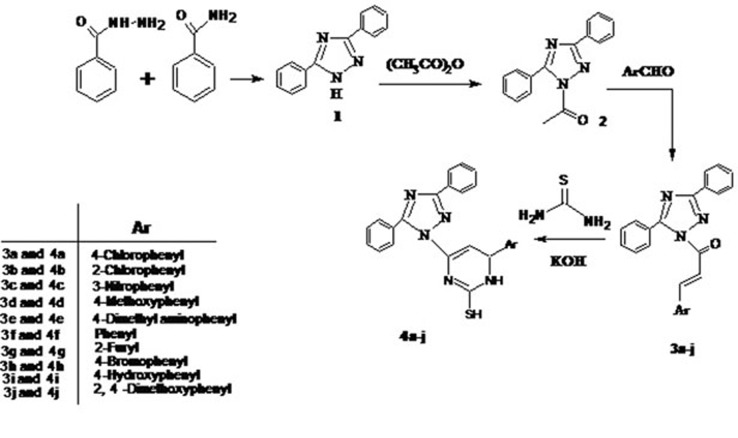
The synthesis of 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol derivatives (4a-j)depicted in Figure 1. The compound1-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)ethanone (2) on condensation with various aromatic aldehydes in NaOH solution yielded the corresponding chalcones (3a-j). These chalcones were further reacted with thiourea in the presence of KOH in ethanol, which led to the formation of dihydropyrimidine derivatives (4a-j) (Figure 2). The structural assessments of compounds 4a-j were carried out by IR, 1H-NMR, Mass spectra and elemental analysis. Structures of compounds 4a-j were confirmed IR, 1H-NMR, mass spectra and elemental analysis. Infrared spectrum of compounds 4a-j showed a sharp absorption band frequencies for-NO2, -Cl, -OCH3, -N-(CH3)2, -OH and -Br groups. Compounds 4a-j showed appropriate 1H-NMR signals for aromatic protons and multiplets were observed in the range of δ 7.06-8.59. The 1H NMR spectra showed a downfield singlet at around 13.4 ppm attributed to SH group. Mass spectra of the compounds 4a-j showed molecular ion peaks with high abundance at m/z in agreement with their molecular formula. Compounds 4a-j evaluated for their in vitro antimicrobial, anticonvulsant and anticancer activity.
Figure 1.
Synthesis of compounds 4a-j.
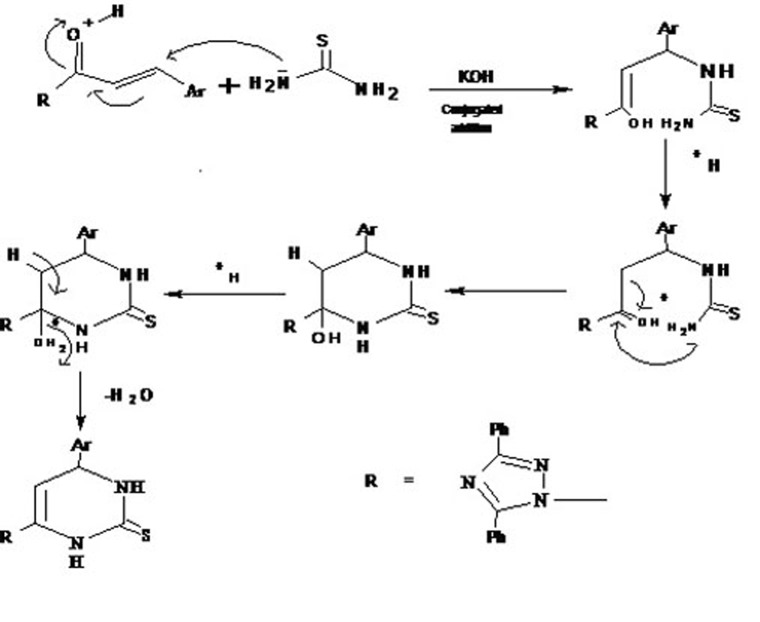
Figure 2.
The possible mechanism involved in the formation of dihydropyrimidine derivatives (4a-j) from the respective chalcones is shown above.
The in vitro antimicrobial activity of tested compounds was determined by agar well method. The results are summarized in Table 1. These antimicrobial data clearly indicated that the presence of methoxy, chloro, nitro and hydroxy substitution on phenyl ring of the pyrimidine nucleus produced remarkable improvement in antimicrobial activity. Phenyl substitution is weakly active among the synthesized compounds. Compound 4c showed significant antibacterial activity against S. aureus and E. coli. Compound 4b showed fungicidal potential against C. albicans and A. niger. The in vitro antimicrobial study clearly reveals that pyrimidine derivatives (4a-j) were found to be significant antimicrobial agents probably due to pharmacologically active pyrimidine nucleus attached with 1, 2, 4-triazole.
The target compounds (4a-j) were tested for anticonvulsant activity by maximal electroshock induced seizure (MES) test. The anticonvulsant and neurotoxicity data of the compounds and standard drugs are presented in Table 2. The compounds that exhibited the most potent anti-MES activity included 4a, 4b, 4c, 4h and 4j which have activity comparable with Phenytoin and Carbamazepine. Minimal motor impairment was measured by the rotorod test. Compounds 4c, 4g, and 4j successfully passed the rotorod test without any sign of neurological deficit, whereas compounds 4d, 4f and 4h exhibited neurological deficit at the dose of 300 mg/kg i.p. Compounds with the electron withdrawing chloro substituent at the ortho and para position and bromo at para position on the phenyl ring of pyrimidine nucleus led to considerable increase in the activity but were relatively toxic. The presence of a bulkier electron donating methoxy group at para and ortho position on the phenyl ring of pyrimidine nucleus results increase in anticonvulsant activity. This may be due to the increase in lipophilicity of the compound. The nitro substituent at the meta position of the phenyl ring found to be equally active with chloro substitution but without sign of neurotoxity. The effect of the hydroxy, phenyl and furyl substitution were found to be less active compounds as compared to other tested compounds.
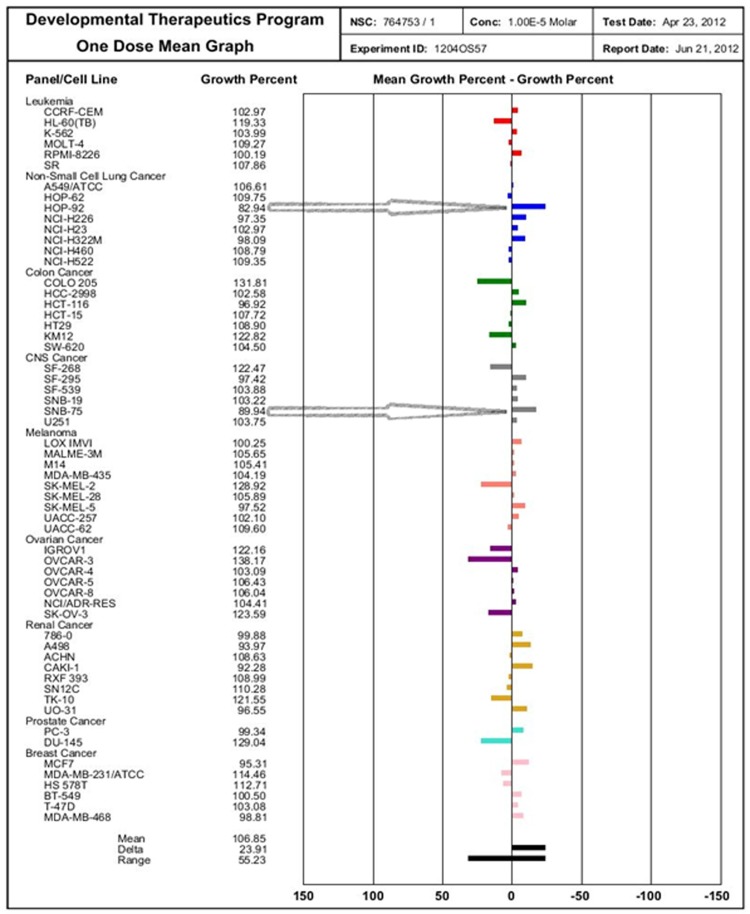
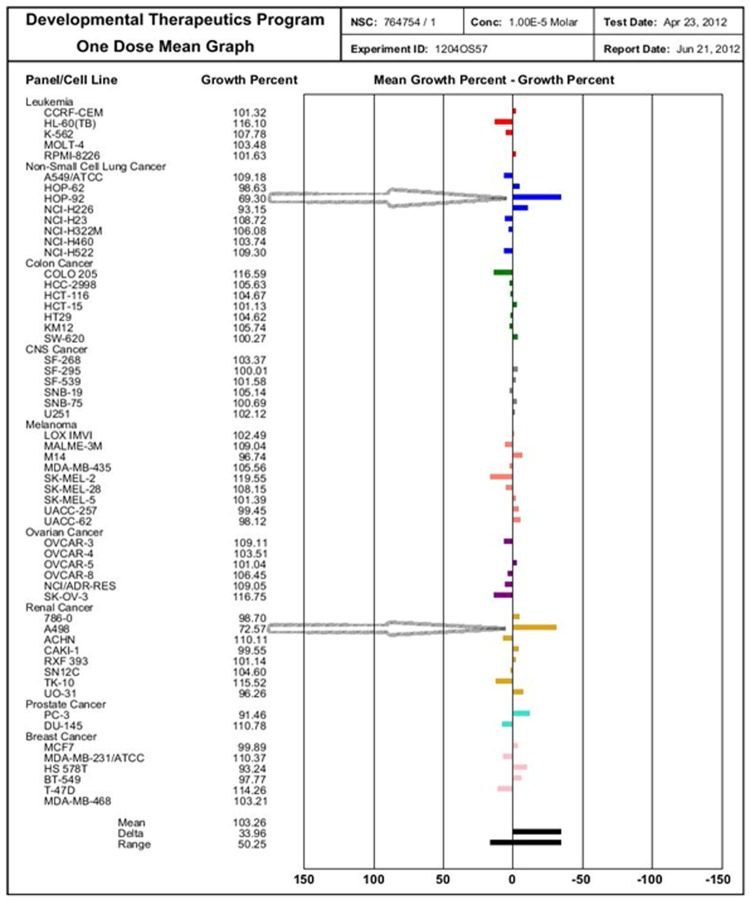
The compounds 4a and 4d were evaluated at single concentration of 10-5 M towards the panel of approximately 60 cancer cell lines derived from nine different cancer types. The mean growth %, range of growth % and growth % relative to most sensitive cell line is depicted in Table 3. The tested compounds showed a broad spectrum of growth inhibitory activity against human tumor cells, as well as some distinctive pattern of selectivity (Figure 3 and Figure 4). Compound 4a was found to be a highly active growth inhibitor of the Non-Small Cell Lung Cancer (HOP-92) and CNS Cancer cell line (SNB-75) with a growth % of most sensitive cell line to be - 27.71, whilst least active over other cell lines. The mean growth % for compound 4a was observed 106.85% and fall in a range - 27.71-55.23. Compound 4d showed selective antitumor sensitivity on Non-Small Cell Lung Cancer (HOP-92) and renal Cancer cell line (A498) with a growth % of most sensitive cell line to be -19.05, whilst least active over other cell lines. The mean growth % for compound 4d was observed 103.26% and fall in a range -19.05-50.25.
Figure 3.
Selected NCI sixty cell screening data highlighting the potency of compound (4a: NSC: 764753/1) againstnon-Small Cell Lung Cancer (HOP-92) and CNS Cancer cell line (SNB-75). Bars to the right of the mean line represent cell lines more sensitive to test compound compared to mean, whereas bars to the left represent less sensitive cell lines.
Figure 4.
Selected NCI sixty cell screening data highlighting the potency of compound (4d: NSC: 764754/1) againstnon-Small Cell Lung Cancer (HOP-92) and renal Cancer cell line (A498). Bars to the right of the mean line represent cell lines more sensitive to test compound compared to mean, whereas bars to the left represent less sensitive cell lines.
Conclusion
In conclusion, new class of pyrimidine derivatives containing 1,2,4-triazole was synthesized and evaluated as antimicrobial, anticonvulsant and anticancer properties. The newly synthesized heterocycles exhibited promising antimicrobial activity against tested microorganisms. Compounds 4b, 4c, 4d, 4i and 4j were found to be the most active antimicrobial The newly synthesized heterocycles exhibited promising antimicrobial activity against tested microorganisms. Compounds 4b, 4c, 4d, 4i and 4j were found to be the most active antimicrobial agents from synthesized series. From the anticonvulsant screening data it was found that of 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol derivatives (4a-j) have encouraging anticonvulsant activity in the MES screen. Compound 4a, 4b, 4c, 4h and 4j found to be effective anticonvulsant agent from tested compounds. In the present study two compounds (4a and 4d) were tested for anticancer activity and most of them displayed antitumor activity on cell lines of Non-Small Cell Lung Cancer, CNS Cancer and renal Cancer.These results suggest that novel triazolechalcones and pyrimidine derivatives are interesting lead molecules for further synthetic and biological evaluation.
Acknowledgments
We are highly thankful to IICT Hyderabad for providing spectral data, NCL Pune for providing micro organisms for this study, National Cancer Institute (NCI) Bethesda, USA for in vitro screening of our compounds in human cancer cell lines. We are also grateful of Principal M.E.S. College Pharmacy, Sonai and Prashant Patil Gadakh, Secreatary, Mula Education Society for providing excellent research facilities for this work.
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Al-soud YA, Al-masoudi NA, Ferwanah AE. Synthesis and properties of new substituted 1,2,4-triazoles: potential antitumor agents. Bioorg Med Chem. 2003;11:1701–8. doi: 10.1016/s0968-0896(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 2.Khanage SG, Mohite PB, Raju SA. Synthesis, Anticancer and Antibacterial Activity of Some Novel 1,2,4-Triazole Derivatives Containing Pyrazole and Tetrazole Rings. Asian J Res Chem. 2011;4:567–73. [Google Scholar]
- 3.Lingappa B, Girisha KS, Kalluraya BN, Rai S, Kumari NS. Regioselective reaction: Novel Mannich bases derived from 3-(4,6-disubstituted-2-thiomethyl)3-amino-5-mercapto-1,2,4-triazoles and their antimicrobial properties. Ind J Chem. 2008;47b:1858–64. [Google Scholar]
- 4.Rao G, Rajasekran S, Attimarad M. Synthesis and Antimicrobial activity of Some 5-phenyl-4-substituted amino-3-mercapto (4H) 1,2,4-triazoles. Ind J Pharm Sci. 2000;6:475–7. [Google Scholar]
- 5.Jalilian AR, Sattari S, Bineshmarvasti M, Shafiee A, Daneshtalab M. Synthesis and in vitro antifungal and cytotoxicity evaluation of thiazolo-4H-1,2,4-triazoles and 1,2,3-thiadiazolo-4H-1,2,4-triazoles. Arch Pharm (Weinheim) 2000;333:347–54. doi: 10.1002/1521-4184(200010)333:10<347::aid-ardp347>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Lazarevic M, Dimova V, Molnar GD, Kakurinov V, Colanceska RK. Synthesis of some n1-aryl/heteroarylaminomethyl/ethyl-1,2,4-triazoles and their antibacterial and antifungal activities. Hetero Comm. 2001;7:577–82. [Google Scholar]
- 7.Chimirri A, Bevacqua F, Gitto R, Quartarone S, Zappala MD, Sarro A. et al. Synthesis and anticonvulsant activity of new 1-H-triazolo[4,5-c][2,3]benzodiaze-pines. Med Chem Res. 1999;9:203–12. [Google Scholar]
- 8. Hunashal RD, Ronad PM, Maddi VS, Satyanarayana D, Kamadod MA. Synthesis, anti-inflammatory and analgesic activity of 2-[4-(substituted benzylideneamino)-5-(substitutedphenoxymethyl)-4H-1,2,4-triazol-3-yl-thio] acetic acid derivatives. Arab J Chem 2011;1-9. [Google Scholar]
- 9.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Study of analgesic activity of novel 1,2,4-triazole derivatives bearing pyrazole and tetrazole moiety. J Pharm Res. 2011;4:3609–11. [Google Scholar]
- 10.Kane MJ, Dudley MW, Sorensen MS, Miller FP. Synthesis of 1,2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents. J Med Chem. 1988;31:1253–8. doi: 10.1021/jm00401a031. [DOI] [PubMed] [Google Scholar]
- 11.Husain MI, Amir M, Singh E. Synthesis and antitubercular activities of [5-(2furyl)-1,2,4-triazoles-3yl thio] acehydrazide derivatives. Ind J Chem. 1987;26B:2512–54. [Google Scholar]
- 12.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Investigation of pyrazole and tetrazole derivatives containing 3,5 disubstituted-4h 1,2,4-triazole as a potential antitubercular and antifungal agent. Bioint Res App Chem. 2012;2:277–83. [Google Scholar]
- 13.Xiao Z, Waters NC, Woodard CL, Li PK. Design and synthesis of pfmrk inhibitors as potential antimalarial agents. Bioorg Med Chem Lett . 2001;11:2875–8. doi: 10.1016/s0960-894x(01)00578-9. [DOI] [PubMed] [Google Scholar]
- 14.Deliwala CV, Mhasalkar MY, Shah MH, Pilankar PD, Nikam ST, Anantanarayan KG. Synthesis and hypoglycaemic activity of 3-aryl(or pyridyl)-5-alkyl amino-1,3,4, thiadiazole and some sulfonyl ureas derivatives of 4h-1,2,4 triazoles. J Med Chem. 1971;14:1000–3. doi: 10.1021/jm00292a035. [DOI] [PubMed] [Google Scholar]
- 15.Cieplik J, Stolarczyk M, Pluta J, Gubrynowicz O, Bryndal I, Lis T. et al. Synthesis and antibacterial properties of pyrimidine derivatives. Ac Polo Pharm Drug Res. 2011;68:57–65. [PubMed] [Google Scholar]
- 16.Mohamed MS, Awad SM, Ahmed NM. Synthesis and antimicrobial activities of new indolyl-pyrimidine derivatives. J App Pharm Sci. 2011;1:76–80. [Google Scholar]
- 17.Richa M, Mishra B, Moorthy N. Synthesis and antimicrobial evaluation of some 3,4-dihydro pyrimidine-2-one derivatives. Tre App Sci Res. 2008;3:203–8. [Google Scholar]
- 18.Morgan JR, Haritakul R, Keller PA. Antimalarial activity of 2,4-diaminopyrimidines. Lett Drug Des Discov. 2008;5:277–80. [Google Scholar]
- 19.Naik TA, Chikhalia KH. Studies on synthesis of pyrimidine derivatives and their pharmacological evaluation. E J Chem. 2007;4:60–6. [Google Scholar]
- 20.Ramesh B, Kulakarni SV. Design, synthesis and anticancer activity of some new pyrimidine derivatives. J Glo Pharma Tech. 2010;2:110–2. [Google Scholar]
- 21.Sondhi SM, Dinodia M, Rani R, Shukla R, Raghubir R. Synthesis, antiinflammatory and analgesic activity evaluation of some pyrimidine derivatives. Ind J Chem. 2009;49b:273–81. [Google Scholar]
- 22.Pore Y, Kuchekar B. Synthesis of novel n1 6-disubstituted 5-cyano-2-thiouracil derivatives as antinociceptive agents. Dig J Nano Biostr. 2008;3:293–8. [Google Scholar]
- 23.Chitre TS, Kathiravan MK, Chothe AS, Rakholiya VK, Asgaonkar KD, Shital M. et al. Synthesis and antitubercular activity of some substituted pyrimidine derivatives. J Pharm Res. 2011;4:1882–3. [Google Scholar]
- 24.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Synthesis and pharmacological evaluation of isoxazole derivatives containing 1,2,4-triazole Moiety. Mar Pharm J. 2012;16:134–40. doi: 10.5681/apb.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krall RI, Penry JK, White BG, Kupferberg HG, Swingard EA. Antiepileptic drug development ii. anticonvulsant drug screening. Epilepsia. 1978;19:409–28. doi: 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 26.Kucukguzel I, Kucukguzel SG, Rollas S, Ozdemir O, Bayrak I, Stables JP. Synthesis of some 3-(aryl/alkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4h-1,2,4-triazole derivatives and their anticonvulsant activity. Farmaco. 2004;59:893–901. doi: 10.1016/j.farmac.2004.07.005. [DOI] [PubMed] [Google Scholar]