Abstract
Purpose: The leaves of Gynura procumbens (Lour.) Merr. has been traditionally used as anticancer. Ethanolic extract of G. procumbens leaves (EGP) showed cytotoxic activity and anticancer activity in animal cancer model. This study was conducted to observe antiproliferative effect using male rat's liver cells induced by 7,12-dimethylbenz(a)antracene (DMBA). Methods: Forty days old Sprague Dawley male rats were divided into 4 groups, (1) 0.5 % CMC Na, (2) 20 mg/kg BW DMBA p.o ten times in three weeks, (3) DMBA+300 mg/kg BW of EGP, and (4) DMBA+750 mg/kg BW of EGP. The extract was dissolved into 0.5 % CMC-and administered daily per oral one week before, during and terminated 1 week after the DMBA induction. After sixthteen week experiment, rat livers were sectioned and stained with Haematoxyllene and Eosin (H&E) and AgNOR. Results: Histopatology profile showed no primary liver tumor on DMBA group. mAgNOR value of DMBA+300 mg/kg BW EGP showed significant antiproliferative effect compared to DMBA group. Conclusion: Ethanolic extract of G. procumbens leaves showed antiproliferative activity on male rats liver induced by DMBA.
Keywords: liver cancer, proliferative, Gynura procumbens, DMBA, AgNOR
Introduction
Liver cancer is the world's fifth largest in man and the seventh largest in women of cancer deaths.1 The number of deaths in the world caused by liver cancer showed more than one million deaths per year. Chemotherapy, surgery, hormonal therapy and radiotherapy has been developed, but remain several problems such as toxic effect on normal cells and drug resistance problem.2 Discoveries and research have been developed to overcome those problem, including using natural products as an alternative for the prevention and treatment of cancers.3
One of the medicinal plants used traditionally as a cure for cancer is Gynura procumbens (Lour.) Merr. Previously, our research group named Cancer Chemoprevention Research Center (CCRC) Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, has explored several anticancer activity of G. procumbens leaves (EGP). Ethanolic extract of G. procumbens (EGP) showed inhibitory effect on carcinogenicity of mice lung tumor induced by benzo(a)pyrene (BAP) and also showed antimutagenic activity on Salmonella typhimurium.4 The fraction XIX-XX of EGP has a cytotoxic activity on HeLa cervical cancer cells (IC50 119 µg/ml), inhibited HeLa cell proliferation and induced apopotosis.5 EGP is also reported to have antiangiogenic effects,6 therefore the plant is potential as antimetastasis and anti-invasion. The Ethyl acetate fraction of G. procumbens leaves ethanolic extract increased the effectiveness of doxorubicin on MCF-7 and T47D breast cancer cells.7 In vivo study of EGP on female rats induced by DMBA has been conducted. EGP performs chemopreventive effect to suppress breast cancer initiation,8 and performs supression of breast cancer development on early state of female rats induced by DMBA.9
The aim of the present investigation was to examine the effect of EGP administration on carcinogenesis of DMBA on male rats liver. We would like to explore the effect of EGP on proliferation of male rats liver induced by DMBA. The results of this study is expected to be the scientific evidence of G. procumbens leaves as a chemopreventive agent and the development of subsequent research in cancer prevention.
Materials and Methods
Materials
G. procumbens leaves were harvested from Ngaglik, Sleman, Yogyakarta and determined in Department of Pharmaceutical Biology, Faculty of Pharmacy UGM. Extract was prepared by the soxhlet extraction procedure using 96% ethanol (Asia Lab, Yogyakarta), and concentrated using rotary evaporator. DMBA (7.12-dimetilbenz [a] anthracene) was obtained from Sigma (Saint Louis, MO). Sodium Carboxymethyl Cellulose (CMC-Na) (E. Merck) was used as suspending agent of the extract.
Animals
Sprague Dawley ale rats (Rattus norvegicus) (40 days old) weighed from 60 to 80 g were obtained from the Laboratory of Pharmacology and Toxicology, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia. Animals were conditioned for one week before use, and were given standard pellet diet and water ad libitum, and kept on a 12:12 h light/dark cycle.
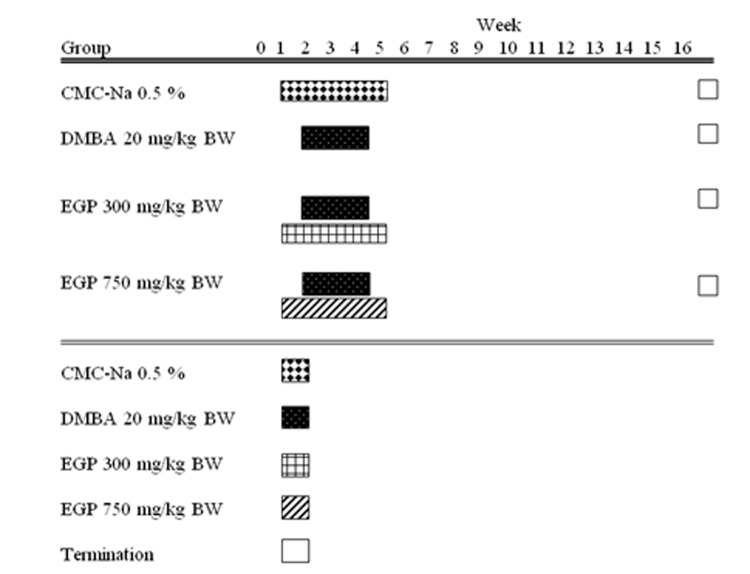
Forty rats were devided into four groups, 10 rats per group (Figure 1). In the beginning of 40 days age, solvent control (group 1) was administered with 0.5% CMC-Na, groups of animal (group 2, 3, and 4) intended for carcinogen were treated per oral with single dose of DMBA (Sigma) (20mg/kgBW), third a week for 3 weeks. DMBA was dissolved in CMC-Na and EGP was dissolved in 0.5% CMC-Na as vehicle. Group 3 and group 4 were administered per oral once a day by 300 mg/kgbw and 750 mg/kgbw, respectively for 5 weeks, started 1 week before DMBA initiation until 1 week after DMBA administration. Body weight were recorded weekly throughout the study. The animal handling protocols of this study were in accordance with the guidelines of the animal care of the Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Gadjah Mada University, Indonesia, and approved by the committee for animal research.
Figure 1.
Experimental time line
H&E staining
At the end of the experiment (16 week) all rats were sacrificed by decapitation as scheduled. At autopsy, liver organ were removed and fixed in 10% buffered formalin. After 12-24 h of fixation, 3-5 μm tissue slices were embedded in paraffin, and stained with hematoxylin and eosin for microscopy.
AgNOR staining
AgNOR staining was performed according to the modified method.10,11 The staining solution was prepared by mixing one part of 2% gelatin in 1% formic acid with two parts of 50% aqueous silver nitrate. All sections were cut to 3 µm in thickness from routinely processed paraffin blocks. Sections were immersed in sodium citrate buffered (pH 6.0) and incubated 20 min in autoclave (120˚C,1.1-1.2 Bar). Sections then were covered with the AgNOR staining solution at room temperature in the dark for 15-20 min. The specimens were then washed with 5% sodium thiosulfate and distilled deionized water, dehydrated through graded ethanol to xylene, and mounted in synthetic medium.
AgNORs, which are appeared as dots both outside and within the nucleoli, were counted according to the description of previous report.12 A minimum one hundred nuclei per specimen were observed randomly in three different views. Mean AgNOR (mAgNOR) was used as a parameter to evaluate antiproliferative activity. mAgNOR is a mean of black dots in a cell, computed from total amount of blackdots (min 100 cells) divided with amount of cells (min 100 cells). All specimens were observed on a binocular microscope (Olympus® DP12 microscope digital camera system, NY) with an immersion oil lens at magnification of x1000.
Statistical analyses
Statistically significant difference of body weight by ANOVA continued with LSD, while antiproliferative activity were evaluated by Kruskal Wallis continued with Mann-Whitney. Significance was set at p < 0.05 for all tests.
Results
Effect of EGP on body weight, survival rate and liver morphology of male rat
There was no direct evidence of toxicity due to EGP treatment. Body weight changes of the animal treated with CMC-Na, DMBA and DMBA+EGP were compared ( Figure 2). In the beginning of the study and in the sixth week, the body weight changes of CMC-Na group was different from the other groups (p<0.05). Final body weight changes showed that DMBA was no significant different from other groups (p<0.05) ( Table 1 and Figure 2). In general, treatment of EGP in low dose induced fluctuative body weight changes than EGP in high dose. The phenomenon is similar to DMBA control group. The survival curves for the DMBA and DMBA+EGP were practically identical before the 4th week of the experiment, then the survival curve of the DMBA group become higher compared to the EGP treatment group (Figure 3). The higher dose of EGP, the lower the lifespan of the rats. There were no macroscopic differences in morphology of liver organ (Figure 4).
Figure 2.
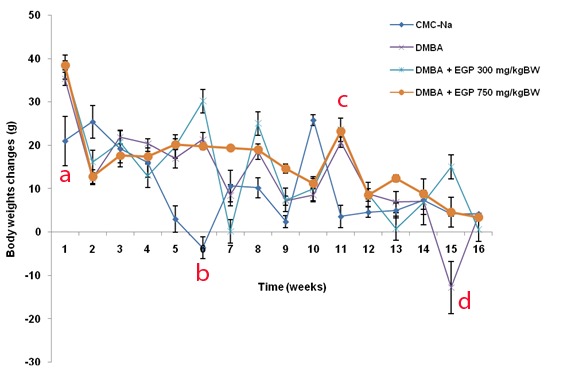
Body weight changes for animals.
a=CMC-Na group significant different from DMBA, DMBA+EGP 300 mg/kgBW, and DMBA+EGP 750 mg/kgBW (p<0.05) in the first week, b= CMC-Na group significant different from DMBA+EGP 300 mg/kgBW (p<0.05) in the sixth week, c=CMC-Na group significant different from DMBA, DMBA+EGP 300 mg/kgBW, and DMBA+EGP 750 mg/kgBW (p<0.05) in the tenth week, d= CMC-Na group significant different from DMBA (p<0.05) in the fiftheen week.
Table 1. Effect of EGP on proliferation of DMBA-induced liver male rats .
| Treatment Group | Final Body Weight Changes (g)1 | mAgNOR2 |
| 0.5 % CMC-Na | 4.20 ± 2.77 | 1.49 ± 0.04 |
| DMBA | 10.00 ± 13.99 | 2.25 ± 0.17 |
| DMBA + 300 mg/kg BW EGP | 0.57 ± 5.79 | 1.72 ± 0.04* |
| DMBA + 750 mg/kg BW EGP | 3.40 ± 2.96 | 1.95 ± 0.14 |
1Values represent means ± SD. 2mAgNOR = mean AgNOR, * are statistically significant different from DMBA groups (p< 0.05) by Kruskal Wallis continued with Mann-Whitney
Figure 3.
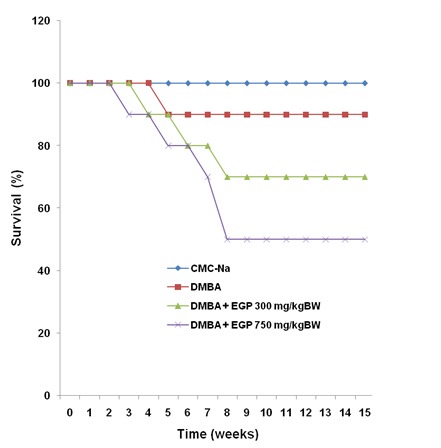
Survival curve of animals.
The y-axis represents the number of rats (%) and the x-axis the time from the start of the experiment (week).
Figure 4.
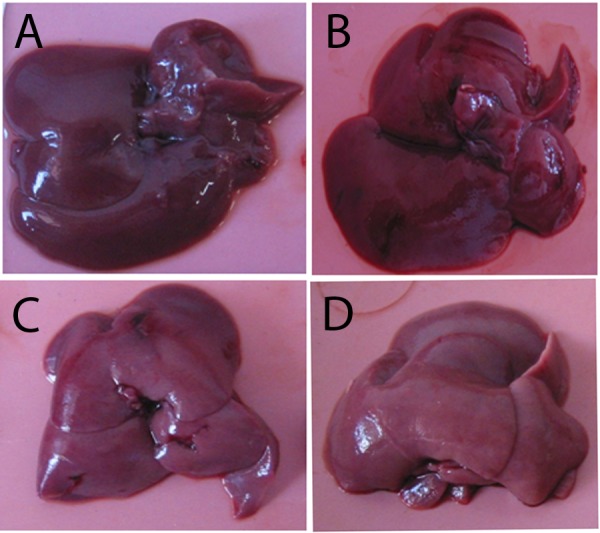
Morphology of liver treated with EGP in 7,12-dimethylbenz[a]nthracene-induced male rats liver.
Rats were divided into 4 groups, (A) 0.5 % CMC-Na, (B) DMBA control group (20 mg/kgbW in CMC-Na), (C) DMBA+ 300 mg/kgBW EGP; (D) DMBA+750 mg/kgbw EGP. At autopsy, liver organ were removed and fixed in 10% buffered formalin.
Histopathological studies of liver
Histopathology profile of liver of DMBA and DMBA+EGP treated rats is depicted in Figure 5. In general, hematoxylin and eosin staining showed no differences in morphology between DMBA and DMBA+EGP. There were no primary liver cancer i all of the rats. There was only one rat in DMBA group that showed lymphoblastic cells in the liver or lymphosarcoma (Figure 6).
Figure 5.
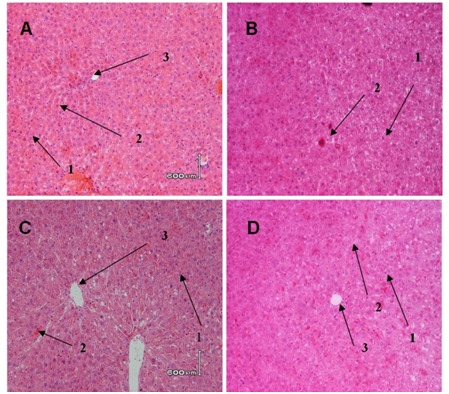
Histological evaluation in liver tissues of control and experimental group.
Rats were divided into 4 groups, (A) 0.5 % CMC-Na, (B) DMBA control group (20 mg/kgbW in CMC-Na), (C) DMBA+ 300 mg/kgBW EGP; (D) DMBA+750 mg/kgbw EGP. At autopsy, liver organ were removed and fixed in 10% buffered formalin. 3-5 μm tissue slices were embedded in paraffin, and stained with hematoxylin and eosin (a1=hepatocyte, 2=sinusoid,3=central venous). Magnification x 400.
Figure 6.
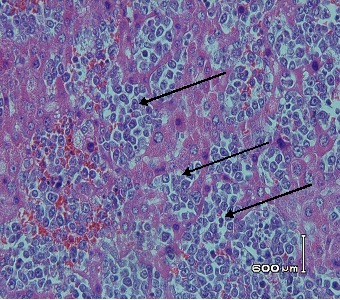
Hematoxylin and eosin stained of lymphoblastic cells in liver tissues
of DMBA control group (20 mg/kgbW in CMC-Na). At autopsy, liver organ were removed and fixed in 10% buffered formalin. 3-5 μm tissue slices were embedded in paraffin, and stained with hematoxylin and eosin. Lymphoblastic cells are pointed by black arrow. Magnification x 400.
Antiproliferative activity of EGP on male rat liver
AgNOR staining on rat liver (Figure 7) showed that DMBA group has higher blackdots than the other groups. mAgNOR score (Table 1) of CMC-Na and DMBA are 1.49 ± 0.04 and 2.25 ± 0.170 respectively. Treatment of EGP 300 mg/kgbw exhibited significant antiproliferative activity of DMBA (Table 1) with mAgNOR score 1.72 ± 0.04 (p<0.05), while treatment EGP 750 mg/kgbw showed no significancy to DMBA group. In general, treatment of EGP inhibit proliferation on rat liver induced by DMBA. The higher dose of EGP, the lower proliferative activity of the extract.
Figure 7.
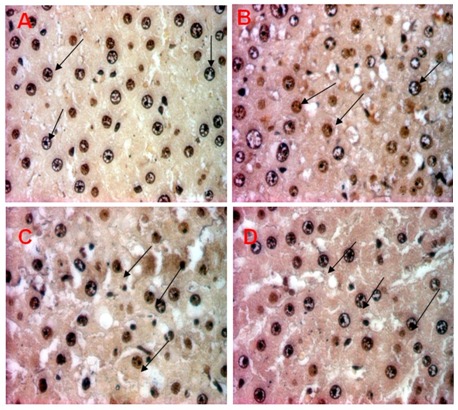
AgNO3 stained liver tissues of control and experimental group.
Rats were divided into 4 groups, (A) 0.5 % CMC-Na, (B) DMBA control group (20 mg/kgbW in CMC-Na), (C) DMBA+ 300 mg/kgBW EGP; (D) DMBA+750 mg/kgbw EGP. At autopsy, liver organ were removed and fixed in 10% buffered formalin. 3-5 μm tissue slices were embedded in paraffin, and stained with AgNO3. Argyrophilic nucleolar organiser regions (AgNORs) are visible as dark dots within the plasma cell nuclei (pointed by black arrow). Magnification x1000.
Discussion
This study explored the effect of G. procumbens leaves ethanolic extract on DMBA induced male rats liver. In this study, EGP was given a week before, during and after induction of DMBA. EGP treatment was intended to prevent metabolic activation of DMBA and also prevent initiatition and progression of liver cancer.
Our present study showed that DMBA induced high proliferation compared to EGP treatment group. Treatment of EGP 300 mg/kgbw is significantly decreased proliferation of liver cells induced by DMBA. The results in similar to previous study 13 that reported treatment of EGP 300 mg/kgbw significantly (p<0.05) inhibited proliferation of mammary glands induced by DMBA. Antiproliferative activity of EGP is mediated by metabolic enzyme, CYP and GST.
DMBA needs to be metabolized to become a reactive metabolite. DMBA is a substrate of the enzyme cytochrome P450 (CYP), CYP1A1 and CYP1B1.14 In the phase I metabolism, DMBA is changed to 8.9-; 5.6-; 3,4-epoxide DMBA by CYP1A115 and 3,4-epoxide DMBA by CYP1B1.15,16 DMBA 3,4-epoxide compounds can undergo several reaction such as hydration and oxidation to form ultimate carcinogens that are highly mutagenic.17 The epoxide metabolite also can interact with glutathione S-transferase (GST) to generate glutathione conjugates that are readily metabolized to the mercapturic acid and will be eliminated from the body .18
Expression of CYP and GST are inducible. CYP expression is regulated by transcription factor named Aryl hydrocarbon Receptor (AhR).19 Some compound are CYP inducer.19 DMBA is an autoinducer of CYP 1A1 expression.20Induction of CYP 1A1 leads to increasing of DMBA reactive metabolite. Expression of GST is also regulated by transcription factor such as Antioxidant-Responsive Element (ARE), Xenobiotik-Responsive Element (XRE), GST-P Enhancer 1 (GPE) or glucocorticoid-Rensonsive Element (GRE).21 Induction of GST expression is important to eliminate DMBA reactive metabolite from the body.
A previous study 20 reported that treatment of DMBA induced high CYP1A1 expression, but low expression of GSTµ on female rat liver. Treatment of EGP 300 mg/kgbw significantly inhibit CYP1A1 and induced expression of GSTµ on female rat liver induced by DMBA. We proposed that the mechanism of EGP antiproliferative activity on male rat liver is via inhibition of CYP1A1 and induction of GST, but the mechanism nedds to be explored further. Our present study showed that the higher concentration of EGP, the lower proliferative activity on rat liver induced by DMBA. This phenomena is similar to the previous study on female rats 8,20,22 The phenomenon is probably due to saturation of enzymatic receptor, but the mechanism need to be clarified more details.
In the present study, EGP inhibited proliferation of rat liver induced by DMBA. We conclude that EGP inhibited carcinogenesis of DMBA on male rats liver. Ethanolic extract of Gynura procumbens is potential to be developed as anticancer.
Conclusion
Ethanolic extract of G. procumbens leaves showed antiproliferative activity on male rats liver induced by DMBA
Acknowledgements
We would like to Sitarina Widyarini, DVM, PhD. (Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta) for helping on analysis of histopathology.
Conflict of interest
Authors declare no conflict of interest.
Abbreviations
Ethanolic extract of G. procumbens leaves (EGP)
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global ancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes mcf-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-akt and its downstream substrates. Mol Cancer Ther. 2007;6:1031–1038. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- 3.Walaszek Z, Hanausek M, Slaga TJ. Mechanisms of chemopreventiion. Suppl. Am. Coll. Phys. 2004;125:128–133. doi: 10.1378/chest.125.5_suppl.128s-a. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyanto SUGIYANTO, Sudarto B, Meiyanto E, Nugroho AE, Jenie UA. Anticarcinogenic activity of plant isolate. Indo J Pharm. 2003;14(4):216–225. [Google Scholar]
- 5.Meiyanto E, Septisetyani EP. Antiproliferative activity and apoptosis induction of phenolic fraction gynura procumbens (lour.) merr. leaves ethanolic extract on hela cells. Artocarpus. 2005;5(2):74–80. [Google Scholar]
- 6.Jenie RI, Meiyanto E. Antiangiogenic effect of sambung nyawa (gynura procumbens (lour.) merr.) leaves ethanolic extract on chick chorio allantois membrane. Indo J Pharm. 2006;17(1):50–55. [Google Scholar]
- 7.Jenie RI, Meiyanto E. Co-chemotherapy of sambung nyawa (gynura procumbens (lour.) merr.) leaves ethanolic extract and doxorubicin on breast cancer cells. Indo J Pharm. 2007;18(2):81–87. [Google Scholar]
- 8.Meiyanto E, Susilowati S, Tasminatun S, Murwanti R, Sugiyanto DAN. Chemopreventive effect of ethanolic extract of gynura procumbens (lour), merr on the carcinogenesis of rat breast cancer development. Indo J Pharm. 2007;18(3):154–161. [Google Scholar]
- 9.Meiyanto E, Tasminatun S, Susilowati S, Murwanti R, Sugiyanto DAN. Suppression of dmba-induced carcinogenesis of breast cancer in post initition stage by ethanolic extract of gynura procumbens (lour), merr leaves. Indo J Pharm. 2007;18(4):169–175. [Google Scholar]
- 10.Pich A, Margaria E, Chiusa L, Bortolin P, Palestro G. Relationship Between AgNORs, MIB-1 and OncogeneExpression in Male Breast Carcinoma and Papillary Superficial Bladder Neoplasm. Oncology Reports. 2003;10:1329–1335. [PubMed] [Google Scholar]
- 11.Bánkfalvi A, Giuffré G, őfner D, Diallo R, Poremba C, Buchwalow IB, Barresi V, Bőcker W, Tuccari G. Relationship between her2 status and proliferation rate in breast cancer assesed by immunohistochemistry, fluorescence in situ hybridisation and standardised agnor analysis. International Journal of Oncology. 2003;23:1285–1292. [PubMed] [Google Scholar]
- 12.Rizali E, Auerkari EI. Teknik pewarnaan silver (agnor) sebagai salah satu cara menentukan aktivitas proliferasi sel tumor dan apoptosis. Jurnal Kedokteran Gigi Indonesia. 2003;10(3):41–45. [Google Scholar]
- 13.Hamid IS. Proliferation activity of gland mammae after treated with gynura procumbens leaves extract and dmba initiation on sprague dawley rats. Veterinaria Medika. 2009;2:1979–1305. [Google Scholar]
- 14.Shimada T, Guengerich FP. Inhibition of human cytochrome p450 1a1-, 1a2-, and 1b1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol. 2006;19:288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- 15.Weimer TL, Ashok PR, Ulrich H, David AS, Craig S, Michael RM, William B, Jerry H, Bailey G. Influence of ß-naphthoflavone on 7,12-dimethylbenz(a)anthracene metabolism, dna adduction, and tumorigenicity in rainbow trout. Toxicol Sci. 2000;57:217–228. doi: 10.1093/toxsci/57.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Heidel SM, Holston K, Jeroen T, Buters M, Gonzales FG, Jefcoate CR, Czupyrynski CJ. Bone marrow stromal cell cytochrome p4501b1 is required for pre-b cell apoptosis induced by 7,12-dimethylbenz(a)antracene. Molecular Pharmacology. 1999;56(6):1317–1323. doi: 10.1124/mol.56.6.1317. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner HE, Vulimiri SV, Uberecken MJRA, Digiovanni J. Role of cytochrome p450 1a1 and 1b1 in the metabolic activation of 7,12-dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem. Res. Toxicol. 2002;15:226–235. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- 18.Yanaida Y, Kohno H, Yoshida K, Hirose Y, Yamada Y, Mori H, Tanaka T. Dietary silymarin supresses 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male f344 rats. Carcinogenesis. 2002;23(5):787–794. doi: 10.1093/carcin/23.5.787. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-antona C, Ingelman-sundberg M. Cytochrome p450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 20.Hamid IS, Sugiyanto Meiyanto E, Widyarini S. CYP1A1 and GSTμ expression of hepatocytes induced by 7,12-dimethylbenz(a)anthracene and the influence of Ethanolic Extract of Gynura procumbens. Indo J Pharm. 2009;20(4):198–206. [Google Scholar]
- 21.Hayes JD, Pulford DJ. The Glutathione S-transferase Supergene Family: Regulation of GST and the Contribution of The Isoenzymes to Cancer Chemoprotection and Drug Resistance. Chem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 22.Hamid IS, Sugiyanto SUGIYANTO, Meiyanto E, Widyarini S. P53 expression in sprague dawley rat mammary after initiation of 7,12-dimethylbenz[a]anthracene (dmba) and give chemoprevention of gynura procumbens. Media Ked Hewan. 2008;24(3):171–176. [Google Scholar]