Abstract
Purpose: Azithromycin (AZI) is a new macrolide antibiotic with a better activity against intracellular gram negative bacteria in comparison with Erythromycin. The purpose of this research was to prepare AZI nanoparticles (NPs) using PLGA polymer and to compare the effectiveness of prepared nanoparticles with untreated AZI solution. Methods: AZI NPs were prepared by Modified Quasi-Emulsion Solvent Diffusion method. The antibacterial activities of prepared NPs in comparison with AZI solution were assayed against indicator bacteria of Escherichia coli (PTCC 1330), Haemophilus influenzae (PTCC 1623) and Streptococcus pneumoniae (PTCC 1240) using agar well diffusion. Inhibition zone diameters (IZD) of nano-formulation were compared to the corresponding untreated AZI. Mean Inhibitory Concentration (MIC) values of AZI were also determined using serial dilution method in nutrient broth medium. Results: Mean IZD of nano-formulations for all indicator bacteria were significantly higher than that of untreated AZI (P<0.01). The enhanced antibacterial efficacy was more dominant in the gram positive species. The MIC values of NPs against the tested bacteria were reduced 8 times in comparison to those of untreated AZI. Conclusion: These results indicated an improved potency of AZI NPs which could be attributed to the modified surface characteristics as well as increased drug adsorption and uptake.
Keywords: Azithromycin, PLGA, Nanoparticle, Modified quasi emulsion solvent- diffusion, Minimum inhibitory concentration
Introduction
Nanoparticles as one of the most recent novel drug delivery carriers have been shown to improve drug efficiency via targeting the delivery of drugs, improving the bioavailability and either sustaining the release of the drugs or solubilizing them for systemic delivery. Additional advantage of this novel technology is the lower enzymatic as well as environmental degradation of pharmaceutical active ingredients. 1-5 The main features of nanoparticles, making them ideal candidates for drug delivery, are small size and use of biodegradable materials in their preparation. Indeed the nano-sized character of these particles causes their extravagation through the endothelium in inflammatory sites, epithelium, tumors, or penetrate microcapillaries and consequently allows for efficient uptake by a variety of cell types and selective drug accumulation at target sites. 2,5-8 The use of biodegradable materials for nanoparticles preparation allows for sustained drug release within the target site over a period of days or even weeks and thus increases the therapeutic benefit, while minimizing side effects. The best-known class of biodegradable materials for controlled release is the poly lactide-co-glycolides (PLGAs). PLGAs are biocompatible and biodegradable polymers that are hydrolytically degraded into nontoxic oligomers or monomers, lactic acid and glycolic acid. 9-12
There have been numerous studies in recent years, especially based on nanoparticle systems, in order to the targeted the delivery of antibiotics toward the infected cells as well as enhancing their physicochemical properties. Fawaz and coworkers showed that Ciprofloxacin-loaded nanoparticles were more active in human macrophages infected with Mycobacterium avium than free drug. 13 In the other study it has been found by Fattal and coworkers that the efficacy of ampicillin is increased by 120-fold in murine salmonellosis by loading the drug into the nanoparticles. 14 Moreover Italia and coworkers proved that biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity. 15
Azithromycin (AZI) is a new macrolide antibiotic (Figure 1) that is similar in structure to erythromycin with a broader gram-negative antibacterial spectrum. This drug acts by binding to the 50s ribosomal subunit of susceptible microorganism and interfering with microbial protein synthesis. 16,17 Antimicrobial spectrum of AZI is wide including aerobic gram-positive microorganism such as Staphylococcus aureus, Streptococcus pneumoniae, aerobic gram-negative microorganism such as Haemophilus influenzae and anaerobic microorganism such as Clostridium perfingens as well as Mycobacteria. It worth to note that Haemophilus influenzae and Streptococcus pneumoniae are responsible for a broad variety of infections such as lower respiratory tract diseases.
Figure 1.
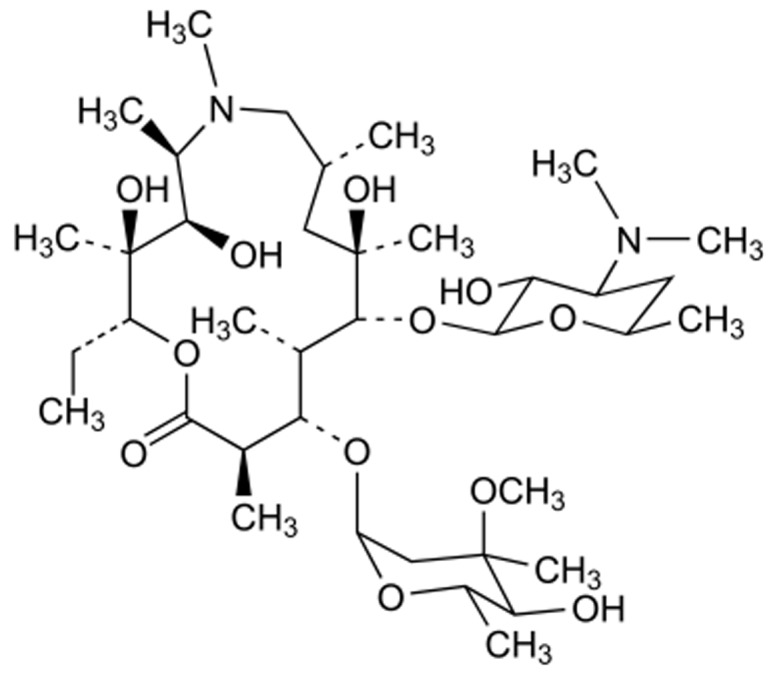
Chemical structure of azithromycin
In our previous study we developed AZI nanoparticles and evaluated their physicochemical properties. 18 In the present work we aimed to evaluate the antibacterial activity of selected formulations with appropriate physicochemical specifications against a group of most important AZI susceptible bacteria including Escherichia coli, Haemophilus influenzae and Streptococcus pneumoniae in comparison with untreated azithromycin.
Materials and Methods
Materials
AZI powder was obtained from Dr Reddy,s pharmaceutical Company, India. Poly (D,L-lactide-co-glycolide) (PLGA) (50:50 D,L-lactide:glycolide) with average molecular weight of 12,000 g/mol (Resomer RG 502), was purchased from boehringer Ingelheim, Germany. Poly vinyl alcohol, PVA, with molecular weight of MW 95000 was obtained from Acros Organics (Geel, Belgium). Escherichia coli (PTCC1330), Haemophilus influenzae (PTCC 1623), Staphylococcus aureus (PTCC 1112) and Streptococcus pneumonia (PTCC 1240) were purchased from Persian Type Culture Collection, Iran. Haemophilus Selective Agar, SoyBean Casein Digest Agar and Antibiotic assay Medium were purchased from Merck (Darmstadt, Germany). Blood Base Agar media was purchased from DIFCO, U.K. All other materials used were of analytical or HPLC grade.
Preparation of nanoparticles
Nanoparticles (Nanospheres) were prepared according to our previously published method. 18 Briefly NPs (nanospheres) with 1:3, 1:2 and 1:1 ratios of the drug to PLGA were prepared by nano-precipitation according to the modified quasi emulsion solvent diffusion technique. AZI and PLGA powders were co-dissolved in internal phase containing acetone, 2.5 ml, at room temperature (25°C). Typically, different ratios of drug and polymer, with the total amount of 100 mg, were co-dissolved in acetone. The resulting organic solution was injected at the constant rate of 0.5 ml/min in aqueous phase (40 ml) containing PVA 95000 (2% w/v), as a stabilizing agent. As water solubility of AZI decreases at higher pH, in order to gain higher drug loading, the pH of aqueous phase was adjusted to 8. The process was carried out under homogenization for 5 min using the Silent Crusher M (Heidolph, Germany). The agitation speed was 13000 rpm in an ice-water bath. Acetone was eliminated at the room temperature under stirring for 12 h. The final nano-suspension was centrifuged (Beckman Centrifuge, AvantiTMJ-25, USA) at 14000 rpm for 30 min and the precipitated NPs were washed twice with water, using the previously described centrifugation approach, and then lyophilized by using a lyophilizer (Christ Alpha 1-4; Germany). Final dry powder was taken out for anti bacterial investigations.
Physicochemical characterization of AZI loaded nanoparticles
The mean particle-size values of NPs were measured by using a laser diffraction particle-size analyzer (Sald 2101, Shimadzu, Japan) equipped with Wing software (version 1.20). The morphology of the NPs were investigated by scanning electron microscopy (SEM) (LEO 440i, Leo Electron Microscopy Ltd, Cambridge, UK) at an accelerating voltage of 20 kV. In order to determine the encapsulation efficiency of NPs, three samples were dissolved in acetone, after evaporation of the acetone the drug was dissolved in 100 ml sterile water. 3,5,19 The mixture was then centrifuged and the supernatant was drawn to measure their AZI contents microbiologically after appropriate dilution. A microbiological assay was adapted from the method described in the official US Pharmacopoeia (USP 2004) monograph. The X-ray powder diffraction (XRPD) of the drug, PLGA, NPs and physical mixture in the 1:2 drug to polymer ratio were recorded using an automated X-ray diffractometer (Siemens D5000, Munich, Germany). Drug release was performed in sink conditions according to our previously published method. 18
Antibacterial activity of the nanoparticle suspensions
The NPs antimicrobial activity was compared with that of the free drug by means of well diffusion method as well as broth serial dilution method using E. coli (PTCC 1330), H. influenzae (PTCC 1623), S. pneumoniae (PTCC 1240) and S. aureus (PTCC 1112). All the bacteria were purchased from Persian type culture collection. The bacteria obtained in lyophilized form were activated according to their manufacturer’s protocol. At first, their stock samples that contained 20٪ glycerol were prepared from activated bacteria and kept at freezer for further uses. The activated E. coli, H. influenzae, S. aureus and S. pneumoniae were cultured in Soy Bean Casein Digest Agar, Haemophilus Selective Agar, Nutrient Broth and Blood Base Agar medium respectively (24 hours before studies).
The antibacterial activity of the selected formulations as well as control samples was evaluated using agar well diffusion and broth dilution method. 20 In agar well diffusion method, firstly appropriate amount of prepared inoculum of each bacterium, were transferred into its selective medium. Soy Bean Casein Digest Agar medium was used for culturing E. coli. Also H. influenzae and S. pneumoniae were transferred on the surface of Haemophilus Selective Agar and Blood Base Agar respectively. Wells with 8-mm diameters were punched on the surface of agar media using a sterile cork borer. Aliquots of 100 µl of each sample (1:3, 1:2 and 1:1 AZI to PLGA nanoparticles) were transferred into the wells. After incubation at 37 °C for 24 hours, the inhibition zones around the wells were measured in using zone reader. In order to evaluate the MIC of NPs the mentioned nanoparticle suspensions were serially diluted. The tubes were inoculated with a 1ml of inoculums of tested bacterium (final concentration of 105-106CFU/ml). After 24 h of incubation at 37°C, the tubes were screened for any evidence of bacterial growth. MIC was defined as the lowest concentration of AZI that completely suppressed the bacterial growth. 21 PLGA nanoparticles without AZI and untreated AZI were applied as positive and negative controls respectively.
Statistical analysis
One-way analysis of variance (ANOVA) was performed for comparison of the results. P values of 0 < 0.01 were considered significant.
Results and discussion
Physicochemical properties of AZI loaded nanoparticles
Based on our previously published paper, the submicron particles ranging from 212 to 252 nm with Narrow range of size (Figure 2), a relatively monodisperse distribution with spherical morphology (Figure 3) were achieved using modified quasi emulsion solvent diffusion technique. Results also indicated a maximum AZI loading of about 78.5٪ with an increasing manner with increase in the proportion of PLGA. This observation may be attributed to the higher viscosity of the internal organic phase for higher PLGA ratios, which in turn would decrease the diffusion coefficient of the drug. Furthermore at higher polymer ratio the extent of drug loss during washing procedure will be less.
Figure 2.
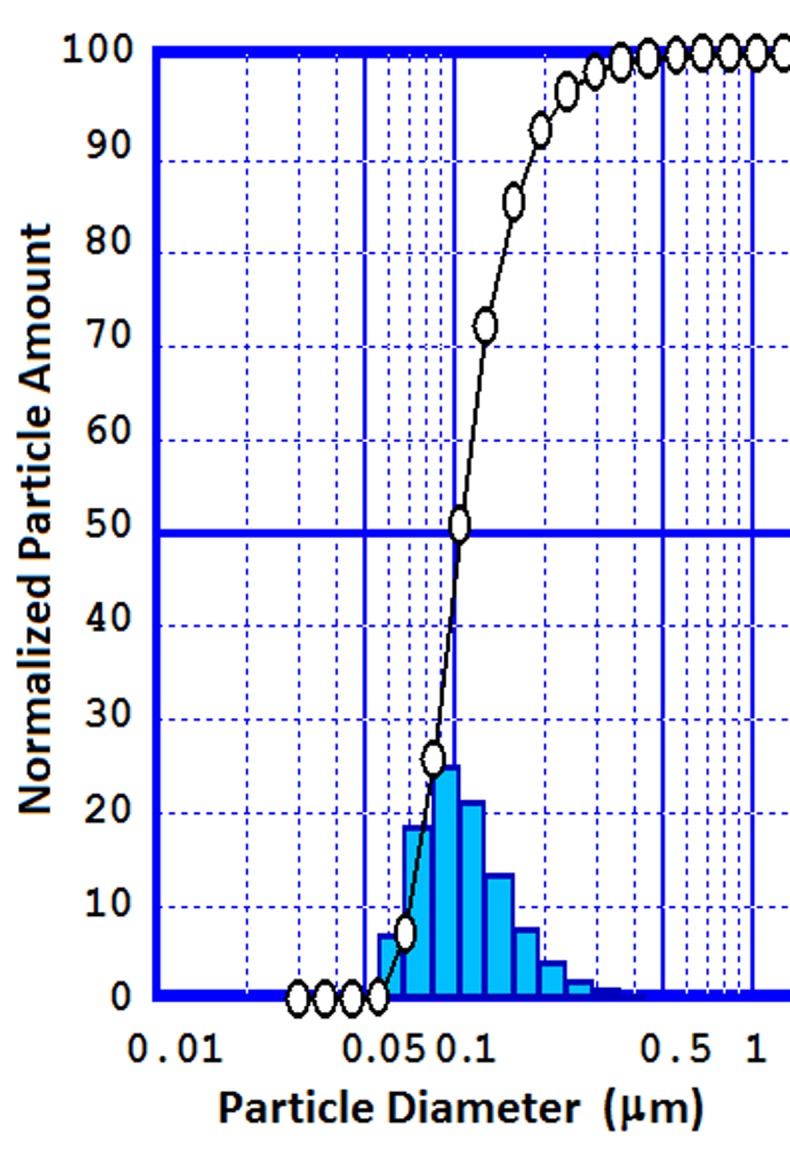
Particle size distribution of nanoparticles preparation with 1:3 ratios of the drug to PLGA ratio.
Figure 3.
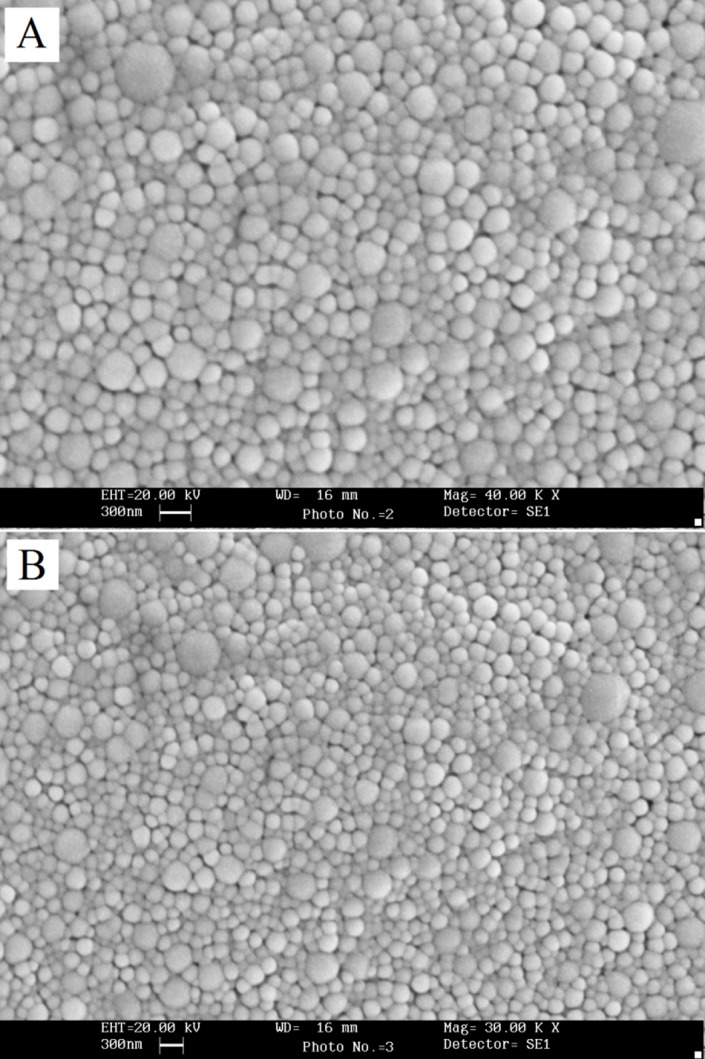
SEM images of azithromycin loaded nanoparticles of the (A) 1:2 (B) 1:3 drug to polymer ratios.
XRPD patterns of NPs were characterized by the complete absence of any diffraction peaks, suggesting a complete amorphization of AZI in the NPs. 18 Based on the results from release study the release rate of AZI from NPs was slower and more sustained than that of intact AZI that may be due to the presence of insoluble polymer (PLGA) in the NPs matrix body which in turn reduces the water penetration, hence dissolution and diffusion 22-24 (data not shown). 18
Antimicrobial activity of AZI loaded nanoparticles
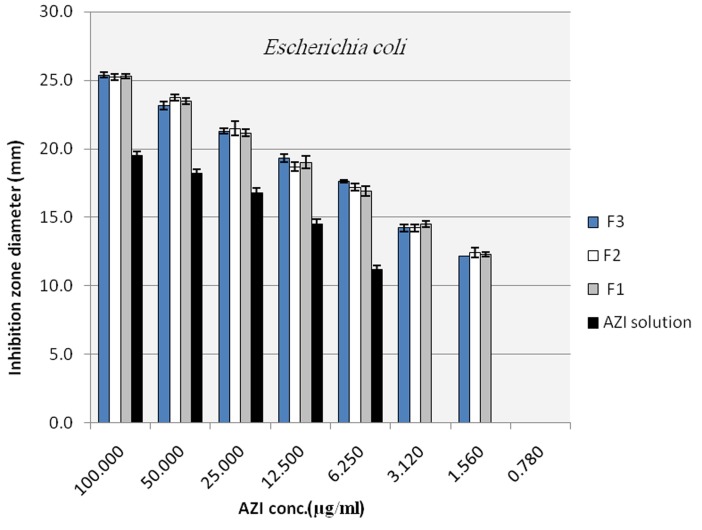
To evaluate the antibacterial activity of the selected nano formulations and untreated AZI samples, agar diffusion method and broth dilution method, that is highly recommended methods for testing of antimicrobial agents against fastidious organisms like Haemophilus spp. and streptococci, were performed based on NCCLS guideline with some modifications as described in material and method section. Mean inhibitory diameters related to each of the nano formulation of AZI were compared to their untreated counterparts in the same concentrations using ANOVA. Figure 4 to Figure 6 illustrate and compare mean inhibitory diameters related to AZI nonoparticles with untreated samples against E. coli, H. influenzae and S. pneumoniae respectively. As it can be seen there are significant differences between inhibitory zone diameters of samples. For instance the concentration of 100 µg/ml of untreated AZI produced mean inhibitory diameter of about 20 mm, whereas AZI nanoparticles showed the same mean inhibitory diameter (20 mm) in the concentration of 12.5 µg/ml indicating 8 times reduction in the AZI concentration to achieve the same antibacterial activity against E. coli (Figure 4). In a same manner as shown in Figure 5 and 6 the activities of AZI nanoparticles were considerably enhanced against H. influenzae and S. pneumoniae compared to their untreated counterparts. As the first inhibitory diameters appear in the concentrations of 25 µg/ml and 3.12 µg/ml for untreated AZI against H. influenzae as well as S. pneumoniae respectively, they shift to 3.12 µg/ml and 0.19 µg/ml when AZI nanoparticles were applied, showing 8 and 16 times reductions in the required AZI concentrations for producing the same action against H. influenzae And S. pneumoniae respectively. Table 1 show the mean MIC of the nano formulation and untreated AZI against E. coli and S. aureus. The lowest inhibitory concentration were about 0.78 µg/ml and 0.39 µg/ml regardless of the ratio of drug:polymer in nanoparticle suspension, whereas the inhibitory concentration were about 6.25 µg/ml and 3.12 µg/ml for the corresponding Untreated AZI solution in E. coli and S. aureus respectively, indicating 8 times reduction in AZI MIC. Blank NPs indicated no antibacterial activity against indicated bacteria. Also the ratio of polymer used had no influence on the antibacterial activity of the nanoparticles, at least for the indicator bacteria examined here (P>0.01). It also worth to note that among three indicator bacteria tested in this study the activity of prepared nano formulations against gram positive S. pneumoniae were remarkably better than gram negative species of E. coli and H. influenza. This could be because of some possible mechanisms; one is the fact that presence of a double membrane could exclude certain structures from penetrating the cell, partially accounting for why gram negative bacteria are generally more resistant to antibiotics than other gram positive bacteria. 25
Figure 4.
Inhibition zone diameter of azithromycin loaded nanoparticles in plates with E.coli inoculums (NANO 1:1 (F1), NANO 1:2 (F2), NANO 1:3 (F3))
Figure 6.
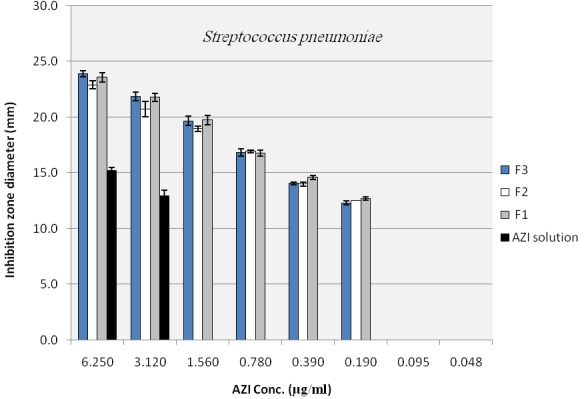
Inhibition zone diameter of azithromycin loaded nanoparticles in plates with S. pneumonia inoculum (NANO 1:1 (F1), NANO 1:2 (F2), NANO 1:3 (F3))
Figure 5.
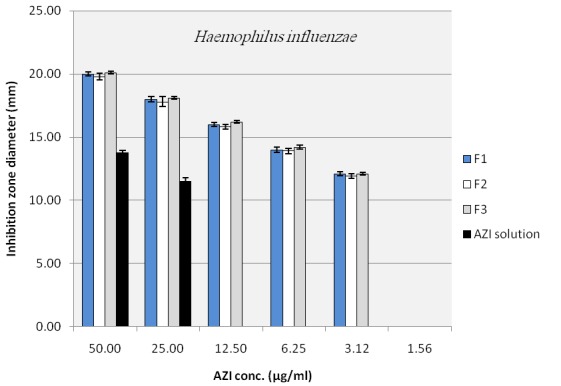
Inhibition zone diameter of azithromycin loaded nanoparticles in plates with H. influenza inoculums (NANO 1:1 (F1), NANO 1:2 (F2), NANO 1:3 (F3))
Table 1. Minimum inhibitory concentration (MIC) of untreated AZI solution (AZI), physical mixtures (PM) and AZI-loaded nanoparticles with different drug:polymer ratios against E. coli and S. aureus.
| MIC (µg/ml) | ||
| Sample | E. coli | S. aureus |
| AZI | 6.25 | 3.12 |
| PM 1:1 | 6.25 | 3.12 |
| PM 1:2 | 6.25 | 3.12 |
| PM 1:3 | 6.25 | 3.12 |
| NANO 1:1 | 0.78 | 0.39 |
| NANO 1:2 | 0.78 | 0.39 |
| NANO 1:3 | 0.78 | 0.39 |
Conclusion
The present study was conducted to evaluate the antibacterial activity of AZI-PLGA nanoparticles against E. coli, H. influenzae, S. aureus and S. pneumoniae which are important species susceptible to AZI. The obtained results indicate that there is a significant enhancement in the efficacy of AZI loaded nano formulations in comparison with untreated AZI in the same concentration. The improved efficacy of pharmaceutical active products when prepared in nano formulations have been repeatedly reported so far. There are also numerous studies to enhance antibiotics efficacies against their indicator microorganisms exploiting the nano technology that showed better activities. 10,13,14 This may be due to the several reasons more dominantly facilitated penetration of drug in nano particle form into the bacterial cells; and better delivery of the drug to its site of action 10 as well as higher stability of the drug in nano encapsulated form against rapid enzymatic/hydrolytic degradation. 26
Adhesion of the NPs to the bacterial cell wall can be another mechanism for greater antibacterial activity of the NPs in comparison with pure drug. This adhesion could provide a sustained antimicrobial activity of the drug incorporated against the target organisms. 27-29
In conclusion, Azithromycin-loaded poly (lactide-co-glycolide) (PLGA) nanoparticles (NPs) were more effective than untreated AZI against E. coli, H. influenzae, S. aureus and S. pneumonia. Therefore, the clinical effectiveness of azithromycin might be enhanced if its nanoparticles are incorporated in the dosage forms e.g. aerosol, ocular, topical and oral preparations as colloidal drug delivery system compared to the conventional formulation of azithromycin present in the market.
Acknowledgments
The authors wish to thank Research Center for Pharmaceutical Nanotechnology, Tabriz, Iran for the financial support of this work.
Conflict of interest
All the authors report no conflicts of interest.
References
- 1.Ge H, Hu Y, Jiang X, Cheng D, Yuan Y, Bi H. et al. Preparation, characterization, and drug release behaviors of drug nimodipine-loaded poly(epsilon-caprolactone)-poly(ethylene oxide)-poly(epsilon-caprolactone) amphiphilic triblock copolymer micelles. J Pharm Sci. 2002;91:1463–73. doi: 10.1002/jps.10143. [DOI] [PubMed] [Google Scholar]
- 2.Ingh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jelvehgari M, Barar J, Valizadeh H, Shadrou S, Nokhodchi A. Formulation, characterization and in vitro evaluation of theophylline-loaded Eudragit RS 100 microspheres prepared by an emulsion-solvent diffusion/evaporation technique. Pharm Dev Technol. 2010;16(6):637–44. doi: 10.3109/10837450.2010.508075. [DOI] [PubMed] [Google Scholar]
- 4.Jelvehgari M, Nokhodchi A, Rezapour M, Valizadeh H. Effect of formulation and processing variables on the characteristics of tolmetin microspheres prepared by double emulsion solvent diffusion method. Indian J Pharm Sci. 2010;72:72–78. doi: 10.4103/0250-474X.62251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelvehgari M, Zakeri-milani P, Siahi-shadbad MR, Loveymi BD, Nokhodchi A, Azari Z. et al. Development of pH-sensitive insulin nanoparticles using eudragit L100-55 and chitosan with different molecular weights. AAPS Pharmscitech. 2010;11:1237–1242. doi: 10.1208/s12249-010-9488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in caco-2 cells is size dependent. Pharm Res. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 7.Hajdo L, Szulc AB, Klajnert B, Bryszewska M. Metabolic limitations of the use of nucleoside analogs in cancer therapy may be overcome by application of nanoparticles as drug carriers: a review. Drug Development Research. 2010;71:383–394. [Google Scholar]
- 8.Sunderland CJ, Steiert M, Talmadge JE, Derfus AM, Barry SE. Targeted nanoparticles for detecting and treating cancer. Drug Development Research. 2006;67:70–93. [Google Scholar]
- 9.Barrera DA, Zylstra E, Lansbury PT, Langer R. Synthesis and RGD peptide modification of a new biodegradable copolymer: poly(lactic acid-co-lysine) J Am Chem Soc. 1993;115:11010–11011. [Google Scholar]
- 10.Esmaeili F, Hosseini-Nasr M, Rad-Malekshahi M, Samadi N, Atyabi F, Dinarvand R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomedicine. 2007;3:161–167. doi: 10.1016/j.nano.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Hou Z, Zhou C, Luo Y, Zhan C, Wang Y, Xie L. et al. PLA nanoparticles loaded with an active lactone form of hydroxycamptothecin: development, optimization, and in vitro–in vivo evaluation in mice bearing H22 solid tumor. Drug Development Research. 2011;72(4):337–345. [Google Scholar]
- 12.Yadav KS, Chuttani K, Mishra AK, Sawant KK. Long circulating nanoparticles of etoposide using PLGA-MPEG and PLGA-pluronic block copolymers: characterization, drug-release, blood-clearance, and biodistribution studies. Drug Development Research. 2010;71:228–239. [Google Scholar]
- 13.Fawaz F, Bonini F, Maugein J, Lagueny AM. Ciprofloxacin-loaded polyisobutylcyanoacrylate nanoparticles: pharmacokinetics and in vitro antimicrobial activity. International Journal of Pharmaceutics. 1998;168:255–259. [Google Scholar]
- 14.Fattal E, Youssef M, Couvreur P, Andremont A. Treatment of experimental salmonellosis in mice with ampicillin-bound nanoparticles. Antimicrob Agents Chemother. 1989;33:1540–1543. doi: 10.1128/aac.33.9.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Italia JL, Yahya MM, Singh D, Ravi Kumar MN. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone. Pharm Res. 2009;26:1324–1331. doi: 10.1007/s11095-009-9841-2. [DOI] [PubMed] [Google Scholar]
- 16.Zakeri-Milani P, Ghanbarzadeh S, Lotfipoor F, Milani M, Valizadeh H. Pharmacokinetic study of two macrolide antibiotic oral suspensions using an optimized bioassay procedure. Journal of Bioequivalence & Bioavailability. 2010;2:111–115. [Google Scholar]
- 17.Zakeri-Milani P, Valizadeh H, Islambulchilar Z, Damani S, Mehtari M. Investigation of the intestinal permeability of ciclosporin using the in situ technique in rats and the relevance of P-glycoprotein. Arzneimittelforschung. 2008;58:188–192. doi: 10.1055/s-0031-1296491. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi G, Valizadeh H, Barzegar-Jalali M, Lotfipour F, Adibkia K, Milani M. et al. Development of azithromycin-plga nanoparticles: physicochemical characterization and antibacterial effect against salmonella typhi. Colloids Surf B Biointerfaces. 2010;80:34–39. doi: 10.1016/j.colsurfb.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Jelvehgari M, Valizadeh H, Rezapour M, Nokhodchi A. Control of encapsulation efficiency in polymeric microparticle system of tolmetin. Pharm Dev Technol. 2010;15:71–79. doi: 10.3109/10837450903002173. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard M7-A5. 5th ed. Wayne, PA: NCCLS; 2000.
- 21.No HK, Young Park N, Ho Lee S, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. International Journal of Food Microbiology. 2002;74:65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 22.Valizadeh H, Nokhodchi A, Qarakhani N, Zakeri-Milani P, Azarmi S, Hassanzadeh D. et al. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin, and Eudragit E100. Drug Dev Ind Pharm. 2004;30:303–317. doi: 10.1081/ddc-120030426. [DOI] [PubMed] [Google Scholar]
- 23.Azarmi S, Farid J, Nokhodchi A, Bahari-saravi SM, Valizadeh H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices. Int J Pharm. 2002;246:171–177. doi: 10.1016/s0378-5173(02)00378-2. [DOI] [PubMed] [Google Scholar]
- 24.Valizadeh H, Zakeri-Milani P, Barzegar-Jalali M, Mohammadi G, Danesh-Bahreini MA, Adibkia K. et al. Preparation and characterization of solid dispersions of piroxicam with hydrophilic carriers. Drug Dev Ind Pharm. 2007;33:45–56. doi: 10.1080/03639040600814965. [DOI] [PubMed] [Google Scholar]
- 25.Lotfipour F, Nazemiyeh H, Fathi-Azad F, Garaei N, Arami S, Talat S. et al. Evaluation of Antibacterial Activities of Some Medicinal Plants from North-West Iran. Iranian journal of basic medicinal sciences. 2008;11:80–85. [Google Scholar]
- 26.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 27.Dillen K, Bridts C, Van Der Veken P, Cos P, Vandervoort J, Augustyns K. et al. Adhesion of PLGA or Eudragit/PLGA nanoparticles to Staphylococcus and Pseudomonas. Int J Pharm. 2008;349:234–240. doi: 10.1016/j.ijpharm.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Lboutounne H, Chaulet JF, Ploton C, Falson F, Pirot F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(epsilon-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release. 2002;82:319–334. doi: 10.1016/s0168-3659(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 29.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]