Abstract
Purpose: The paper tried to systematically investigate the in vitro antioxidant activity of Rhizoma Cibotii (RC) for the first time. Method: The methanol extract from RC (MERC) was prepared then systematically investigated by various antioxidant assays, including: DPPH• (1, 1-diphenyl-2-picrylhydrazyl radical), ABTS•+ (3-ethylbenzthiazoline-6- sulfonic acid diammonium salt radical), •O2- (superoxide anion radical), •OH (hydroxyl radical) scavenging assays, Fe3+ reducing power, Cu2+ reducing power assays, compared with positive controls Trolox (± -6 -hydroxyl -2,5, 7, 8-tetramethlychromane-2-carboxylic acid) and BHA (butylated hydroxyanisole). Its total phenolics and caffeic acid content were also measured by Folin-Ciocalteu method and HPLC, respectively. Result: MERC exhibited effective antioxidant activity in dose-dependent manners and its IC50 values were calculated as 44.2 ± 0.62, 19.84 ± 0.31, 137.66 ± 2.90, 22.94 ± 0.90, 289.73 ± 46.17, 53.52 ± 1.51 µg /mL,for DPPH•, ABTS•+, •O2-, •OH scavenging assays, Fe3+ reducing power, Cu2+ reducing power assays, respectively. Its total phenolics content was 50.88 ± 1.24 mg CAE /g and the caffeic acid content was 1.82 ± 0.19 mg/g. Conclusion: Rhizoma Cibotii has effective in vitro antioxidant activity which may attribute to its total phenolics, among which caffeic acid can be considered as one of the active components. The pharmacological effects or healthcare functions of whole RC may result from the synergistic effects caused by the combination of its components and its antioxidant effect plays an important role in the synergistic effects.
Keywords: Rhizoma Cibotii, antioxidant activity, radical scavenging, total phenolics, HPLC, caffeic acid
Introduction
Rhizoma Cibotii (RC), the root of Cibotium barometz (L.) J. Sm. in Dicksoniaceae, is a commonly used Chinese herbal medicine. From the viewpoint of traditional Chinese medicine (TCM), RC has the efficacies of tonifying the liver and the kidney, strengthening bones and tendons, relaxing the meridian, dispelling wind-dampness.1 Hence, it is usually used as the main ingredient with other Chinese herbal medicine to pickle wine for drinking for body building and disease treatment like waist sour, rheumatism and so on. In modern clinical applications, RC is used to treat lumbar and leg pain, numbness, hemiplegia, leucorrhea spermatorrhea, women bleeding and kinds of tumors such as osteoma, osteosarcoma, brain tumors, and multiple myeloma.2,3
Phytochemical study on RC indicated that RC mainly contained fatty acid (e.g. oleic acid, palmitic acid, and octadecaonic acid),4,5 phenolic acids (e.g. caffeic acid, protocatechuic acid),6,7 flavonoids (e.g. kaempferol, onychin)8,9and so on. Pharmacological investigations revealed that palmitic acid has anti-inflammatory effect and octadecaonic acid can lower blood lipid4, water-soluble phenolic acids such as caffeic acid and protocatechuic acid are the active constituents for rheumatoid arthritis and osteoarthritis10, flavonoids like kaempferol and onychin possess antioxidant activity and anticancer effects.11-13
Despite that the components in RC possess various pharmacological effects, according to the free radical biology & medical, the pharmacological effects or healthcare functions of Chinese medicine of whole RC may closely related to it antioxidant activity.14 However, to our knowledge, the comprehensive evaluation of antioxidant activity of RC has not been reported yet. Therefore, this paper tries to evaluate its in vitro antioxidant activity for the first time by various methods, including DPPH•, ABTS•+, •O2- and •OH radical-scavenging, Fe3+reducing power and Cu2+ reducing power assays. Its total phenolics and caffeic acid content were also measured by chemical methods.
Obviously, the study is helpful to explain the mechanism of pharmacological effects or healthcare functions of RC.
Materials and Methods
Plant material
Rhizoma Cibotii was purchased from Guangzhou University of TCM Yanghe Interlink Limited Company and identified by Prof. Tang Shuhui.
Chemicals and reagents
DPPH• (1,1-Diphenyl-2-picrylhydrazyl radical), pyrogallol, Trolox ( ± -6 -hydroxyl -2,5, 7, 8-tetramethlychromane-2-carboxylic acid), BHA (butylated hydroxyanisole) and neocuproine (2,9-dimethyl-1,10-phenanthroline) were purchased from Sigma Co.; ABTS diammonium salt [2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid diammonium salt)] , and D-2-deoxyribose were obtained from Amresco Co.; HPLC-grade acetonitrile was purchased from Kermel Co. (Tianjin, China); Caffeic acid was purchased from NICPBP; All other chemicals were of analytical grade.
Preparation of plant extract
The plant was powdered then extracted with methanol by Soxhlet extractor for 12 hours. The extract was concentrated under reduced pressure to a constant weight then stored at 4℃.
DPPH• scavenging assay
DPPH• radical-scavenging activity was determined as previously described by Li.15 Briefly, 1 mL of DPPH• solution (0.1 mol/L) was mixed with 0.5 mL of various concentrations of samples dissolved in 95% ethanol. The mixture was kept at room temperature for 30 min, and then the absorbance at 519 nm was measured on a spectrophotometer (Unico 2100, Shanghai, China), using 95% ethanol as the blank. Trolox and BHA were used as the positive controls and the percentage DPPH• inhibition of the test samples was calculated: Inhibition % = (1 – A0/As) × 100. Where As is the absorbance in the presence of RC or positive controls, while A0 is the absorbance in the absence of RC and positive controls.
ABTS•+ scavenging assay
The scavenging activity of ABTS•+ was measured by a previously described method 15. The ABTS•+ was produced by mixing 0.35 mL of ABTS diammonium salt (7.4 mmol/L) with 0.35 mL of potassium persulfate (2.6 mmol/L). The mixture was kept in the dark at room temperature for 12 h to allow completion of radical generation, and then diluted with 95% ethanol (about 1:50) so that its absorbance at 734 nm was 0.70 ± 0.02 measured on a spectrophotometer (Unico 2100, Shanghai, China). To determine the scavenging activity, 1.2 mL of ABTS•+ reagent was mixed with 0.3 mL of sample or negative control (95% ethanol), and the absorbance at 734 nm was measured 6 min after the initial mixing, using 95% ethanol as the blank. The percentage inhibition of the samples was calculated as: Inhibition % = (1 – A/A0) ×100%. Where A0 is the absorbance at 734 nm of the negative control, A is the absorbance at 734 nm of the mixture with sample or positive controls.
Superoxide anion (•O2-) radical-scavenging assay
Measurement of superoxide anion scavenging activity of RC was based on the pyrogallol autoxidation method of Marklund S and Marklund G,16 as described by Li et al.15 Briefly, a sample solution (1 mg/m L, x μL, where x = 0, 50, 100, 150, 200, 250 μL) was mixed with Tris-HCl buffer (2920 - x μL, 0.05 mol/L, pH 8.2) containing EDTA (1 mmol/L) and pyrogallol (80 μL, 6 mmol/L), then shaken rapidly at room temperature. The absorbance at 325 nm of the mixture was measured (Unico 2100, Shanghai, China) against the Tris-HCl buffer as blank per 30 s for 5 min. Trolox and BHA were used as the positive controls. The slope of the correlation of absorbance with time was calculated. The reaction mixture without sample was used as the control. The •O2 -scavenging ability was calculated as: (1 - Slope of sample/ Slope of control) × 100 %.
Hydroxyl (•OH) radical-scavenging assay
The scavenging activity on the hydroxyl radical was investigated by the deoxyribose method17, with some modification. Our preliminary experiments demonstrated that most organic solvents especially alcohol can promote the inhibition percentage value. Hence, the inhibition of the hydroxyl radical was evaluated as follows: all test samples (2 mg/mL) were first dissolved in methanol and 10-110 μL sample solution was taken into mini tubes, and the methanol then removed at 80 ºC to eliminate its interference. The reactions were performed in 0.2 M phosphate buffer (pH 7.4), containing 2.8 mmol/L deoxyribose, 2.8 mmol/L H2O2, 25 μM FeCl3, 80 μM Na2EDTA, and the test sample (20-220 μg). The reaction was started by adding ascorbic acid to a final concentration of 100 μM, and the reaction mixture (800 μL in total) was incubated for 20 min at 50ºC in a water bath. After incubation, the color was developed by addition of 0.5 mL 2-thiobarbituric acid (1 g/100 mL) followed by 0.5 mL trichloroacetic acid (5 g/100 mL) and heating in an oven at 105ºC for 15 min. The sample was cooled and its absorbance was measured at 532 nm against buffer (as blank). The reaction mixture without test sample was used as control. The scavenging activity on hydroxyl radicals was expressed as: Inhibition % = (1 - A/Ac) × 100%. Where Ac is the absorbance at 532 nm of control (without sample), and A is the absorbance at 532 nm of the reaction mixture containing sample.
Reducing power assay
Ferric ions (Fe3+) reducing power
Ferric cyanide (Fe3+) reducing power was determined by the method of Oyaizu 18, as described by Li et al.15. Sample solutions (1 mg/mL, x mL, x = 20, 40, 60, 80, 100, 120 μL) were mixed with Na2HPO4/ KH2PO4buffer (350-x μL, 0.2 M, pH 6.6) and K3Fe(CN)6 (250 μL, 1 g/100 mL). The mixture was incubated at 50ºC for 20 min, before 250 μL of trichloroacetic acid (10 g/100 mL) was added, and the mixture centrifuged at 3000 g for 10 min. The supernatant (400 μL) was recovered, mixed with distilled water (400 μL) and FeCl3 (400 μL, 0.1 g/100 mL) and placed immediately into the spectrophotometer (Unico 2100, Shanghai, China), and the timer started. The absorbance at 700 nm was measured at 90 s. Samples were analyzed in groups of three samples, and when the analysis of one group was finished, the next group of three samples were mixed with FeCl3 to avoid oxidization by air. Trolox and BHA were used as the positive controls, and an increased absorbance indicated increased reducing power. The percentage reducing power of the sample as compared with the maximum absorbance tested, which appeared in BHA at 83.3 μg/mL, was calculated by using the formula: (As / Am) ×100. Here, Am; ;=maximum absorbance tested , As = absorbance of sample.
Cupricions (Cu2+) reducing power
The cupric ions (Cu2+) reducing power capacity was measured by Gülçin 19, with slight modification. Briefly, 125 μL CuSO4 aqueous solution (10 mmol/L), 125 μL neocuproine ethanolic solution (7.5 mmol/L) and 500 μL CH3COONH4 buffer solution (100 mmol/L, pH 7.0) were added to test tubes with different volumes of MERC(1 mg/mL, 20-100 μL). Then, the total volume was adjusted with the buffer to 1 mL and mixed vigorously. Absorbance against a buffer blank was measured at 450 nm after 30 min. Increased absorbance of the reaction mixture indicates an increase of reduction capability. Trolox and BHA were used as the positive controls. The percentage reducing power of the sample as compared with the maximum absorbance tested, which appeared in BHA at 100 μg/mL, was calculated by using the formula: (As /Am) ×100. Here, Am= maximum absorbance tested, As = absorbance of sample.
Determination of total phenolics
The total phenolics content of MERC was determined using a modified Folin-Ciocalteu colorimetric method20, with CA (caffeic acid) as a standard. The MERC (5 mg/mL, 500μL) was mixed with the Folin-Ciocalteu reagent (0.25 mmol/L, 500 μL) and 15% sodium carbonate solution (Na2CO3 ,1.0 mL). The mixture was centrifuged at 3500 g for 3 min after incubation for 30 minutes at room temperature. The absorbance of supernatant was determined at 760nm and the total phenolics content was expressed as caffeic acid equivalent (CAE) in milligram per gram dry extract.
Caffeic acid determination using HPLC
HPLC analysis was performed using Techcomp LC2000, Dikma Diamonsil C18(250 mm×4.6 mm, 5 μm particle size). The mobile phase consisted of solvent A (acetonitrile) and solvent B (0.1 % acetic acid in water), the flow rate was 0.5 mL/min, injection volume was 10 µL and absorption was measured at 323 nm. The content of caffeic acid in the MERC was obtained on the basis of the standard calibration curve.
Statistical analysis
Results were reported as the mean ± standard error of three measurements and analyzed by SPSS (Version 10.0). The IC50 values were calculated by linear regression analysis and all linear regression in this paper was analyzed by Origin 6.0 professional software. One-way analysis of variance (ANOVA) and multiple comparisons were carried out to test any significant difference between the means. A P value of less than 0.05 was considered to be significant.
Results
Scavenging ability on DPPH•
As DPPH• is a stable free radical, it is widely used for radical scavenging assay.19 The radical can dissolve in methanol or ethanol, and their colors show characteristic absorption at 519 nm. When an antioxidant scavenges the free radical by hydrogen donation, the colors in the DPPH• assay solutions become lighter.14 As presented in Figure 1, the radical-scavenging effect of MERC on DPPH• was in a concentration-dependent manner and had an effective capacity at high concentration. The scavenging effect of MERC and the positive controls on the DPPH radical decreased in the order of Trolox(96.8% ) ≈ BHA(93.1.5%) > RC (79.6%), at the concentration of 80 µg/mL. The IC50 values of RC, Trolox and BHA were 44.26 ± 0.62, 4.02 ± 0.71, and 2.03 ± 0.28 µg /mL, respectively (table 1).
Figure 1.
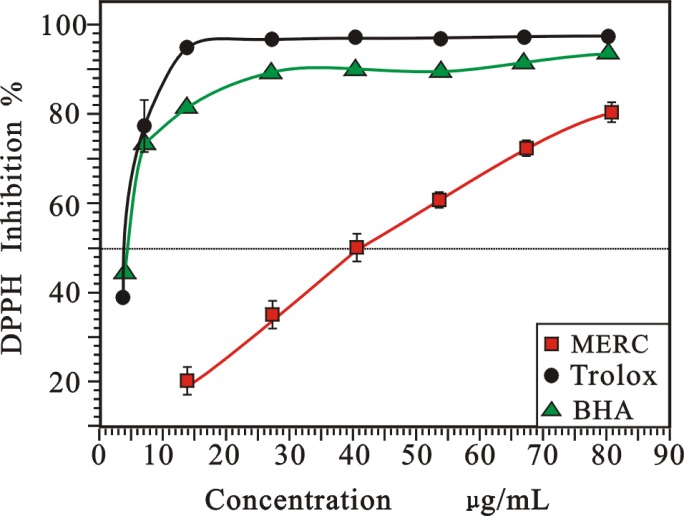
Radical-scavenging activity on DPPH• of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Table 1. The IC50 values of MERC and positive controls (µg/mL) .
| Assays | Rhizoma Cibotii | BHA | Trolox |
| DPPH | 44.26 ± 0.62a,b | 2.03 ± 0.28 | 4.02 ± 0.71 |
| ABTS | 19.84 ± 0.31a,b | 2.03 ± 0.28 | 1.49 ± 0.00 |
| • O 2+ | 137.66 ± 2.90a,b | 2568.62 ± 11.78 | 799.11 ± 72.30 |
| • OH | 22.94 ± 0.90a,b | 26.39 ± 0.28 | 41.84 ± 3.79 |
| Reducing power (Fe3+) | 289.73 ± 46.17a,b | 34.14 ± 0.26 | 44.96 ± 0.60 |
| Reducing power (Cu2+) | 53.52 ± 1.51a,b | 5.74 ± 0.23 | 11.32 ± 0.09 |
All values were mean ± SD (n=3). Results were analyzed by ANOVA. Value with a significantly different from Trolox (P < 0.05); Value with b significantly different from BHA (P < 0.05). MERC, methanolic extract from Rhizoma Cibotii.
Scavenging ability on ABTS•+
Unlike the reactions with DPPH•, which involve H-atom transfer, the reactions with ABTS+involve an electron-transfer process. Generation of the ABTS •+ cation forms the basis of one of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity of pure substances, aqueous mixtures.21 As presented in Figure 2, the scavenging ability of MERC on ABTS•+ was enhancing along with its concentration and was similar to the positive controls at high concentration. As listed in Table 1, the IC50 of MERC, BHA and Trolox were 19.84 ± 0.31, 2.03 ± 0.28 and 1.49 ± 0.00 µg/mL, respectively. Although the IC50 of RC was; ;higher than BHA and Trolox, it still showed an effective antioxidant capacity.
Figure 2 .
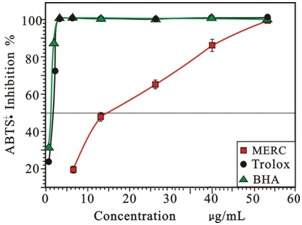
Radical-scavenging activity on •ABTS+ of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Scavenging ability on •O2-
Superoxide anion (•O2-) is one of the most important free radicals in living cells. As Figure 3 shown, the •O2- radical-scavenging effectof MERC was in a dose-response manner. As listed in table 1, the IC50 of MERC, BHA and Trolox were 137.66±2.90, 2568.62±11.78, 799.11±72.30 µg/mL, respectively. So, MERC exhibited stronger •O2--scavengingactivity than the positive controls.
Figure 3 .
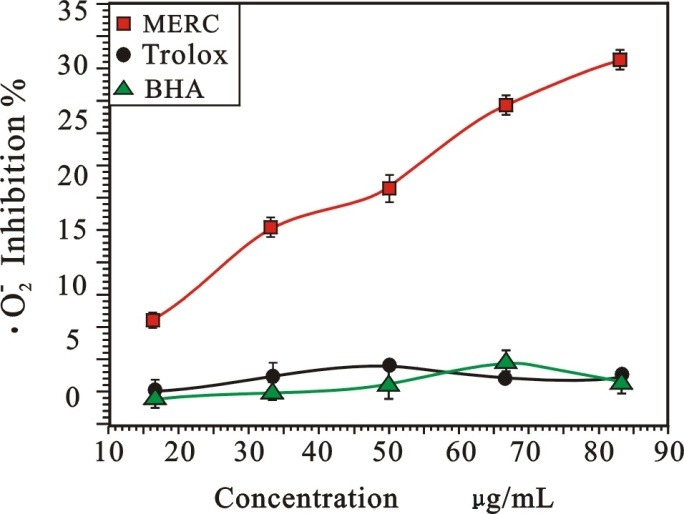
Radical-scavenging activity on •O2- of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Scavenging ability on •OH
Hydroxyl radical (•OH) is also one of the most important free radicals in living cells. However, as the most dangerous form of ROS, it has a high reactivity with a very half-life of approx. 10-9 s in vivo.22 In the study, the •OH scavenging activity was evaluated by the Fenton reaction using the deoxyribose assay. A mixture of Fe3+-EDTA, hydrogen peroxide (H2O2), and ascorbic acid were used to generate hydroxyl radicals (•OH). The response degrades D-2-deoxyribose into fragments which under heating with 2-thiobarbituric acid at acidic solution, are detected at 540 nm because they generate a pink chromogen.Figure 4 revealed that the percentage •OH scavenging effect of MERC and the positive controls (Trolox and BHA) were in concentration-dependent manners. Their relative scavenging activity on •OH decreased in the order of BHA ≈ RC > Trolox. Their IC50(showed in table 1) were 22.94 ± 0.90, 26.39 ± 0.28 and 41.84 ± 3.79 µg/mL, respectively.
Figure 4.
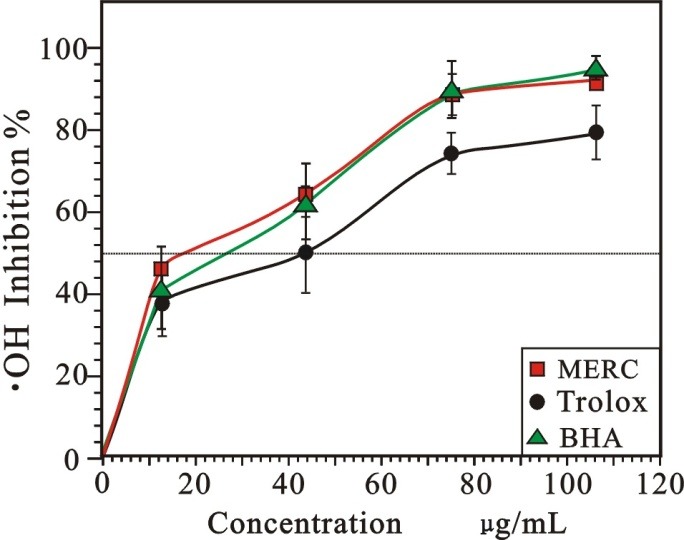
Radical-scavenging activity on •OH of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Reducing power
Many reports have revealed that there is a direct correlation between antioxidant activities and reducing power.23 Hence, reducing capacity may serve as a significant indicator of its potential antioxidant activity. As shown in Figure 5, it can be seen that the reducing power percentage values of MERC and the positive controls (BHA and Trolox) increased with the increasing of sample concentration in the range of the tested concentration. Their relative reducing powers on Cu2+ were as follows: BHA >Trolox > MERC. Their IC50 were 34.14 ± 0.26, 44.96 ± 0.60, 289.73 ± 46.17 µg/mL, respectively. The similar result could be also observed in Cu2+ reducing power assay as presented in Figure 6. The IC50 of MERC, BHA and Trolox were 523.52 ± 1.51, 5.74 ± 0.23 and 11.32 ± 0.09 µg/mL, respectively.
Figure 5 .
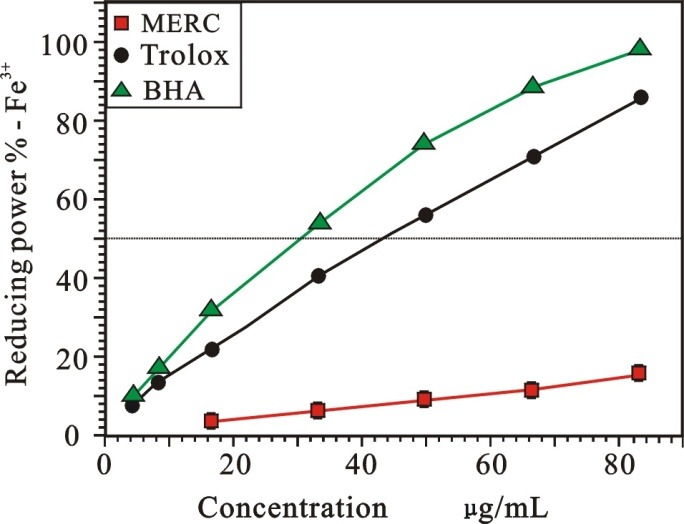
Reducing activity on Fe3+ of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Figure 6 .
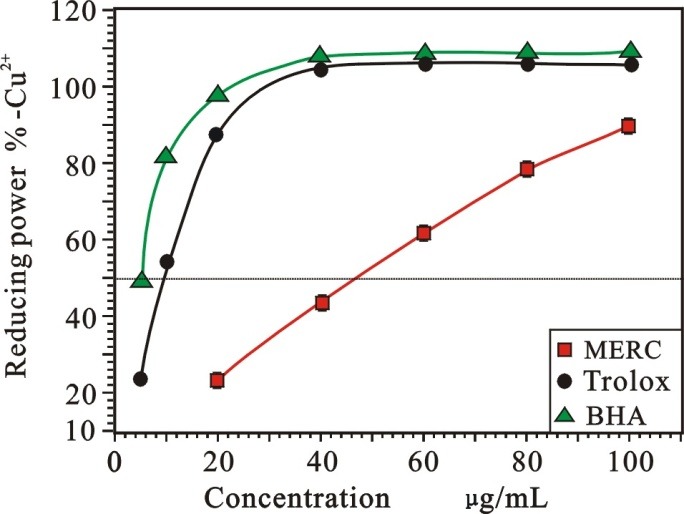
Reducing activity on Cu2+of MERC and positive controls.
(Each value is expressed as mean ± standard deviation, n =3. MERC, methanolic extract from Rhizoma Cibotii.)
Total phenolics content
In this study, Folin-Ciocalteu method was used for determining total phenol content. The principle of the assay is described as follows: Folin-Ciocalteu reagent can oxidate the total phenols compounds quantificationally and is reduced to a blue compound and the color depth is positively related with the total phenols content. In the assay, caffeic acid was used as the standard and the result showed a good liner relationship, according to the linear regression equation: y = 0.0653x +0.102 (R=0.994,x for sample amount, y for absorbance). The total phenolics content of MERC was 50.88 ± 1.24 mg CAE/ g.
Identification and quantification of caffeic acid by HPLC
Sample was identified by the peck symmetry and retention time, by comparison with reference substance at the same conditions. Chromatograms of caffeic acid and MERC were shown in Figure 7 & 8. According to the linear regression equation y = 51374.17x -197426.85 (R=0.9992, x for sample amount, y for peak area), caffeic acid contents in MERC was calculated as 1.82 ± 0.19 mg/g.
Figure 7.
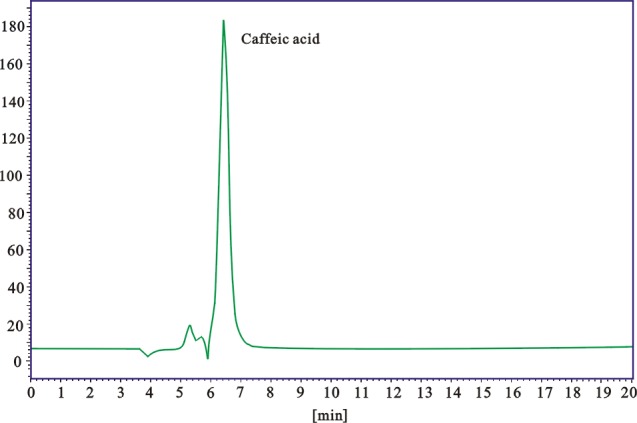
HPLC chromatogram of caffeic acid
Figure 8.
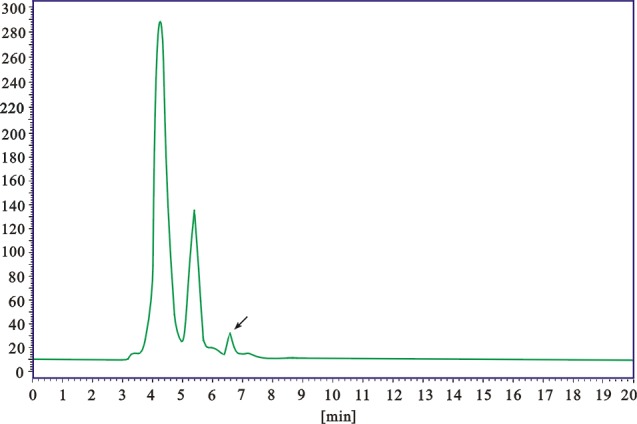
HPLC chromatogram of MERC (methanolic extract from Rhizoma Cibotii)
Discussion
Our results showed that MERC and the standards dose-dependently increased the radical inhibition (or reducing power) values in all antioxidant assays, including DPPH•, ABTS•+, •O2-, •OH, Fe3+ & Cu2+ reducing power assays, suggesting that RC had effective radical-scavenging activity or reducing powers.
On the other hand, a great deal of studies proved that the antioxidant activity of plants may likely be attributed to the phenolic content in it. Our data suggested that RC contained relatively high levels of total phenolics (50.88 ± 1.24 mg CAE/g). As previously mentioned, phytochemical study on RC had indicated the presence of phenolic compounds, including caffeic acid, protocatechuic acid, kaempferol, and onychin (Figure 9).
Figure 9.
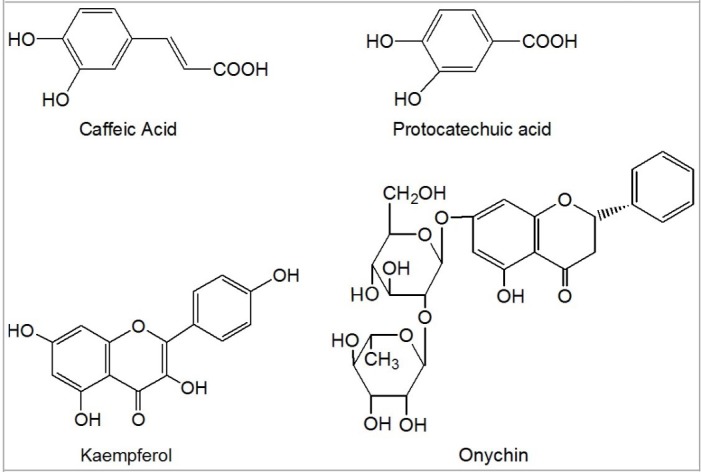
Chemical structure of caffeic acid, protocatechuic acid, kaempferol, and onychin
Since caffeic acid has been proved to be extremely versatile in pharmacological activity,24 it was analyzed by HPLC method in the present study. The results revealed that the caffeic acid contents in MERC was calculated as 1.82 ± 0.19 mg/g and a higher ratio (1.82: 50.88) of caffeic acid vs. total phenolics content suggested that caffeic acid was one of main phenolic compounds in MERC.
As described previously, besides phenolic compounds, other compounds were also found in RC and pharmacological investigations revealed that those compounds possessed different pharmacological effects, therefore, we assumed that the pharmacological effects or healthcare functions of whole RC is likely due to the synergistic effects caused by the combination of its components. Undoubtedly, antioxidant effect plays an important role in the synergistic effects.
Conclusion
As a traditional Chinese medicine herb, Rhizoma Cibotii possesses an effective in vitro antioxidant activity which can contribute to its medicinal or health-care effects. Its antioxidant activity may result from the radical-scavenging & reducing power, and can attribute to the total phenolics, among which caffeic acid is regarded as one of bioactive compounds.
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.China Pharmacopoeia Committee: Chinese Pharmacopoeia (VolumeⅠ). Beijing: Chemical Industry Press; 2010.
- 2.Xu ZY, Chen ZD, Chen ZL, Hou LB. The progress in research of cibotium barometz. Journal of Chinese Medicine Materials. 2000;23:160–161. [Google Scholar]
- 3.Zhao Y, Zhu JY, Zhang GJ. Chinese Medicine (Basis and application of TCM). Beijing: People’s Medical Publishing House; 2007.
- 4.Jia TZ, Zhang JP. Comparative study on essential oil in rhizoma cibotii and processed product. China Journal of Chinese Material Medica. 1996;21:216–217. [PubMed] [Google Scholar]
- 5.Xu ZY, Chen ZD, Chen ZL, Hou LB, Zhang K. Studies on the chemical constituents of cibotium barometz(ⅱ) Pharmaceutical Journal of Chinese People’s Liberation Army. 2000;16:65–68. [Google Scholar]
- 6.Zhang CL, Wang ZX. Studies on the constituents of cibotium barometz (l) j. sm. rhizome. Chinese Journal of Medicinal Chemistry. 2001;11:279–280. [Google Scholar]
- 7.Cheng QH, Yang ZL, Hu YM. Studies on the chemical constituents of rhizoma cibotii. Progress in Pharmaceutical Sciences. 2003;27:298–299. [Google Scholar]
- 8.Xu ZY, Yan Y, Chen ZD, Chen ZL, Zhang K. Studies on the chemical constituents of cibotium barometz (ⅱ) Pharmaceutical Journal of Chinese People’s Liberation Army. 2004;20:337–339. [Google Scholar]
- 9.Hu YW, Yu JL. Advances in study on chemical constituents and pharmacological effects of rhizoma cibotii. Lishizhen Mdicine and Medica Medica Research. 2006;17:275–276. [Google Scholar]
- 10.Yuan Z, Yu JT, Su SW. Determination of protocatechuic acid and caffeic acid in rhizoma cibotii and rhizoma pteris by tlcs. Journal of Shenyang Pharmaceutical University. 2000;17:338–340. [Google Scholar]
- 11.Yang FX, Zhu BY, Zheng X, Hu JF, Ge XL, Huang HL, Liao DF. Protective Effect of Onychin on Lipid Peroxidation in Experimental Liver Damage of Mice. Journal of Nanhua University (Medical Edition) 2002; 30:4-6. [Google Scholar]
- 12.Kim J K, Choi S J, Cho H Y. Protective effects of kaempferol (3,4',5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci Biotechnol Biochem. 2010;74:397–401. doi: 10.1271/bbb.90585. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Zhao CC, Han WN, Liu FZ. Effects of flavonoids on myocardial apoptosis. Chinese Pharmacological Bulletin. 2008;24:635–639. [Google Scholar]
- 14.Ling GT: Antioxidant food and health (Chinese). Beijing: Chemical Industry Press; 2004.
- 15.Li XC, Wu XT, Huang L. Correlation between antioxidant activities and phenolic contents of radix angelica sinensis (danggui) Molecules. 2009;14:5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 17.Kitts DD, Arosha AROSHA, N W, Chun H. Antioxidant properties of a north american ginseng extract. Molecular and Cellular Biochemistry. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 18.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese Journal of Nutrition. 1986;44:307–315. [Google Scholar]
- 19.Gülçin I. Antioxidant properties of resveratrol: a structure-activity insight. Innovative food science and emerging technologies. 2010;11:210–218. [Google Scholar]
- 20.Lacikova L, Muselik J, Msterova I, Grancai D. Antioxidant activity and total phenols in different extracts of four staphylea l. species. Molecules. 2007;12:98–102. doi: 10.3390/12010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-evans C. antioxidant activity applying an improved abts+ radical cation decolorization assay . Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of rumex crispus l. extracts. Journal of Agricultural and Food Chemistry. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 24.Touaibia M, Jean-francois JEAN-FRANCOIS, Doiron J. Caffeic acid, a versatile pharmacophore: an overview. Mini Reviews in Medicinal Chemistry. 2011;11:695–713. doi: 10.2174/138955711796268750. [DOI] [PubMed] [Google Scholar]


