Abstract
Purpose: Celecoxib is nonsteroiddal anti-inflammatory drug that has been used extensively to treat patients with arthritis. The aim of the present study was to formulate and characterize liposomal vesicles loaded with celecoxib. Methods: Liposomes were prepared by thin film method using soya lecithin and cholesterol. The release of drug was determined using a dialysis membrane method. Liposomes were characterized by Differential Scanning Calorimetery (DSC), Transmission Electron Microscopy (TEM) and their particle size was also determined. Results: The results showed that the drug encapsulation efficiency was 67.34% and there was 67.16% release after 0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 24 h. Results of particle size determination showed a mean size of 677nm and nanoparticles were spherical as shown by TEM. The DSC curve of lecithin, cholesterol and celecoxib were different from celecoxib containing liposome. Conclusion: The results of characterization of the vesicles indicated the potential application of celecoxib loaded liposome as carrier system.
Keywords: Celecoxib, Liposome, Thin film method, Differential scanning calorimeter, Transmission electron microscopy, Particle size
Introduction
Celecoxib is a non-steroiddal anti-inflammatory drug (NSAID) that acts on cyclooxygenase-2 (COX-2). It has been used extensively to safely treatment patients with arthritis and also treatment patients with familial adenomatous polyposis and to induce apoptosis in colon, stomach, prostate cancer cells and have antitumor activity in lung, colon and pancreatic cancer.1,2
Liposomes are used as drug carrier for a wide range of drugs including analgesics and anti-inflammatory drug.3 Liposome is a spherical vesicle composed of a bilayer membrane 4 and commercially important drug delivery system for their biodegradability and biocompatibility.5liposomes have been used to administer drugs by several routs such as oral, parenteral and topical.4 Several studies have showed that encapsulation of drug in liposome may enhance the drug permeation into the skin.5
The aim of this study was to prepare and evaluate celecoxib loaded liposomes and to study their in vitro release behavior.
Materials and Methods
Soya lecithin and cholesterol were purchased from Acros, USA and Merck, Germany respectively. Celecoxib was kindly donated by Exir pharmaceutical Co., Iran. All of the solvents were of the analytical grade.
Preparation of liposome
Liposomes were prepared by thin film method. Briefly soya lecithin and cholesterol were dissolved in chloform-methanol (1:1) and 5 mg celecoxib was added in the solution, then the mixture was evaporated in a rotary evaporator. The thin film formed in the round-bottomed flask was hydrated with phosphate buffer. The suspension was agitated by vortex for 30 min and then sonicated for an hour.4,5
Evaluation of the loading efficacy
The liposomal suspension was centrifuged at 20000 rpm for 30 min. The supernatant was analyzed at 260 nm using spectrophotometer (Biochrom WAP Biowave II).
In vitro drug release studies
In vitro celecoxib release from the liposome was determined using dialysis membrane method. 5 g of each formulation was put in a dialysis bag (BETAGEN, width 40 mm). The receptor phase was 25 ml ethanol 80% and was continually using a magnet stirred at 37˚C. An aliquot of 2 ml of sample was withdrawn from each batch at definite time intervals (0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 24 h) and replaced with the same amount of ethanol to maintain sink condition. Then, the concentration of drug released was monitored using a UV spectrophotometer at 260 nm.
Differential Scanning Calorimetry (DSC)
The calorimetric analysis was performed in order to determine the differences in properties of lecithin and cholesterol previously structured in the liposome and the effect of the celecoxib on these properties. The DSC curves were recorded using a DSC-1 Mettler Toledo oven with a temperature range of 30 to 200˚C for 6 min.
Particle size determination)
The average diameter and polydispersity index (PDI) of liposomes were determined using a particle sizer Qudix, ScatterO Scope I system (Korea) at 25 ˚C.
Transmission Electron Microscopy (TEM)
The morphology of the liposomes were observed under Transmission Electron Microscope (Philips- CM-10) operated at HT-100 KV. The samples were stained with 25% (w/v) Formuar and placed on copper grids with films for viewing
Results
The encapsulation of celecoxib in liposome was 67.34%. Celecoxib release from the liposome formulation was 67.16%. Sonication time was affected the liposomal particle size. By increasing the sonication time from 30 min to 1 h, the particle size decreases from 969 nm to 677 nm that had shown in Figure 1a and b (P>0.05), but the particles had polydispersity as shown in Figure 2 a, b, c and d respectively. So it suggested the sonication time is an important parameter in the liposome preparation because the increasing the time of sonication is necessary to decrease the polydispersity. The PDI the liposomes were 2.5 ± 0.0.
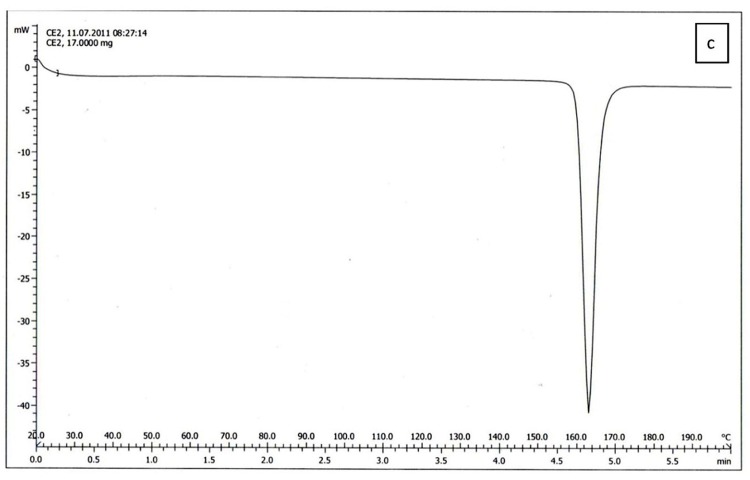
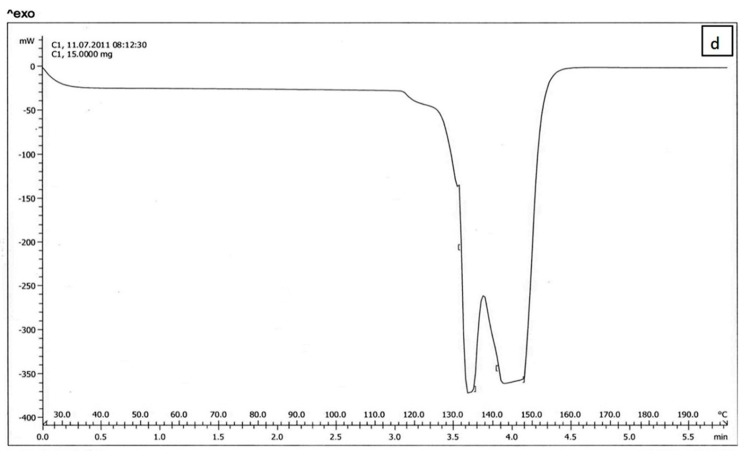
The results of DSC of cholesterol, lecithin, celecoxib and celecoxib containing liposome are shown in Figure 2a, b, C and d respectively. The DSC curve of cholesterol, lecithin and celecoxib containing liposome showed two endothermic peaks in the regions 70-80 and 110-120˚C for cholesterol, 190-200˚C for lecithin and 130-140, 140-150˚C for celecoxib containing liposome (Figure 2a, b, d ), while the DSC curve of celecoxib showed single endothermic peak at 160-170˚C (Figure 2c).
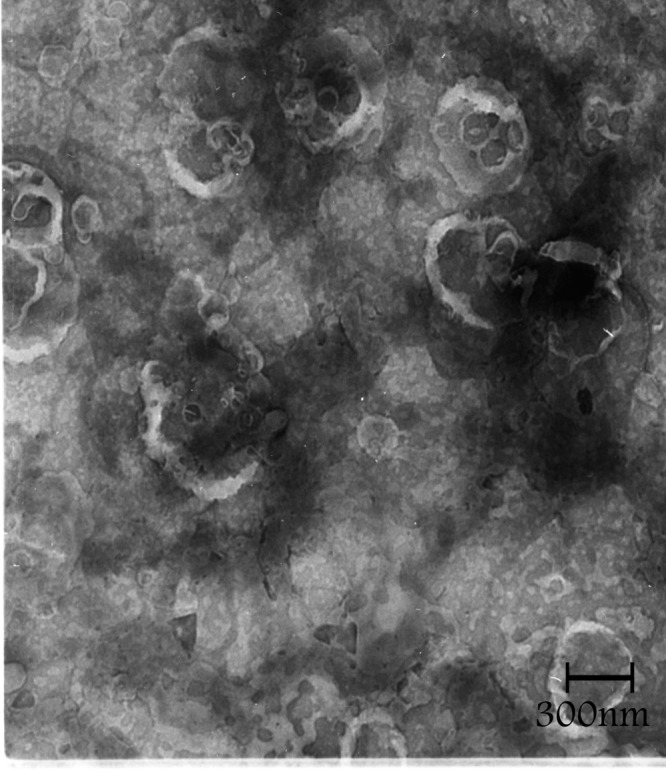
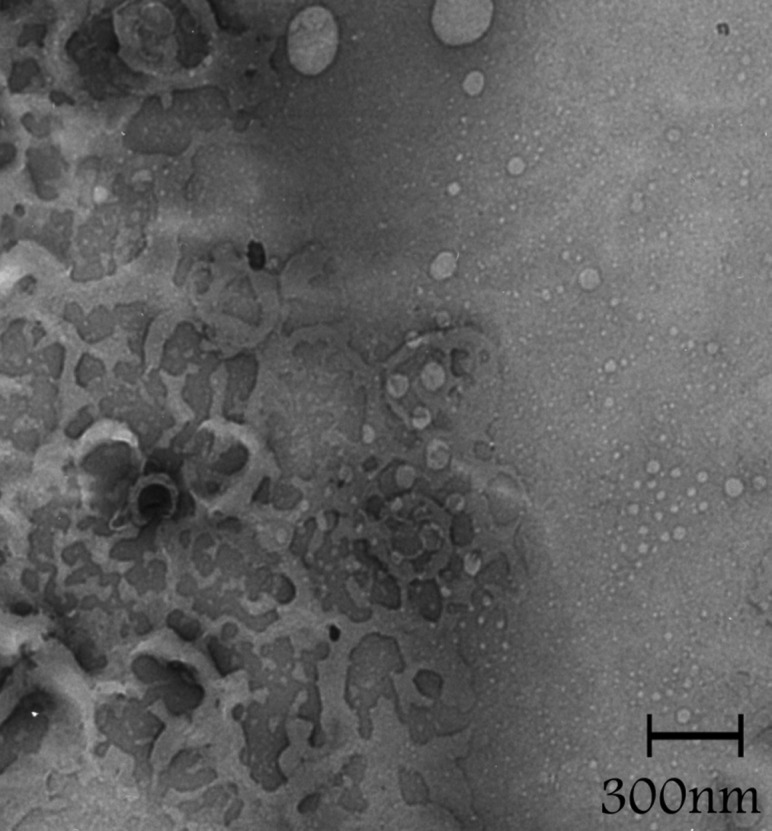
Figure 3, shows the TEM photograph of liposome, denoting shape of the nanoparticles. It is evident that the particles are spherical in shape and also shows that the liposomes have polydispersity and monodispersity.
Figure 3.TEM image in different fields of the celecoxib containing liposomes prepared by thin film method (a-d).
a.
b.
c.
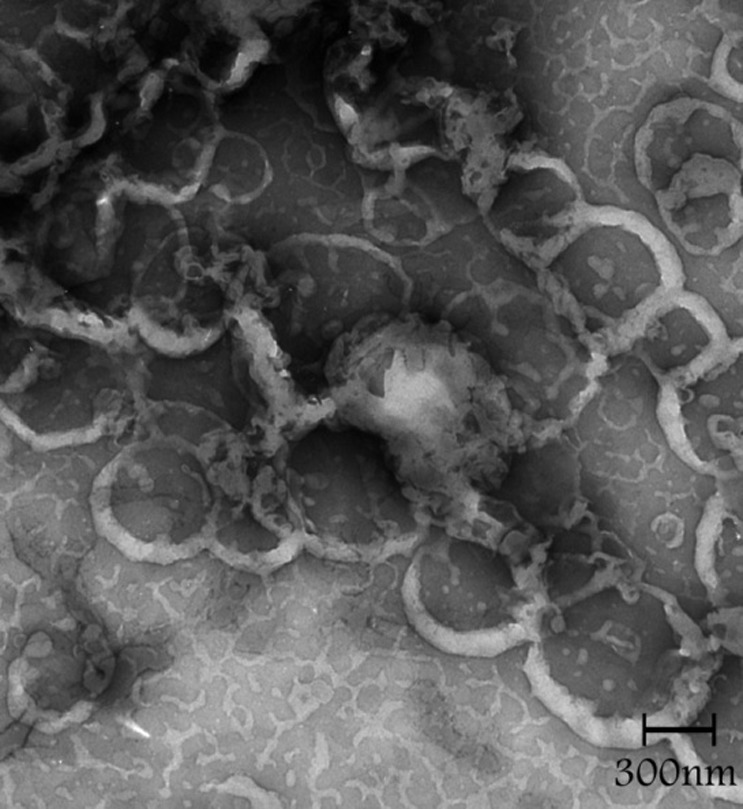
d.
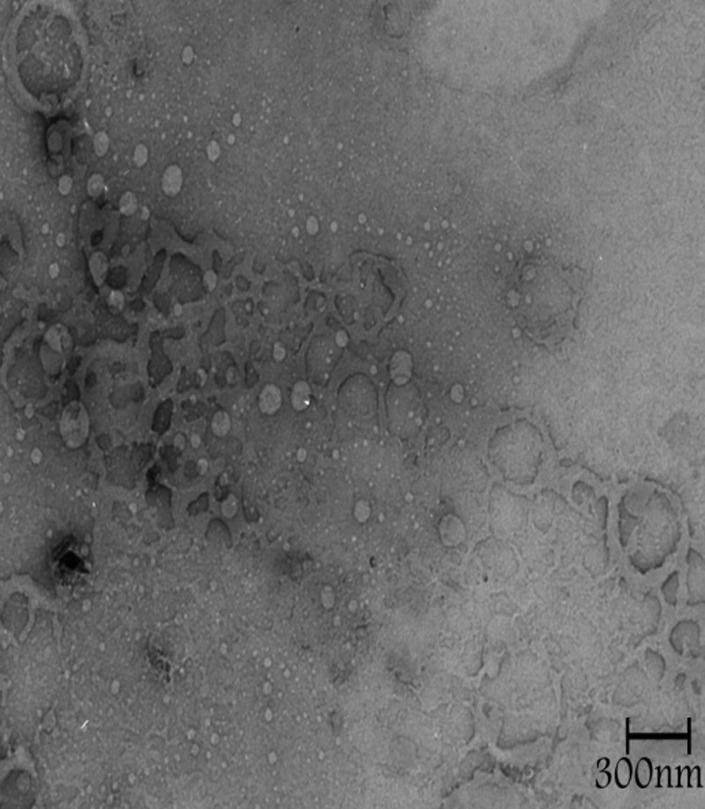
Discussion
Celecoxib is a new anti-inflammatory drug that acts on cyclooxygenase-2 and also has been shown to have antitumor properties.1,2 Liposomes are used as drug carriers for a wide range of drugs and are importante in pharmaceutical field due to decrease of side effects.3 Liposome incorporates hydrophilic drugs through an aqueous core or entraps hydrophobic drugs using phospholipid bilayers which surround the aqueous core.6
According to our results, the loading drug and release drug was 67.34% and 67.16% respectively. Many other studies indicated that the liposomes were suitable systems for loading drugs, for example, Glavas et al showed that the percentage of Lidocaine HCL encapsulation into liposome gels was over 72%.7 Another study showed that cyproterone acetate containing liposomes had 74±6.11% loading efficiency and the liposomal formulation has better penetration potential than conventional cyproterone acetate formulation.5 In another study, the results of the skin retention studies showed fairly high miconazol retention with liposomes when compared with commercial creams of the drug.8 Also, quantitative analysis of encapsulated carvacrol in liposome vesicles showed that only a small amount 4.16% of the starting quantity of carvacrol was incorporated.9
The results of DSC curve of lecithin, cholesterol and celecoxib were very different from celecoxib containing liposomes. On the other hand, the DSC curve of lecithin, cholesterol can be dependent on the previous organization of these molecules to form liposome bilayer and also the results of Figure 2. indicated that celecoxib can interact with lecithin and cholesterol during the preparation of the liposomes. When celecoxibe was added to lipid phase during the liposome preparation, The DSC curve was shifted. The absence of flattened peaks indicated the homogeneity in the lipids forming the liposome.
Figure 2.a) Differential Scanning Calorimetry of cholesterol.b) Differential Scanning Calorimetry of lecithin.c) Differential Scanning Calorimetry of celecoxib.d) Differential Scanning Calorimetry of celecoxib containing liposome.
a.
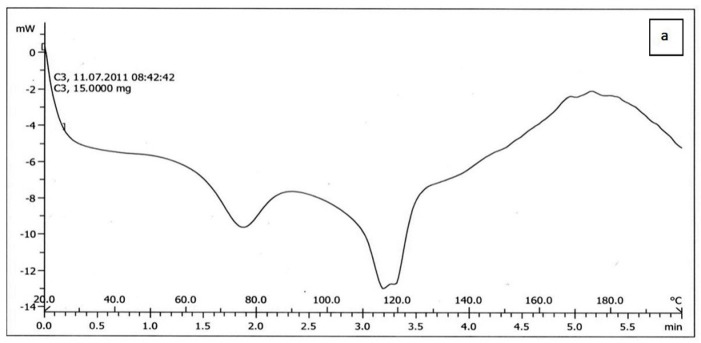
b.
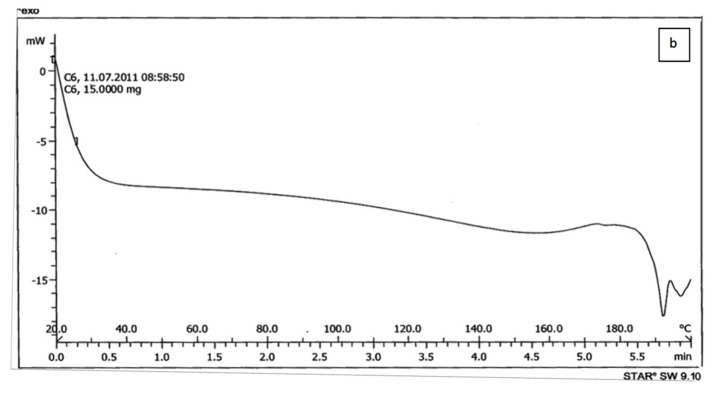
c.
d.
The sonication time is an important parameter in the liposome preparation because the size of liposome decreased (Figure 1). Reduction in the particle size is a key factor for improving the performance of poorly soluble drugs.10 The results of a study demonstrated that the size of the drug loaded liposome decreased significantly from 188 ±1.2 nm to 73 ±7.8 nm when the drug-lipid ratio was decreased from 1:10 to 1:1.6 TEM image analysis of celecoxib containing liposome showed the spherical structure. TEM imaging showed the small vesicles began to emerge until finally aggregated and vesicle formed the multilamellar vesicle (Figure 3a). Also Figure 3b , c and d showed liposomes of various shapes and TEM image confirmed that liposomes are vesicle having phospholipids bilayer. Many other TEM studied also indicated that TEM is a suitable instrument for investigating the morphology of liposomes prepared by thin film method.4
Figure 1.a) Particle size of liposomes containing celecoxib after 30 min sonication b) Particle size of liposomes containing celecoxib after 1 h sonication.
a.
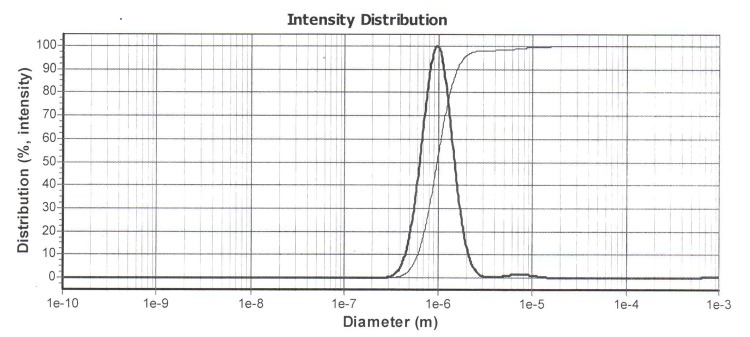
b.
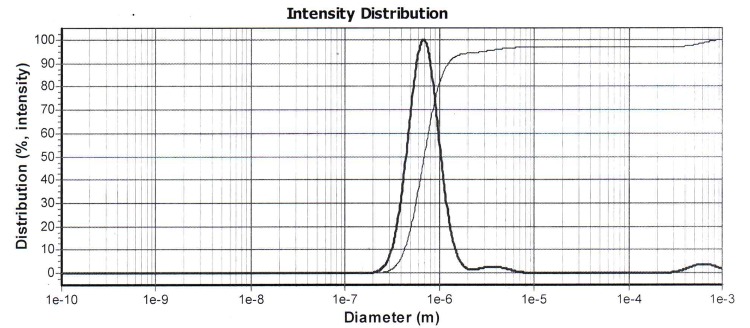
Conclusion
Loaded of celecoxib in liposomes were found to have the appropriate size and exhibited improved release properties. Also, celecoxib containing liposome would be a suitable means for protecting and permeation of the drug.
Acknowledgments
The work was financially supported by Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Yang HM, Kim HS, Park KW, You HJ, Jeon SI, Youn SW. et al. Celecoxib, a cyclooxygenase-2 inhibitor, reduces neointimal hyperplasia through inhibition of akt signaling. Circulation. 2004;110(3):301–8. doi: 10.1161/01.CIR.0000135467.43430.16. [DOI] [PubMed] [Google Scholar]
- 2.Guirguis M.S, Sattari S, Jamali F. Pharmacokinetics of celecoxib in the presence and absence of interferon induced acute inflammation in the rat: application of a novel hplc assay. J. Pharm Pharmaceut Sci. 2001;4(1):1–6. [PubMed] [Google Scholar]
- 3.Lopes L.B, Scarpa V.M, Pereira L.N, De Oliveria L.C, Gomes Oliveria A. Interaction of sodium diclofenac with freeze-dried soya phosphatidylcholine and unilamellar liposomes. Brazilian J of Pharmaceutical Sciences. 2006;42(4):497–504. [Google Scholar]
- 4.Chetanachan P, Akarachalanon P, Worawirunwong D, Dararutana P, Bangtrakulonoth A, Bunjop M, Kongmuang S. Ultrastructural characterization of liposome using transmission electron microscope. Advanced Materials Research. 2008;55-57:709–711. [Google Scholar]
- 5.Mohammadi Samani S, Montaseri H, Jamshidnejad M. Preparation and evaluation of cyproterone acetate liposome for topical drug delivery. Iranian J of Pharmaceutical Sciences. 2009;5(4):199–204. [Google Scholar]
- 6.Ramana LN, Sethuraman S, Rauga U, Krishnan UM. Development of a liposomal nanodelivery system for nevirapine. J of Biomedical Science. 2010;17(57):1–9. doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodov M.G, Simonska M, Goracinova K. Formulation and characterization of topical liposome gel bearing lidocaine hcl. Bullention of the Chemists and Technologists of Macedonia. 2005;24(1):59–65. [Google Scholar]
- 8.Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. International Journal of Pharmaceutic. 2001;228(1-2):43–52. doi: 10.1016/s0378-5173(01)00810-9. [DOI] [PubMed] [Google Scholar]
- 9.Liolios CC, Gortzi O, Lalas S, Tsaknis J, Chinou I. Liposome incorporation of carvacrol and thymol isolated from the essential oil of origanum dictammus l. and invitro antimicrobial activity. Food Chemistry. 2009;112:77–83. [Google Scholar]
- 10.Luo Y, Chen D, Ren L, Zhao X, Qin J. Solid lipid nanoparticles for enhancing vinpocetin´s oral bioavailability. J Controlled Release. 2006;114:53–56. doi: 10.1016/j.jconrel.2006.05.010. [DOI] [PubMed] [Google Scholar]