Abstract
Since its introduction capillary electrophoresis has shown great potential in areas where electrophoretic techniques have rarely been used before, including here the analysis of pharmaceutical substances. The large majority of pharmaceutical substances are neutral from electrophoretic point of view, consequently separations by the classic capillary zone electrophoresis; where separation is based on the differences between the own electrophoretic mobilities of the analytes; are hard to achieve. Micellar electrokinetic capillary chromatography, a hybrid method that combines chromatographic and electrophoretic separation principles, extends the applicability of capillary electrophoretic methods to neutral analytes. In micellar electrokinetic capillary chromatography, surfactants are added to the buffer solution in concentration above their critical micellar concentrations, consequently micelles are formed; micelles that undergo electrophoretic migration like any other charged particle. The separation is based on the differential partitioning of an analyte between the two-phase system: the mobile aqueous phase and micellar pseudostationary phase. The present paper aims to summarize the basic aspects regarding separation principles and practical applications of micellar electrokinetic capillary chromatography, with particular attention to those relevant in pharmaceutical analysis.
Keywords: Capillary electrophoresis, Micellar electrokinetic, Capillary chromatography, Pharmaceutical analysis
Introduction
Capillary electrophoresis (CE) is an instrumental evolution of traditional electrophoretic techniques, where separation occurs in fused-silica capillaries and involves application of high voltages across buffer filled capillaries in order to achieve separation. Due to its speed of analysis, high efficiency, automated analytical equipment, low reagents and sample consumption and rapid method development, CE has gained momentum in pharmaceutical analysis, being regarded today as an alternative and also a complementary technique to the more frequently used high performance liquid chromatography (HPLC).
CE is actually a range of separation techniques based on different separation principles: capillary zone electrophoresis–CZE (based on the differences between the electrohoretic mobilities of the analytes), micellar electrokinetic capillary chromatography–MEKC (separation of neutral compounds using surfactant micelles), capillary gel electrophoresis–CGE (filtration of analytes through a gel network), capillary isoelectric focusing–CIEF (separation of zwitterionic analytes within a pH gradient), capillary electrochromatography–CEC (separation of analytes in a capillary filled with a chromatographic stationary phase).1-4
Various CE techniques offer various possibilities for pharmaceutical analysis. Depending on the complexity of the sample, the nature of its components, on the intended application and the nature of the analytes, each of these techniques will provide various advantages for the separation and detection of different pharmaceutical substances.
Substances of pharmaceutical interest are usually neutral from electrophoretic point of view and also frequently the separation of substances with very similar structural and physico-chemical properties is required. Being based on differences between the electrophoretic mobilities of the analytes, the classic CZE method is not suited for the separation of neutral substances, which migrate towards the detector with the same velocity as the electro-osmotic flow (EOF). MEKC is an electrophoretic technique developed in the early 90 by Shigeru Terabe that extends the applicability of CE to neutral analytes, which cannot be separated using simple free solution CE.
The same instrumentation that is used for CZE is used for MEKC, which demonstrates the versatility and adaptability of the method. MEKC differs from CZE because it uses an ionic micellar solution instead of the simple buffer salt solution. MEKC can be used for the separation of both ionic and neutral substances while CZE typically separates only ionic substances. Thereby MEKC has a great advantage over CZE in the separation of mixture containing both ionic and neutral analytes.1,2,5
The separation principle of MEKC is based on the differential partition of the analytes between micelles and water while CZE is based on the differences between the own electrophoretic mobility of the analytes.
Separation Principle
MEKC is based on the addition to the buffer solution of a micellar “pseudostationary” phase, which interacts with the analytes according to partitioning mechanisms, just like in a chromatographic method. The “pseudostationary” phase is composed of a surfactant added to the buffer solution in a concentration above its critical micellar concentration (CMC). In this system, EOF acts like a chromatographic “mobile phase”. From a “chromatographic point of view”, the EOF’s “plug-like” flow profile is almost ideal as it minimizes band broadening, which can occur during the separation process.5-7
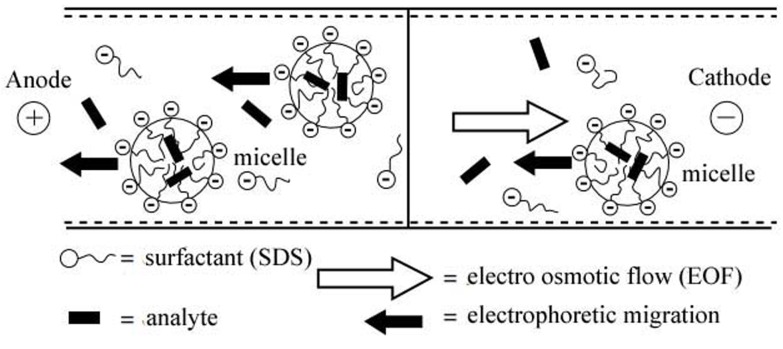
The most commonly used surfactant sodium dodecyl sulfate (SDS), an anionic surfactant. The anionic SDS micelles are electrostatically attracted towards the anode. The EOF transports the bulk solution towards the negative electrode due to the negative charge on the internal surface of the silica capillaries. But the EOF is usually stronger than the electrophoretic migration of the micelles and therefore the micelles will migrate also toward the negative electrode with a retarded velocity (Figure 1).5-8,10
Figure 1.
Schematic of the separation principle in MEKC5
When a neutral analyte is injected into the micellar solution, a fraction is incorporated into the micelle, while the remaining fraction of the analyte migrates with the electroosmotic velocity. Consequently, micelles decrease selectively the migration of neutral solutes they interact with (by partitioning mechanism), which otherwise would migrate with the same velocity as the EOF.
The separation depends on the individual partitioning equilibrium of the different analytes between the micellar and the aqueous phase. The greater percentage of analyte is distributed into the micelle, the slower it will migrate. Therefore, analytes that have greater affinity for the micelles exhibit slower migration velocities compared with analytes that are mostly distributed in the bulk solution.2,5,8,10,11
With SDS micelles, the general migration order will be exactly the opposite as in ECZ: anions, neutral analytes and cations. Anions will remain mostly in the bulk solution due to electrostatic repulsions from the micelle; neutral molecules will be separated exclusively due to their hydrophobicity; while cations will migrate last due to the strong electrostatic attraction. This generalization regarding the migration order can be sometimes useful, but strong hydrophobic interaction between analytes and micelles can overcome repulsions and attractions. Likewise, the own electrophoretic mobilities of the analytes can also modify the migration order.9,12
Analytes which are highly retained by the micelle will have longer migration times, while analytes which have limited interactions with the micelle will have migration times close to the EOF (t0). Very hydrophobic compounds may be totally included into the micelle and will migrate with the micelles velocity (tmc). Methanol is not retained by the micelles and migrates with t0 being used as marker for the EOF, while a dye Sudan III is totally included into the micelle and can be used as a micellar marker. The period between the migration time of the bulk solution and the migration time of the micelle is often referred to in the literature as migration time window (Figure 2).5,8,13
Figure 2.
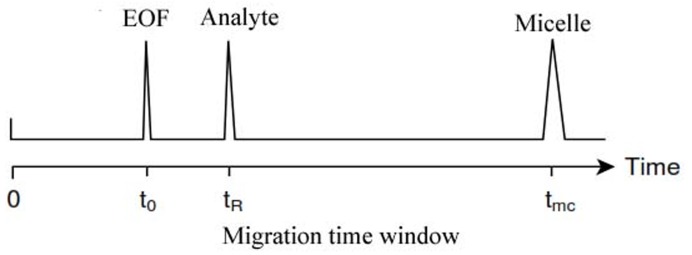
Migration time window in a MEKC separation1
A relatively recent development in MEKC has been to perform separations in the absence of EOF. This may be achieved using coated capillaries or at low pH values. This could be especially useful in the separation of acidic analytes, which would ionized at high pH values and would not interact with the negatively charged SDS micelle.15
Cationic surfactants can be used in MEKC to reverse the charge on the capillary wall, by absorption on the capillary wall surface through a mechanism involving electrostatic attraction between the positively charged ammonium moieties and the negatively charged Si-O-groups; when a reversal of the EOF takes place.9
Micelles and Surfactants
Surfactants are molecules with detergent properties, which are composed of a hydrophilic water-soluble head group and a hydrophobic water-insoluble hydrocarbon chain group.
Although a large number of surfactants are commercially available, a limited number are widely used in MEKC separations. The surfactants suitable for MEKC must be soluble in the buffer solution to form micelles and the micellar solution must be homogeneous, UV transparent and also have a low viscosity.
There are four major classes of surfactants: anionic, cationic, zwitterionic and nonionic (Table 1). Of these, ionic surfactants are generally used in MEKC. Every surfactant has a characteristic CMC and aggregation number (the number of surfactant molecules necessary to form a micelle). Another important parameter is the Kraft point, which represents the minimum temperature where the solubility of surfactants increases steeply due to the formation of micelles.1,5,8,9
Table 1. Surfactants classes and properties1.
| Surfactant | Type | CMC* | n |
| Sodium dodecyl sulphate (SDS) | anionic | 8.1 x 10-3 | 62 |
| Sodium tetradecylsulphate (STS) | Anionic | 2.1 x 10-3 | 138 |
| Sodium dodecanesulphate | anionic | 7.2 x 10-3 | 54 |
| Sodium cholate | anionic | 13-15 x 10-3 | 2-4 |
| Cetyltrimethylammonium bromide (CTAB) | cationic | 0.92 x 10-3 | 61 |
| Dodecyltrimethylammonium bromide | cationic | 15 x 10-3 | 56 |
| Brij - 35 | nonionic | 0.1 x 10-3 | 40 |
| Sulfobetaine | zwitterionic | 3.3 x 10-3 | 55 |
Nonionic surfactants do not posses electrophoretic mobility and cannot be used, as “pseudostationary phase” in conventional MEKC, however can be useful for the separation of charged analytes. This technique using nonionic micelles can be classified as an extension of MEKC.9,16
Micelles are amphiphilic aggregates of surfactants. Above a specific surfactant concentration, the surfactant molecules begin to self-aggregate, forming micelles, spherical aggregates that exhibit electrophoretic migration like any other charged particle. Micelles are long chain molecules and are characterized as possessing a long hydrophobic tails and a hydrophilic head group. Generally micelles are formed in aqueous solution with the hydrophobic tails oriented towards the center of the aggregated molecules and the hydrophilic heads pointing outward into the aqueous solution.6,9,10,12,14
Micelle formation is a very dynamic process, as micelle disaggregate and reconstruct continuously, composing the “pseudostationary phase” which can include hydrophobic analytes.
Micellar solutions can solubilize hydrophobic compounds which otherwise would be insoluble in water. Micelles have the ability to interact with the analytes at molecular level based on hydrophobic and electrostatic interactions. Even neutral analytes can bind to micelles due to the very strong solubilization power of the hydrophobic core.8,9
The micelles used in MEKC are charged on the surface, so an analyte with the opposite charge will strongly interact with the micelle through electrostatic forces while an analyte with the same charge will interact weakly due to the electrostatic repulsion. Therefore the use of a cationic or an anionic surfactant will result in an entirely different result.
The micellar phase can be modified by adding two different surfactants to form a mixed micelle; addition of an ionic and a nonionic surfactant can provide different selectivity in separation. A mixed micelle has a lower surface charge and a larger size; consequently its electrophoretic mobility will be lower than the one of a simple ionic micelle.8,9,12
Some surfactants like bile salts are chiral and can be used for enantiomers separation.16
Buffer Additives
Since MEKC is often applied in the separation of analytes with very similar hydrophobicities and chemical characteristics, sometimes is useful to extend the concept of using a “mobile phase” and a “pseudostationary phase” to the use of buffer additives such as organic modifiers and cyclodextrines.
Organic solvents (methanol, acetonitrile) are used in CZE in order to increase solubility of the analytes, but their role in MEKC is more complex and profound. Organic solvents reduce EOF, consequently increase the migration times and migration time window of the analytes. Also, organic additives reduce the hydrophobic interactions between the micelle and the analyte and can be useful in the separation of analytes which otherwise are almost completely incorporated in micelles. The addition of organic solvents will increase the migration velocity of these hydrophobic analytes, by reducing the partition coefficient between the micelle and the bulk solution. However high concentration of organic solvents may break down the micellar structure, consequently concentrations above 25-30% should be avoided.1,5,6,8,11,13
Cyclodextrines (CD) are cyclic oligosaccharides with truncated cylindrical molecular shapes, having an external hydrophilic surface and an internal hydrophobic cavity, in which they can include other compounds by hydrophobic interactions. The inclusion mechanism is sterically selective, because analytes must fit the size of the cavity, the diameter of which depends on the number of glucose units in the CD structure.17
There is a wide range of both natural and derivatised CD commercially available. The native CD, α-, β-, and γ-CD possess different numbers of glucose sub-units, six, seven and eight respectively. These surface hydroxyl groups can be chemically replaced with groups such as hydroxypropy and dimethyl groups. Ionic chargeable CD offers the possibility of separation of neutral drug enantiomers or enhanced separation of ionic drugs. Several CE specific derivatised CDs have been produced with amino, sulfate or carboxylic groups.18
Because of the chirality of the hydroxyls in the glucose molecules that form the rim of the CD cavity, the inclusion complex formation will be chirally selective. If the enantiomers of a compound have different binding constants, then chiral separation is possible by adding the proper CD in the buffer electrolyte.16
CDs are neutral from electrophoretic point of view, and are not incorporated in micelles, because of the hydrophilic nature of the outside surface of the molecules. Therefore, an analyte included in the CD will migrate with the same velocity as the EOF. The addition of cyclodextrines reduces the apparent distribution coefficient of the analytes between the two phases.
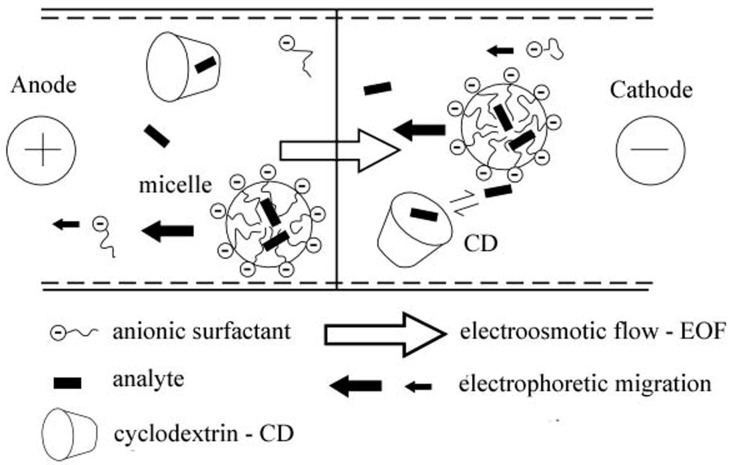
Hydrophobic analytes can become incorporated into either the CD cavity or the micelle. Effectively the addition of the CD establishes two “pseudo stationary” phases in the electrolyte, which can reduce analysis times and offer the possibility of improved separation. CDs have advantages over organic solvents, as they are UV transparent and non-volatile. The schematic principle in cyclodextrin modified micellar electrokinetic chromatography is presented (CD-MEKC) (Figure 3).1,5,16,17
Figure 3.
Schematic of the separation principle in CD – MEKC5
Theoretical Aspects
In MEKC we can define the capacity factor (k) similarly as in chromatography:5
k = nmc / naq
wherenmc and naqare the amount of analyte incorporated into the micelle and in the aqueous respectively. It can be calculated from the migration time of the analyte (tR), of the EOF (t0) and of the micelle (tmc):5
k = tR-t0 / t0(1- tR/tmc)
When k = 0, the migration time of the analyte is equal to t0, which means that the analyte does not interact with the micelle; and when k is infinity, the migration time of the analyte is equal to tmc, which means that the analyte is totally incorporated into the micelle.
The capacity factor is a fundamental term in chromatography while the electrophoretic mobility is characteristic to the electrophoretic process. In ECZ the migration velocity (v) of the analyte is expressed as:5,7,19
v = (μeo + μa) E
whereμeo and μa are the electrophoretic mobilities of the EOF and analyte respectively and E is the electric field strength. We can apply this equation to MEKC by defining the effective electrophoretic mobility of a neutral analyte (μna) as:5,7,19
μna = μmc k/1+k
whereμmc is the electrophoretic mobility of the micelle and k/1+k represents the fraction of analyte incorporated into the micelle. Thus, the velocity of a neutral analyte in MEKC is given as:5,7
v = (μeo + μna) E
The capacity factor provides quantitative information about the analyte distribution between the two phases, while the electrophoretic mobility only gives qualitative information about it.
The resolution equation in MEKC can be given by the following equation:1,5,19,20
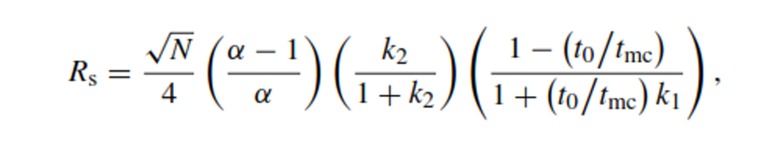
where N is the theoretical plate number, α the separation factor between the two analytes and k1 and k2 their capacity factor.
The separation factor (α) is determined by the micellar solubilization process and is influenced by the chemical nature of both the micellar and aqueous phase. Various surfactant systems can be used as well as mixed micelles, possessing different solubilization characteristics, in order to control migration behavior of the analytes and optimize selectivity.
Method Development Guidelines
Micellar solutions exhibit a relatively high conductivity, so a capillary with small diameter could be the right choice to prevent excessive Joule heating. A longer capillary can be useful in the case of large amount sample solutions to obtain a better separation at the expense of time.1
The micellar solution is prepared by dissolving the surfactant in the buffer solution in a concentration above its CMC. Popular buffer solutions often used to prepare the micellar solution are phosphate, borate, or tris (hydroxymethyl) aminomethane (Tris). Concentrations between 25 and 100 mM are normally employed for both the surfactant and buffer. High concentrations will result in relatively high viscosities and high currents and should be avoided. It is essential that the pH of the buffer remains constant, being a critical parameter in the separation of ionizable analytes.1,13
It should be noted that the counter ion of the ionic surfactant is exchanged by the counter ion of the buffer electrolyte and, consequently, the character of the micelle may be changed.1
The sample solution can be prepared in any solvent; but water will be the first choice solvent if the analyte is soluble in it. When the sample contains high concentrations of an organic solvent, the peaks may be split due to incomplete mixing of the sample solution with the running solution.1
The applied voltage must be kept to a level that doesn’t generate excessive current, the limit being determined by the capacity of the electrophoretic system to dissipate Joule heating. A compromise is, therefore, necessary between high Joule heating and fast separation time.1
It is also necessary to control the capillary temperature because MEKC methods are even more sensitive to temperature variation than CZE methods. The distribution coefficients are highly dependent on temperature; an increase in temperature will cause a decrease in the distribution coefficient of the analytes between the two phases. It is well known that an increase in temperature reduces viscosity of the buffer, which increases both electrophoretic and EOF velocities, reducing the migration time.1,5,13
When preliminary runs show unsatisfactory separation, several analytical parameters may need to be adjusted in order to optimize separation. It is useful to estimate the retention factors by measuring t0 and tmc. If problems occur in measuring tmcdue to the difficulty observing the micelle marker peak, it is advisable to simply assume that tmcis four time longer than t0. The optimum value of the capacity factor k can be estimated as being equal to (tmc/t0)1/2. 1,5,8,13
If optimum k values do not provide acceptable resolution, further tuning may be required; the use of additives such as organic solvents or CDs may be needed, or the use of alternate surfactants may prove effective. The selection of additives is extensive, but the first step is recommended to be the addition of an organic solvent in low concentration. If the analytes have closely related structures, addition of a CD derivative may prove effective. There are many different CD derivatives available, but initially ß-CD or γ-CD can be tested. Further steps can be performed by selecting other CD derivatives if necessary. Another choice is the modification of the micelle by using mixed micelles, in particular, addition of a nonionic, adding cosurfactants, or by adding an organic counter ion.1,5,8,13
One of the issues that still remains unsolved in MEKC is the improvement in reproducibility in quantitative analysis, including migration time and peak height or peak area, a problem characteristic for every CE technique.2,5
MEKC Applications in the Analysis of Pharmaceutical Substances
In principle MEKC is used for the analysis of neutral compounds, or when analyzing mixtures of neutral and charged solutes. But MEKC conditions are also employed when selectivity requirements for a separation exceed the simple mobility differences obtainable in CZE.
MEKC can be especially useful for the determination of drugs in samples having a high protein content (clinical samples, biofluids) reducing the disadvantageous matrix effects caused by organic materials, while CZE through its simplicity and operation stability could be advantageous for pharmaceutical determinations. MEKC can be usually applied in simultaneous separation from complex mixtures of pharmaceutical substances with very similar structural and physico-chemical characteristics.
Many reports have been published detailing the use of MEKC for pharmaceutical applications; Table 2 presents briefly selected pharmaceutical applications and the description of the electrophoretic conditions.
Table 2. Applications of MEKC in the analysis of different pharmaceutical substances .
| Pharmaceutical class |
Substances | Electrophoretic conditions | Reference |
| Penicillins | Amoxicillin, Ampicillin, Benzylpenicillin, Phenoxymethypenicillin, Oxacillin, Cloxacilin |
40 mM sodium tetraborate + 100 mM SDS, pH – 9.3 voltage: + 10kV, temperature: 20 0C, UV detection 210 nm |
21 |
| Penicillins | Amoxicillin, Ampicillin, Benzylpenicillin, Phenoxymethypenicillin, Oxacillin, Cloxacilin, Dicloxacillin, Nafcillin, Piperacillin |
26 mM sodium tetraborate + 100 mM SDS, pH – 8.5 voltage: + 20kV, temperature: 30 0C, UV detection 220 nm |
22 |
| Cephalosporins | Cefazoline, Cefuroxime, Ceftriaxone, Cefoperazone, Ceftazidime |
20 mM sodium tetraborate + 15 mM disodium hydrogenophosphate + 50 mM SDS pH – 6.5, voltage: + 18kV, temperature: 200C, UV detection 214 nm |
23 |
| Macrolides | Erythromycin, Tylosin and related substances | 80 mM sodium phosphate + 20 mM sodium cholate + 7 mMcetyltrimethylammonium bromide, pH – 7.5, voltage: + 15kV, temperature: 250C, UV detection 280 nm |
24 |
| Aminoglycosides | Gentamicin, Sisomicin, Netilmicin, Kanamycin, Amikacin, Tobramycin |
100 mM sodium tetraborate + 20 mM sodium deoxycholate + 15 mM beta-cyclodextrin, pH – 10 voltage: + 20kV, temperature: 250C |
25 |
| Tetracyclines | Tetracycline, Oxytetracicline, Democlocycline, Chlortetracycline, Doxycicline, Minocycline |
15 mM ammonium acetate + 20 mM SDS, pH – 6.5, voltage: + 15kV, temperature: 250C |
26 |
| Sulfonamides | Sulfanilamide, Sulfathiazole, Sulfamethoxazole, Sulfaguanidine, Sulfadiazine |
13.32 mM disodium hydrogen phosphate, 6.67 mM potassium dihydrogen phosphate + 40 mM SDS, pH – 7.5, voltage: + 21kV, temperature: 250C fluorescence detection |
27 |
| Sulfonamides | Sulfamethazine, Sulfamerazine, Sulfathiazole, Sulfachloropyridazine, Sulfamethoxazole, Sulfacarbamide, Sulfaguanidine |
15 mM sodium tetraborate + 25 mM SDS + 20% methanol, pH – 9.3, voltage: + 20kV, temperature: 220C, UV detection 200 nm |
28 |
| Fluoroquinolones | Norfloxacin, Ciprofloxacin, Ofloxacin, Enrofloxacin, Danofloxacin |
25 mM sodium carbonate + 100 mM SDS, pH – 9.2, voltage: + 20kV, temperature: 300C, UV detection 280 nm | 29 |
| Antifungal azoles | Fluconazole, Voriconazole, Itraconazole, Posaconazole | 25 mM phosphoric acid + 100 mM SDS + 13 % acetonitrile + 13 % tetrahydrofuran, pH – 2.2 |
30 |
| Barbiturates | Phenobarbital, Amobarbital, Pentobarbital, Secobarbital, Butabarbital |
10 mM sodium tetraborate + 10 mM disodium hydrogenophosphate + 100 mM SDS + 15% acetonitrile. pH - 8.5, voltage: + 20 kV, UV detection 214 nm |
31 |
| Benzodiazepines | Flunitrazepam, Diazepam, Midazolam, Clonazepam, Bromazepam, Temazepam, Oxazepam, Lorazepam |
25 mM phosphate/borate + 75 mM SDS, pH – 9.3 | 32 |
| Benzodiazepines | Alprazolam, Bromazepam, Chlordiazepoxide, Diazepam, Flunitrazepam, Medazepam, Oxazepam, Nitrazepam |
25 mM sodium tetraborate + 50 mM SDS + 12% methanol, pH – 9.3, voltage: + 25kV, temperature: 20 0C, UV detection 214 nm |
33 |
| Phenotiazines | Promethazino, Ethopropazine, Trimeprazine, Methoprimeprazine, Thioridazine |
80 mM citric acid + 10 mMtetradecyltrimethylammonium bromide + 7 mM ß -CD (9 mM HP ß-CD), pH – 3.5, voltage: + 20kV, temperature: 25 0C, UV detection 254 nm enantiomer separation |
34 |
| Tricyclic antidepressants |
Imipramine, Amitriptyline, Desipramine, Nortriptyline, Doxepin, Trimipramine |
37.5 mM phosphate + 25 mMdodecyltrimethylammonium bromide + 2 M urea, pH – 8, voltage: + 25kV |
35 |
| Xanthines | Caffeine, Theobromine, Theophylline, Pentoxifylline |
20 mM sodium tetraborate + 100 mM SDS, pH – 9.3, voltage: + 30kV, temperature: 25 0C, UV detection 274 nm |
36 |
Another application of MEKC is the chiral separation of optically active pharmaceutical substances. Enantiomer separation by MEKC involves the addition of a chiral agent such as chiral surfactants, crown ethers, or CDs to the background electrolyte with chiral/achiral micelles. Chiral MEKC with chiral surfactants is an important separation mode for chiral compounds, with chiral surfactants including also naturally occurring compounds such as bile salts, amino acids or glucose.37
Chiral separation in MEKC is affected by the affinity of the enantiomers toward the micelles, and the concentration of the micellar phase, which depends on the aggregation properties of the chiral surfactants.1
MEKC can be used for the separation of structural related impurities from the main active drug, and has been proven an alternative to HPLC for quantitation of compounds and the determination of drug-related impurities.1
The structurally related impurities of a drug will possess similar structural and physico-chemical characteristics to the main component, which makes their separation and determination a challenging task. The high separation efficiencies possible for CE often allows a small degree of selectivity to provide an acceptable resolution. The separation and determination of drug-related impurities using CE has been extensively studied, and the method performance and validation data obtained clearly shows that CE methods are successful applications in this area.
During the early phase and later phase of drug development, knowledge of physiochemical properties of pharmaceutical compounds is important in order to predict their bioavailability and blood-brain. Physicochemical properties such as acid dissociation constant (pKa), octanol–water partition-coefficient (logP), solubility, permeability, and protein binding are closely related to drug absorption, distribution, metabolism, and excretion. The pKa determination of acids and bases by CE is based on measuring the electrophoretic mobility of charged species associated with the acid–baseequilibria as a function of pH. CE techniques using “pseudostationary” phases in the background electrolyte, like MEKC, allow the measurement of log Pvalues because of the partitioning of solutes between the micellar and the aqueous phase. CE methods and especially MEKC has been applied successfully for physicochemical analysis of many pharmaceutical compounds and has many advantages over the traditional methods of log P and pKa determination.38,39
Concluding remarks
The use of CE methods in pharmaceutical analysis has become increasingly popular in recent years. The wide range of applications for which the use of this method has proved to be successful includes identification of pharmaceutical substances, assay of drugs, determination of drug-related impurities, physicochemical measurements of drug molecules or chiral separations.11,19,20
For pharmaceutical analysis, the range of applications for which CE can be used is extensive, possibly eclipsing the applications of the more frequently used HPLC. CE also offers a number of advantages over HPLC and other analytical techniques: the rapid development of the analysis method, its analysis speed, reduced consumable and solvent expenses, low sample amount, simplicity of operations, and a greater possibility of implementation of a single set of method conditions for the analysis of several different samples.1
MEKC is a branch of CE, which has become during the years one of the most popular techniques in CE due to its high resolving power and capability of separating both ionic and neutral analytes. MEKC is the most flexible of all CE techniques, offering the greatest selectivity to the widest range of compounds and can be considered the separation method of choice when performing CE analysis for pharmaceutical substances.
MEKC combines the separation mechanism of chromatography with the electrophoretic and electroosmotic movement of analytes and solutions for the separation of constituents in a sample.
In the last 10-15 years MEKC gained popularity among separation scientist as pointed out by the relatively large numbers of article published, therefore this papers main objective is to present the guiding principles regarding the full availability of the technique.
Conflict of interests
There is no conflict of interest in this study.
References
- 1.Schmitt-Kopplin P. Capillary electrophoresis – Methods and Protocols. Totowa, NJ: Humana Press; 2008. [DOI] [PubMed] [Google Scholar]
- 2.Altria KD. Analysis of pharmaceuticals by capillary electrophoresis.Chromatographia CE Series, Volume 2, Friedr. Braunschweig/Wiesbaden:Vieweg & Sohn Verlagsgesellschaft mbH; 1998.
- 3.European Pharmacopoeia. 7th ed. Strasbourg: Council of Europe; 2010.
- 4.British Pharmacopoeia. London: Her Majesty's Stationary Office; 2009.
- 5.Landers JP. Handbook of capillary and microchip electrophoresis and associated microtechniques. Boca Raton: CRC Press; 2008. [Google Scholar]
- 6.Altria KD. Overview of capillary electrophoresis and capillary electrochromatography. J Chromatogr A . 1999;856(1-2):443–63. doi: 10.1016/s0021-9673(99)00830-4. [DOI] [PubMed] [Google Scholar]
- 7.Tagliaro F, Manetto G, Crivellente F, Smith FP. A brief introduction to capillary electrophoresis. Forensic Sci Int . 1998;98:75–88. [Google Scholar]
- 8.Altria KD, McLean R. Development and optimisation of a generic micellar electrokinetic capillary chromatography method to support analysis of a wide range of pharmaceuticals and excipients. J Pharm Biomed Anal . 1998;18(4-5):807–13. doi: 10.1016/s0731-7085(98)00219-2. [DOI] [PubMed] [Google Scholar]
- 9.Muijselaar PG, Otsuka K, Terabe S. Micelles as pseudo-stationary phases in micellar electrokinetic chromatography. J Chromatogr A . 1997; 780:41–61. doi: 10.1016/s0021-9673(97)00632-8. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi SA, Do DP, Saleh AM. Fundamentals of micellar electrokinetic chromatography. Eur J Chem . 2011; 2(2):276–281. [Google Scholar]
- 11.Deyl Z, Miksik I, Tagliaro F. Advances in capillary electrophoresis. Forensic Sci Int . 1998; 92(2-3):89–124. doi: 10.1016/s0379-0738(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 12.Riekkola ML, Wiedmer SK, Valko IE, Siren H. Selectivity in capillary electrophoresis in the presence of micelles, chiral selectors and non-aqueous media. J Chromatogr A . 1997; 792(1-2):13–35. doi: 10.1016/s0021-9673(97)00728-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja S, Jimidar MI. Capillary electrophoresis methods for pharmaceutical analysis. London: Academic Press; 2008. [Google Scholar]
- 14.Bojiţă M, Roman L, Săndulescu R, Oprean R. Analiza şi controlul medicamentelor, volume 2: Metode instrumentale în analiza şi controlul medicamentelor. Deva: Editura Intelcredo; 2003. pp. 240-88.
- 15.Altria KD. Enhanced pharmaceutical analysis by ce using dynamic surface coating system. J Pharm Biomed Anal . 2003; 31(3):447–53. doi: 10.1016/s0731-7085(02)00728-8. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka K, Terabe S. Enantiomer separation of drugs by micellar electrokinetic chromatography using chiral surfactants. J Chromatogr A . 2000; 875(1-2):163–78. doi: 10.1016/s0021-9673(99)01167-x. [DOI] [PubMed] [Google Scholar]
- 17.Fanali S. Enantioselective determination by capillary electrophoresis with cyclodextrins as chiral selectors. J Chromatogr A . 2000; 875(1-2):89–122. doi: 10.1016/s0021-9673(99)01309-6. [DOI] [PubMed] [Google Scholar]
- 18.Gubitz G, Schmid MG. Chiral separation principles in capillary electrophoresis. J Chromatogr A . 1997; 792:179–225. [Google Scholar]
- 19.Altria KD, Kelly MA, Clark BJ. Current applications in the analysis of pharmaceuticals by capillary electrophoresisI. Trend Anal Chem . 1998; 17(4):204–213. [Google Scholar]
- 20.Altria KD, Kelly MA, Clark BJ. Current applications in the analysis of pharmaceuticals by capillary electrophoresisII. Trend Anal Chem . 1998; 17(4):214–26. [Google Scholar]
- 21.Nozal L, Arce L, Rios A, Valcarcel M. Development of a screening method for analytical control of antibiotic residues by micellar electrokinetic capillary chromatography. Anal Chim Acta . 2004; 523(1):21–8. [Google Scholar]
- 22.Perez MI, Rodriguez LC, Cruces-Blanco C. Analysis of different beta-lactams antibiotics in pharmaceutical preparations using micellar electrokinetic capillary chromatography. J Pharm Biomed Anal . 2007; 43(2):746–52. doi: 10.1016/j.jpba.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Pajchel G, Tyski S. Adaptation of capillary electrophoresis to the determination of selected cephalosporins for injection. J Chromatogr A . 2000; 895(1-2):27–31. doi: 10.1016/s0021-9673(00)00588-4. [DOI] [PubMed] [Google Scholar]
- 24.Tobback K, Li YM, Pizarro NA, De Smedt I, Smeets T, Van Schepdael A. et al. Micellar electrokinetic capillary chromatography of macrolide antibioticsSeparation of tylosin, erythromycin and their related substances. J Chromatogr A . 1999; 857(1-2):313–20. doi: 10.1016/s0021-9673(99)00770-0. [DOI] [PubMed] [Google Scholar]
- 25.Wienen F, Holzgrabe U. A new micellar electrokinetic capillary chromatography method for separation of the components of the aminoglycoside antibiotics. Electrophoresis . 2003;24(17):2948–57. doi: 10.1002/elps.200305529. [DOI] [PubMed] [Google Scholar]
- 26.Chen YC, Lin CE. Migration behavior and separation of tetracycline antibiotics by micellar electrokinetic chromatography. J Chromatogr A . 1998; 802(1):95–105. doi: 10.1016/s0021-9673(97)01181-3. [DOI] [PubMed] [Google Scholar]
- 27.Lamba S, Sanghi SK, Asthana A, Shelke M. Rapid determination of sulfonamides in milk using micellar electrokinetic chromatography with fluorescence detection. Anal Chim Acta . 2005; 552(1):110–5. [Google Scholar]
- 28.Kowalski P, Plenis A, Oledzka I, Konieczna L. Optimization and validation of the micellar electrokinetic capillary chromatographic method for simultaneous determination of sulfonamide and amphenicol-type drugs in poultry tissue. J Pharm Biomed Anal . 2011; 54(1):160–7. doi: 10.1016/j.jpba.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt-Kopplin P, Burhenne J, Freitag D, Spiteller M, Kettrup A. Development of capillary electrophoresis methods for the analysis of fluoroquinolones and application to the study of the influence of humic substances on their photodegradation in aqueous phase. J Chromatogr A . 1999; 837(1):253–65. [Google Scholar]
- 30.Lin SC, Liu HY, Lin SW, Yao M, Wu UI, Kuo HP. et al. Simultaneous determination of triazole antifungal drugs in human plasma by sweeping-micellar electrokinetic chromatography. Anal Bioanal Chem . 2012; 404(1):217–28. doi: 10.1007/s00216-012-6087-3. [DOI] [PubMed] [Google Scholar]
- 31.Ferslew KE, Hagardorn AN, McCormick WF. Application of micellar electrokinetic capillary chromatography to forensic analysis of barbiturates in biological fluids. J Forensic Sci . 1995; 40(2):245–9. [PubMed] [Google Scholar]
- 32.Schafroth M, Thormann W, Allemann D. Micellar electrokinetic capillary chromatography of benzodiazepines in human urine. Electrophoresis . 1994; 15(1):72–8. doi: 10.1002/elps.1150150111. [DOI] [PubMed] [Google Scholar]
- 33.Hancu G, Gaspar A, Gyeresi A. Separation of 1,4-benzodiazepines by micellar elektrokinetic capillary chromatography. J Biochem Biophys Methods . 2007; 69(3):251–9. doi: 10.1016/j.jbbm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Lin CE, Chen KH, Hsiao YY, Liao WS, Chen CC. Enantioseparation of phenothiazines in cyclodextrin-modified micellar electrokinetic chromatography. J Chromatogr A . 2002; 971(1-2):261–6. doi: 10.1016/s0021-9673(02)01044-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee KJ, Lee JJ, Moon DC. Determination of tricyclic antidepressants in human plasma by micellar electrokinetic capillary chromatography. J Chromatogr . 1993; 616(1):135–43. doi: 10.1016/0378-4347(93)80480-r. [DOI] [PubMed] [Google Scholar]
- 36.Blanco M, Valverde I. Electrophoretic behaviour of pharmacologically active alkylxanthines. J Chromatogr A . 2002; 950(1-2):293–9. doi: 10.1016/s0021-9673(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 37.Gubitz G, Schmid MG. Chiral separation by capillary electromigration techniques. J Chromatogr A . 2008; 1204(2):140–56. doi: 10.1016/j.chroma.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 38.Kibbey CE, Poole SK, Robinson B, Jackson JD, Durham D. An integrated process for measuring the physicochemical properties of drug candidates in a preclinical discovery environment. J Pharm Sci . 2001; 90(8):1164–75. doi: 10.1002/jps.1070. [DOI] [PubMed] [Google Scholar]
- 39.Jia ZJ. Physicochemical profiling by capillary electrophoresis. Curr Pharm Anal . 2005; 1:41–56. [Google Scholar]