Abstract
Purpose: Telomerase is expressed in most cancers, including breast cancer. Curcumin, a polyphenolic compound that obtained from the herb of Curcuma longa, has many anticancer effects. But, its effect is low due to poor water solubility. In order to improve its solubility and drug delivery, we have utilized a β-cyclodextrin-curcumin inclusion complex. Methods: To evaluate cytotoxic effects of cyclodextrin-curcumin and free curcumin, MTT assay was done. Cells were treated with equal concentration of cyclodextrin-curcumin and free curcumin. Telomerase gene expression level in two groups was compared by Real-time PCR. Results: MTT assay demonstrated that β-cyclodextrin-curcumin enhanced curcumin delivery in T47D breast cancer cells. The level of telomerase gene expression in cells treated with cyclodextrin-curcumin was lower than that of cells treated with free curcumin (P=0.001). Conclusion: Results are suggesting that cyclodextrin-curcumin complex can be more effective than free curcumin in inhibition of telomerase expression.
Keywords: Anti cancer drug, Target therapy, Telomerase, Breast cancer, Drug delivery
Introduction
Telomerase activity is observed in more than 85% in the most cancer cells.1 Telomerase is active in 74% of breast Carcinomas2,3 Therefore, targeting the telomerase in this cancer could be promising step in its treatment.4 For this purpose it is better to use natural compounds such as Curcumin (CUR). Curcumin (commonly known as turmeric) is obtained from Curcuma longa.5 Curcumin has many pharmacological applications such as anticancer, with low or no intrinsic toxicity.6 Despite all these, curcumin suffers from low water solubility and bioavailability.7 To improve its solubility, β-Cyclodextrin was used for encapsulation of curcumin. β-Cyclodextrin is a semi-natural compound with low toxicity, which could enhance drug bioavailability.7 To the best of our knowledge, comparison of inhibitory effect of free CUR and CD-CUR on hTERT gene expression in T47D cell line has never been done so far. Anti-telomerase effect of curcumin has been studied previously in lung cancer cell line8,9 and preparation of β-cyclodextrin-curcumin inclusion complex for improvement of curcumin stability and solubility has been studied.7 In our previous study we showed the inhibitory effect of β-Cyclodextrin-curcumin in telomerase gene expression in breast cancer cell line.10 Rajeswari et al reported that cyclodextrin enhance the bioavailability of insoluble drugs by increasing the drug solubility.11 Yadav and coworkers showed that Cyclodextrin-complexed curcumin had superior attributes compared with free curcumin for cellular uptake.12 Murali et al found that CD-CUR inhibit the growth of prostate cells higher than free curcumin.9 Therefore in this study we investigated the effect of β-Cyclodextrin-curcumin (CD-CUR) and free CUR (CUR) on human telomerase reverse transcriptase (hTERT) gene expression. Anticancer effect of free CUR and CD-CUR in breast cancer cell line, T47-D was compared. The level of telomerase gene expression after 24 h exposure was measured by Real-time PCR.
Materials and Methods
Cell culture and cell line
T47D cell line (breast cancer epithelial like cell line) was prepared from Pasteur Institute cell bank of Iran, code: C203. This cell line was cultured in RPMI1640 (Gibco, Invitrogen, UK) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Invitrogen, UK), 2 mg/ml sodium bicarbonate, 0.05 mg/ml penicillin G (Serva co, Germany), 0.08 mg/ml streptomycin (Merck co, Germany) and incubated in 37°C with humidified air containing 5% CO2.
In vitro cytotoxicity (MTT assay)
Cells in the exponential phase of growth were exposed to CD-CUR inclusion complex or free CUR. Cytotoxic effect of CD-CUR inclusion complex and free CUR was studied by 24, 48 and 72 h MTT assay. 2 × 103 cell/well was plated in a 96-well plate (Coastar from Corning, NY). After 24 h incubation, cells were treated with different concentrations (5-100 μM) of CD-CUR and free CUR for 24, 48 and 72 h in the quadruplicate manner. Media containing equivalent amounts of CD in PBS or DMSO was used as control. After different exposure duration, medium was removed and then fed of the cells with 200 μL of fresh medium. Cells were incubated for 24 h, then 50 μL of 2 mg/ml MTT (Sigma co, Germany) dissolved in PBS was added to each well and plats were covered with aluminum foil and incubated for 4 h. In the next step, wells’ content was removed and 200 μL pure DMSO and 25 μL Sorensen’s glycine buffer were added. Finally amount of formazan was determined by measuring the absorbance at 570 nm using an ELISA plate reader (with a reference wavelength of 630 nm).
Cell treatment
After determining of IC50, 2.5 × 105 cells in 25 cm2 flasks were treated with 3 concentrations lower than IC50 of 24h CD-CUR (5, 10 and 15 μM). Culture flasks were incubated for 24 h. For control cells, the same volume of 10%DMSO without CUR and CD-CUR was added to flask of control cells. An equivalent amount of CD in PBS was used as another control. Culture flasks were incubated in 37°C containing 5% CO2 with humidified atmosphere incubator for 24 h exposure duration.
Real-Time PCR (qRT-PCR) Assay
After the RNA extraction (by the TRIZOL kit, Cinnagene, Iran), cDNA synthesized according the instructions (First Strand cDNA Synthesis Kit fermentase, K1622).
For real-time PCR, according to our previous study10, hTERT primers (Genbank accession: NM_198255, bp 2165-2362) and beta actin primers (Genbank accession: NM_001101, bp 787-917) were used. For hTERT, a 198 bp amplicon and for beta actin a 131 bp amplicon were generated in a 25 µl reaction mixture that contained: 5 pmole of the forward and reverse PCR primers of hTERT (5’CCGCCTGAGCT- GTACTTTGT3’, 5’ CAGGTGAGCCACGAACTGT3’ respectively) or for beta actin (5’TCCCTGGAGAAGAGCTACG3’, 5’GTAGTTT- CGTGGATGCCACA3’ respectively), 2X PCR Master Mix Syber Green I, and 2µl of the cDNA was used. Each DNA sample was divided so that hTERT and beta actin could be amplified, in parallel, and in duplicated from equal amounts of starting cDNA separately. 25 µl reactions contained the following final concentrations: 1X of MaximaTM SYBR Green/ROX qPCR Master Mix (including Maxima TM Hot start Taq Polymerase, Maxima TM SYBR Green qPCR Buffer, SYBR Green I and ROX Passive reference dye), 5pmole of each primer and 2µl of the cDNA. Negative controls were prepared each time, consisting of 2 μl ddH2O instead of the cDNA template. The sample tubes were placed into the (Rotor-Gene 6000, Corbet) with the following settings as manufacture protocol.
Results and Discussion
Cell toxicity studies
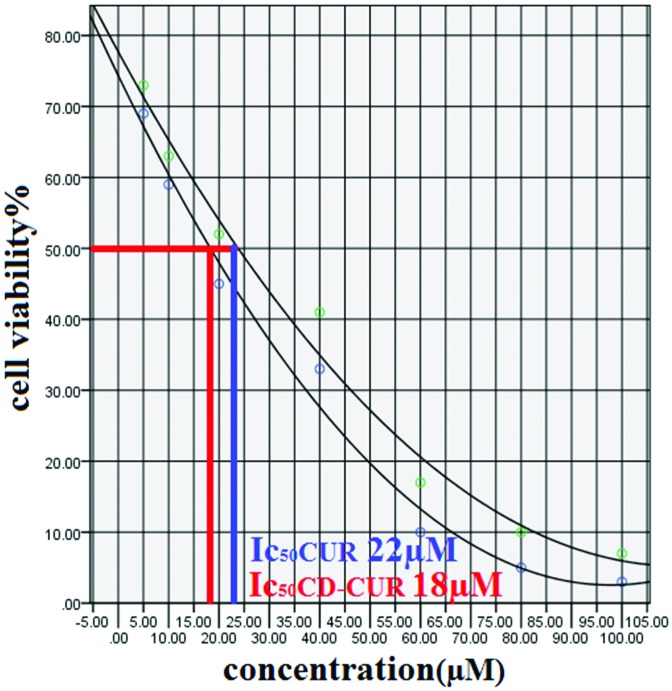
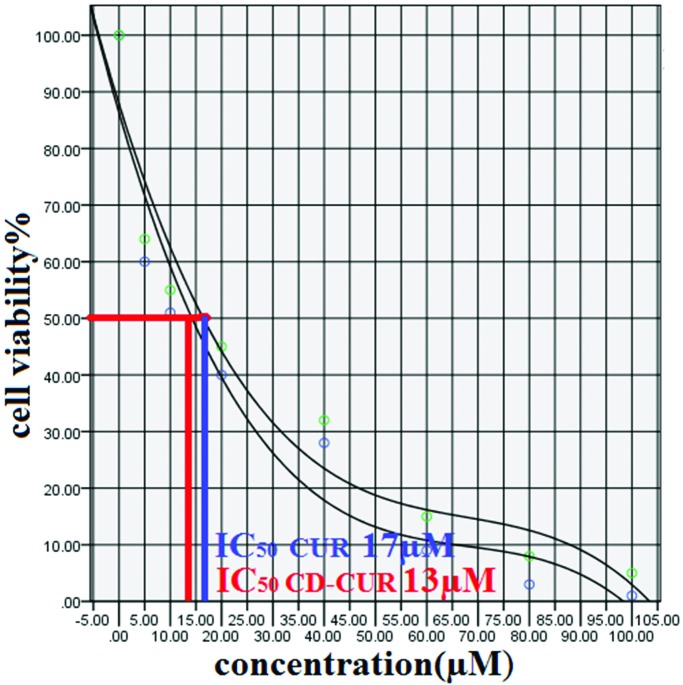
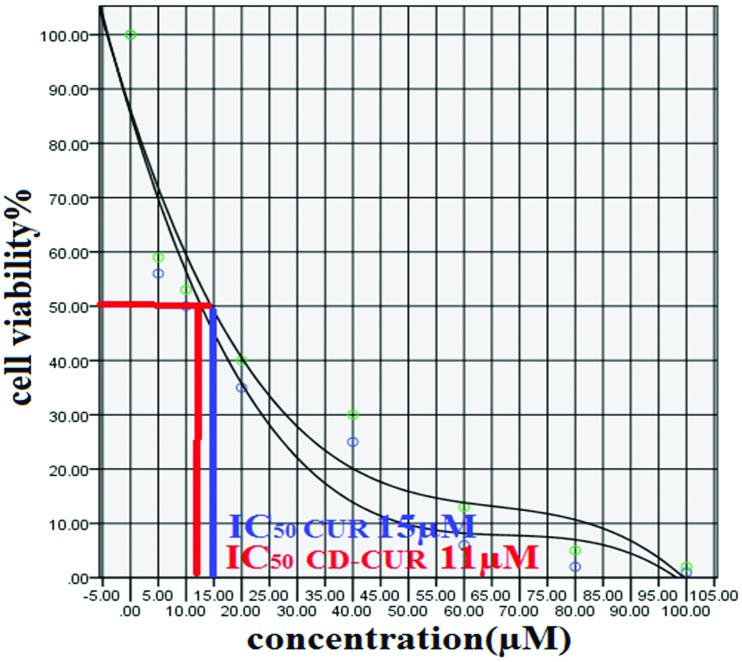
In this study to evaluate the cytotoxic effect (MTT assay) of CD-CUR and free CUR, T47D breast cancer cell line were treated with different concentration (5-100µM) of CD-CUR and free CUR for 24, 48 and 72h. IC50 after 24 h treatment with CD-CUR and CUR was 18 µM and 22 µM respectively (Figure 1). Drug free CD as well as DMSO 2% showed an absorbance value equivalent of 90 and 92% of control respectively suggesting that CD and DMSO 2% have low effect on the cells. Cells treated with concentrations more than 60 µM of CD-CUR and free CUR for 48 and 72h were died completely. IC50 after 48 h treatment with CD-CUR and CUR was 13 and 17µM respectively (Figure 2 and 3). Considering the fact that IC50 values for different treatment durations are not similar, it might be concluded that the effect of CD-CUR and CUR on T47D cell line is time-depending. Moreover IC50 show that effect of CD-CUR on cells is more than free CUR, Demonstrating the enhanced uptake of CD-CUR with respect to CUR.
Figure 1.
MTT assay graph of 24 h cell treatment with CD-CUR and CUR.
Figure 2.
MTT assay graph of 48 h cell treatment with CD-CUR and CUR.
Figure 3.
MTT assay graph of 72 h cell treatment with CD-CUR and CUR.
Real-time PCR
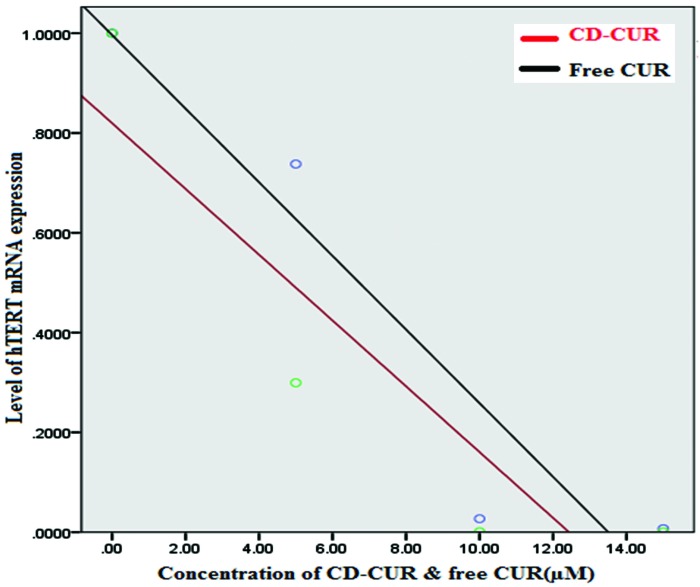
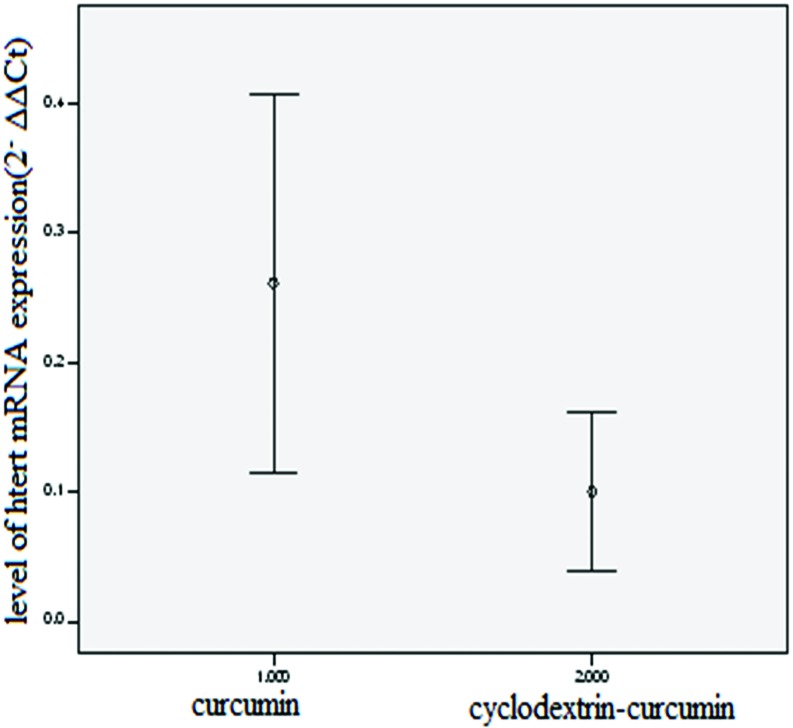
Figure 4 and 5 demonstrate the results of telomerase gene expression study at T47D breast cancer cell line after 24 h of CD-CUR and free CUR exposure. β-Cyclodextrin-curcumin suppresses cell proliferation in T47D breast cancer cells. Cells were treated with curcumin (CUR) or CD-CUR for 24 h. Cell proliferation was determined by MTT assay and normalized to cells treated with equivalent amounts of respective controls (DMSO for curcumin and cyclodextrin for CD-CUR). The level of hTERT mRNA was normalized to mRNA levels of the housekeeping gene, Beta actin, within each sample. The differences of 2–ΔΔCt values were calculated. Increasing 2–ΔΔCt amount resulted in enhanced expression of mRNA levels. Data analyses of real-time PCR showed that with increasing the concentration of CD-CUR and free CUR, a decreasing trend is observed in mRNA levels of hTERT. Compared to CUR, in the same concentration, CD-CUR resulted in a lower mRNA level of hTERT and lower level of hTERT mRNA expression (Figure 5). The difference between two groups was statistically significant (p=0.001, n=4) (Figure 6).
Figure 4 .
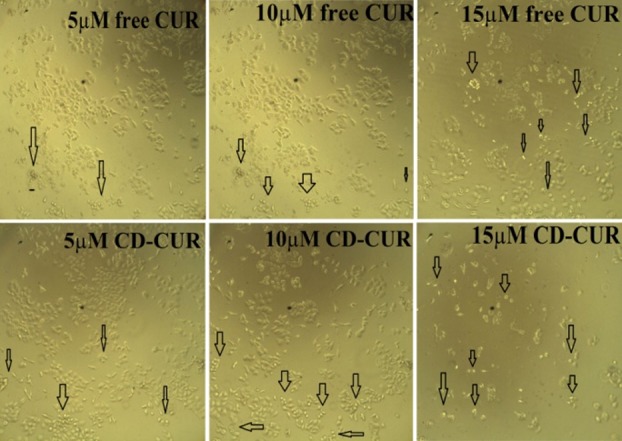
β-Cyclodextrin-curcumin and free curcumin treatment cells (Images were taken by Phase contrast microscope).
Figure 5 .
Level of hTERT mRNA expression in cells treated with CD-CUR or free CUR.
Figure 6 .
comparision of hTERT mRNA expression in curcumin as well as cyclodextrin-curcumin treated cells. The difference between two groups was statistically significant (p=0.001, n=4).
Conclusion
Our results showed that CD-CUR and free CUR had inhibitory effect on T47D breast cancer cell line. This inhibition was time and dose dependent. β-cyclodextrin-curcumin resulted in higher cell toxicity than free curcumin in breast cancer cell line. There was a significant difference between cells treated with CD-CUR and cells treated with free CUR in the levels of telomerase gene expression. The quantity of telomerase was decreased in the cells treated with CD-CUR in comparison to cells treated with free CUR (P=0.001). It may be concluded that relative to free CUR, CD-CUR inclusion complex inhibit the telomerase gene expression in T47D cell line more effectively.
Acknowledgement
This study was supported by grants from Drug Applied Research Center of Tabriz University of Medical Sciences. We thank thereby Drug Applied Research Center of Tabriz University of Medical Sciences for funding this research.
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.Tarkanyi I, Aradi J. Pharmacological intervention strategies for affecting telomerase activity: Future prospects to treat cancer and degenerative disease. Biochimie . 2008;90(1):156–72. doi: 10.1016/j.biochi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick KL, Ogunkolade W, Elkak AE, Bustin S, Jenkins P, Ghilchick M. et al. hTERT expression in human breast cancer and non-cancerous breast tissue: correlation with tumour stage and c-Myc expression. Breast Cancer Res Treat . 2003;77(3):277–84. doi: 10.1023/a:1021849217054. [DOI] [PubMed] [Google Scholar]
- 3.Tian N, Gaines Wilson J, Benjamin Zhan F. Female breast cancer mortality clusters within racial groups in the united states. Health Place . 2010;16(2):209–18. doi: 10.1016/j.healthplace.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Nicole MB. Targeting telomerase and telomeres with small molecule anticancer compounds. MMG 445 Basic Biotechnology eJournal . 2008;4:72–9. [Google Scholar]
- 5.Daybe FV. Uber Curcumin. Den Farbstoff der Curcumawurzzel Ber . 1870;3:609. [Google Scholar]
- 6.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett . 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The indian solid gold. Adv Exp Med Biol . 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 8.Sharma RA, Gescher A, Steward WP. Curcumin: the story so far. Eur J Cancer . 2005; 41(13):1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Yallapu MM, Jaggi M, Chauhan SC. Beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces . 2010;79(1):113–25. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Kazemi F, Zarghami N, Fekri-aval S, Monfaredan A. β-Cyclodextrin-curcumin complex inhibit telomerase gene expression in T47-D breast cancer cell line. Afr J Biotechnol . 2011; 83(10):19481–88. [Google Scholar]
- 11.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech . 2005;6(2):E329–57. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav R, Sahdeo P, Ramaswamy K, Javaraj R, Chaturvedi M, Lauri V. et al. Cyclodextrin-complexd curcumin exhibits antiinflamatory and anti proliferative activity. Biochem pharmacol . 2010; 80(7):1021–32. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]