Abstract
Purpose: The objective of this study was to prepare a suitable form of nanofiber for indomethacin using polymers Eudragit RS100 (ERS) and Eudragit S100 (ES) and to evaluate the effect of some variables on the characteristics of resulted electrospunnanofibers. Methods: Electrospinning process was used for preparation of nanofibers. Different solutions of combinations of ERS, ES and indomethacin in various solvents and different ratios were prepared. The spinning solutions were loaded in 10 mL syringes. The feeding rate was fixed by a syringe pump at 2.0 mL/h and a high voltage supply at range 10-18 kV was applied for electrospinning. Electrospunnanofibers were collected and evaluated by scanning electron microscopy, differential scanning calorimetry and FTIR for possible interaction between materials used in nanofibers. The effect of solvent and viscosity on the characteristics of nanofibers also was investigated. Results: Fiber formation was successful using a solvent ethanol and mixture of ERS and ES. Increase in viscosity of ethanolic solutions of ERS followed by addition of ES in the solution led to preparation of smooth fibers with larger diameters and less amounts of beads. DSC analysis of fibers certified that indomethacin is evenly distributed in the nanofibers in an amorphous state. FTIR analysis did not indicate significant interaction between drug and polymer. Conclusion: It was shown that drug-loaded ERS and ES nanofibers could be prepared by exact selection of range of variables such as type of solvent, drug: polymer ratio and solution viscosity and the optimized formulations could be useful for colonic drug delivery.
Keywords: Electrospun, Nanofiber, Indomethacin, Eudragit
Introduction
In recent years, drug delivery to colon has gathered a lot of attentions both from pharmaceutical industry and academia. Colonic drug delivery is significantly important not just for the delivery of protein and peptide drugs but also for treatment of diseases associated with colon such as colon cancer, ulcerative Colitis and diarrhea. Colon is believed to be suitable adsorption site especially for poorly absorbed drugs mostly because of its long retention.1
Different colon targeted drug delivery systems have been tried where pH, time, pressure dependent and microbially triggered systems are the primary approaches for colon drug delivery.2,3 Recent researches are mainly based on the combination of two or even more colon-target drug delivery methods. This methodology decreases the effect of physiological changes of gastrointestinal tract and thus facilitates the prediction of drug releasing process in different conditions.
Apart from large diversity in colonic drug delivery systems, nanofibers containing drugs have been less considered in colon-target delivery systems. Various approaches can be used for preparation of nanofibers. Electrospinning is one of the most reliable techniques for nanofiber formation. In this method an electrical charge is applied to draw very fine fiber in nanoscale from a liquid. These electrospun fibers have a high surface to volume ratio which makes them promising candidate in adsorption of less-soluble drugs.4-6 Electrospinning is mostly applied in tissue engineering,7 implement materials, wound dressing, prosthesis8 and drug delivery.9
Different parameters significantly affect the process namely: molecular weight, solution characteristics (viscosity, surface tension and conductivity), electric potential, concentration, distance between the capillary and collector screen, temperature, humidity and air velocity in the chamber.10
A lot of researches have revealed that indomethacin can be effective in colon cancer treatment. However, indomethacin as a non-steroidal anti-inflammatory drug (NSAIDS) has a lot of adverse effects on gastrointestinal tract. Therefore this drug was chosen in colon-targeted drug delivery process. On the other hand, indomethacin is a less soluble drug which makes that a promising candidate for electrospunnanofibers.
The objective of this study was to evaluate the effect of two factors (ratio of Eudragit S100:Eudragit RS100 and ratio of drug:polymer) on morphological characteristics of indomethacin nanofibers and optimize formulation variables such as viscosity of electropinning solutions and type of solvents in orderto obtain the best colonic drug delivery system for indomethacin.
Materials and Methods
Materials
Indomethacin (Darupakhsh Pharmaceutical manufacturing company, Tehran, Iran),EudragitS100 (ES) and Eudragit RS100 (ERS) (Rohm Pharma, GmbH, Germany), sodium chloride and potassium di-hydrogen phosphate (Merck, Germany) were obtained from the indicated sources.
Preparation of Electrospinning Solutions
25% (w/v) solution of Eudragit RS and 15% (w/v) dispersion of drug were prepared in water. Then electrospining solution with proportion of 1:1(v/v) for drug and polymer was made. The same work was carried out by ethanol as the solvent. On the other hand,25% (w/v) solutions of polymethacrylates (ERS and ES) and 15% (w/v) solution of drug were prepared in ethanol as a good solvent. The ratios of ES:ERS were 30:70, 50:50 and 70:30. Then electrospining solution with ratio of 1:1(v/v) for drug and polymers was made. Further formulations with The ratios of 20:80, 80:20 and 100:0 for ES:ERS and the electrospining solution with ratios of drug: polymer at range 1:1,1.5:1, 2:1, and 2.5:1(v/v) were also prepared. Finally, regarding the characteristics and reproducibility of preliminary formulations the least and most levels were considered to design a series of runs according to a 32 full factorial design.The ratio of drug to polymer and ES:ERS was considered as the independent variables.Table 1 summarizes the independent variables.The resulted formulations of factorial design are listed in Table 2.
Table 1. Experimental design: factors and responses.
| Independent variables | -1 | 0 | +1 |
| X1: Ratio of Eudragit S100: RS100 | 20:80 | 60:40 | 100:0 |
| X2: Drug:polymer ratio | 1:1 | 1.5:1 | 2:1 |
Table 2. Composition of experimental formulations (runs).
| Formulation | X1 | X2 |
| F1 | -1 | -1 |
| F2 | -1 | 0 |
| F3 | -1 | +1 |
| F4 | 0 | -1 |
| F5 | 0 | 0 |
| F6 | 0 | +1 |
| F7 | +1 | -1 |
| F8 | +1 | 0 |
| F9 | +1 | +1 |
Electrospinning Process
The spinning solutions were loaded in 10 mL syringes. The feeding rate was controlled by a syringe pump (Cole-Pham®, USA) and was fixed at 2.0 mL/h. A high voltage supply fixed at 10-18 kV was applied,and a piece of aluminum foil was used to collect the ultrafine fibers with a horizontal distance of 15cm from the needle tip.Electrospunnanofibers were collected and stored in desiccator for more studies.
Scanning Electron Microscopy
The surface morphologies of electrospun fibers were assessed using a LEO - rp-1455scanning electron microscope (SEM). Prior to the examination, the samples were silver sputter-coated under argon to render them electrically conductive. The pictures were then taken at an excitation voltage of 15 kV.
Differential Scanning Calorimetry
DSC analyses were carried out using a Mettler-Ms603s differential scanning calorimeter. Sealed samples were heated at 30 ºC/min from 20 to280 ºC.
Fourier Transformed Infrared Spectroscopy
FTIR was conducted using a Nicolet-Nexus 670 FTIR spectrometer. The samples were prepared using the KBr disk method (2mg sample in 200mg KBr) and the scanning range was 500–4000cm−1 with a resolution of 2 cm−1.
Results and Discussion
Preparations ofDrug-Loaded Nanofibers
Suitable selection of solvent is one of the most important factors for successful preparation of electrospun polymer nanofibers.11-13 The solvent should be able to dissolve the drug easily as well as maintaining electrospinnability of polymer solutions.Eudragit RS100 could be electrospun into fibers when methanol or ethanol was used as the solvent.14 In our study, ERS aqueous solutions were unspinnable.Only discrete droplets were observed when they were subjected to the electrospinning process.
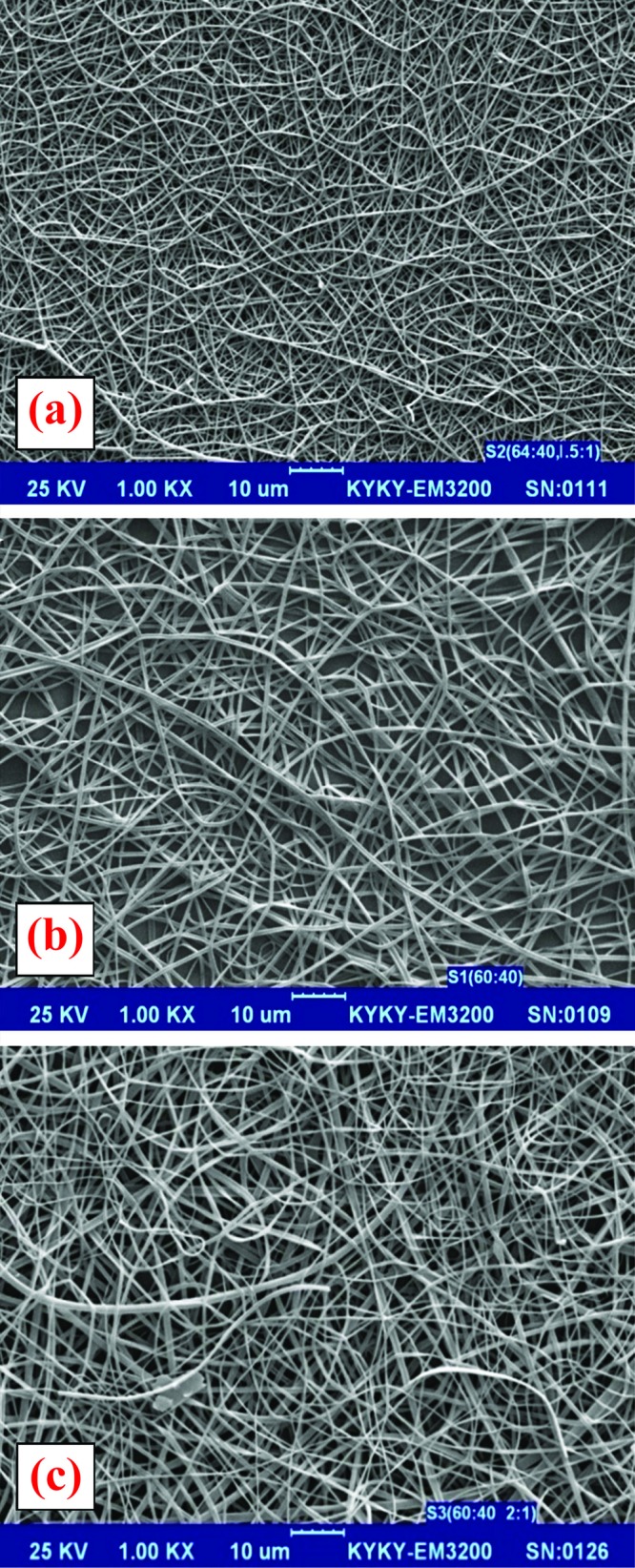
Nanofibers from ERS in ethanol showed discrete beads and/or beaded fibers when the viscosity of the solution was low. The formation of half-hollowed beads was thought to be a result of the evaporation of the solvent from the beads. Further increase in solution viscosity by addition of ES resulted in the formation of smooth fibers with larger diameters (Figure 1).
Figure 1.

SEM images of formulations with ES:ERS in the ratios of ; (a) 30:70, (b) 50:50 and (c) 70:30 (magnification ×1000).
SEM images of drug loaded nanofibers with different amounts of indomethacin and various ratios of ES:ERS asserted that nanofiber formation is just possible with ratios of drug:polymer in ranges 1:1, 1.5:1 and 2:1 and ratio of 2.5:1 did not form suitable nanofiber in all ratios of polymers. Also, nanofiber formation in formulations with drug: polymer ratios of 2:1 and 1.5:1 was only occurred when the ratio of ES:ERS was in range 20:80 and 100:0. This result could be to the decrease in viscosity of electrospinning solutions affected by increase in amount of drug which in consequence disrupted nanofiber formation process. SEM images of drug loaded nanonfibers are presented in Figure 2. Solution viscosity plays an important role in determining the fiber size and morphology during spinning of polymeric fibers.When the solution viscosity decreases surface tension has the overcoming influence on fiber morphology with the final results of decrease in fiber diameters and bead formation. Correlation between the polymer viscosity and/or concentration and fibers formed from electrospinning has been surveyed in a number of studies.15-19 Chowdhury et al. investigated the effect of experimental parameters such as polymer concentration, viscosity and surface tension on the morphology of electrospun Nylon 6 fibers. They found that increase in the concentration and viscosity and lowering surface tension manages to formation of the uniform nanofibers.20
Figure 2.
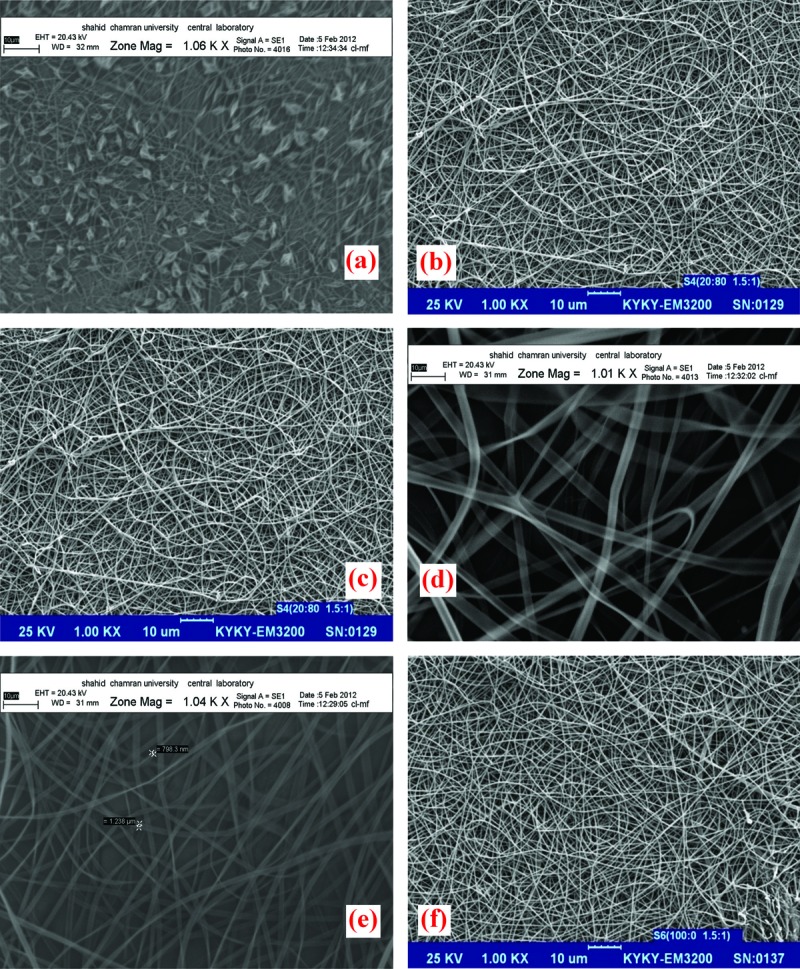
SEM images of formulations; range of ES:ERS and drug:polymer was (a) 20:80 and 1:1, (b) 20:80 and 1.5:1, (c) 20:80 and 2:1, (d) 80:20 and 1:1, (e) 100:0 and 1:1, (f) 100:0 and 1.5:1.
Finally, according to preformulation studies ratios of 1:1, 1.5:1 and 2:1 for drug: polymer and 20:80, 60:40 and 100:0 for ES:ERS were selected to design 9 formulations based on full factorial design. Figure 3 shows SEM images of formulations containing ES:ERS at the ratio 60:40 ES:ERS and drug: polymer at ranges 1:1, 1.5:1 and 2:1.Comparing SEM Figures 2 and 3 it can be seen that addition of ES could lead to the formation of smooth fibers with larger diameters and low beads which could be illustrated by increase in viscosity of electrospinning solutions.
Figure 3.
SEM images of formulations with ES:ERS in the ratio of 60:40 and different ratios of drug:polymer; (a) 1:1 ratio, (b) 1.5:1 ratio, and (c) 2:1 ratio.
Physical State of Components in the Nanofibers
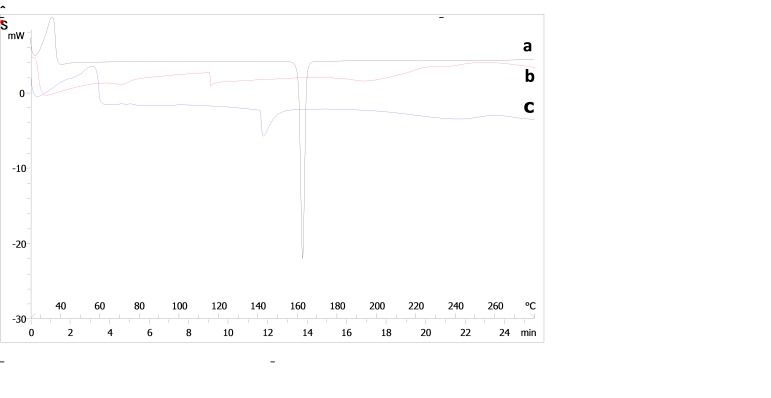
DSC thermograms of drug and Eudragits are shown in Figure 4. The DSC curve of pure indomethacin indicated a single endothermic response corresponding to a melting point of 179 ºC (Figure 4a). Thecomposed of pure ERS exhibited a single endothermic response in 115 ºC, suggesting that Eudragit RS is in amorphous state (Figure 4b). On the other hand, ES showed a single endothermic response in 142 ºC (Figure 4c). Figure 5 illustrates thermograms of formulations resulted from factorial design. According to Figure 5, all formulations exhibited a broadband wide endotherm ranging from 190 to 240 ◦C which could be due to polymer melting. In addition, melting point peak of indomethacin was removed in all formulation and it may be caused by the presence of Eudragits that resulted in a loss of crystalline content of indomethacin. The presence of an endothermic peak at 60°C in some formulations (F1, F3, F4 and F5) could be due to lowering of Tg of Eudragits by addition of drug to the formulation composition. This phenomenon was more obvious in formulations containing Eudragit RS. Plasticizing effect of NSAIDs and increase in macromolecular mobilities of polymeric chains due to presence of these drugs has been previously demonstrated.21,22 DSC studies demonstrated that distribution of drug molecules in the nanonfiber structure was occurred with change in state of drug from crystallinity to amorph status.
Figure 4.
DSC thermograms of (a) drug, (b) Eudragit RS100 and (c) Eudragit S100.
Figure 5.
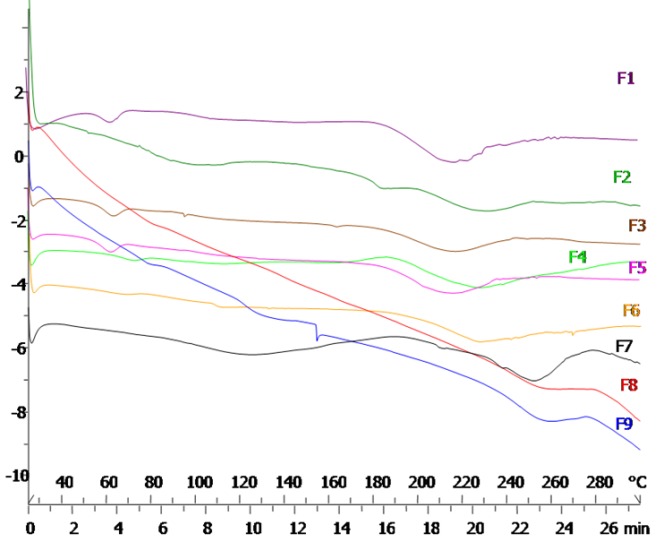
DSC thermograms of formulations resulted from factorial design
Compatibility of Nanofiber Components
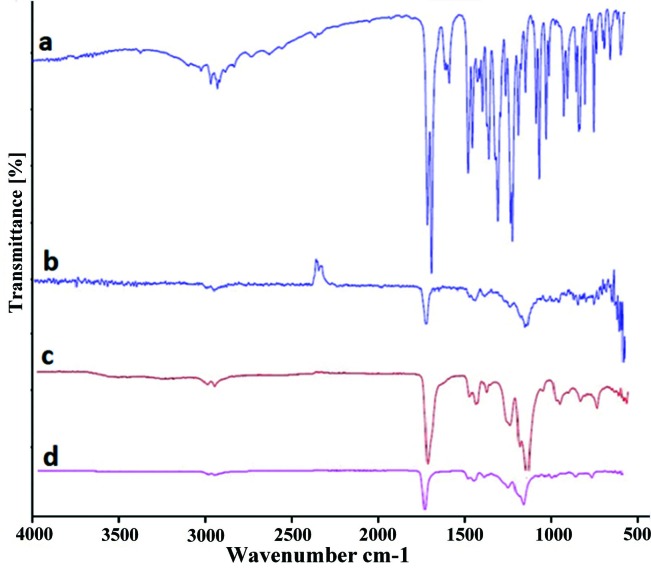
FTIR spectra of drug, polymers and formulation F1 was shown in Figure 6. Accordingly, the spectrum of indomethacin showed bands characteristic of secondary carbonyl groups (C=O) at 1714 cm−1, (C=O amid) in 1690 cm-1, phenyl groups (C=C stretch vibration) at 1523cm−1 and (O-H stretch vibration) at 3022cm−1.The spectrum of ERS had a broad band characteristic of groups carbonyl (C=O) at 1723 cm−, and ester linkages (C-O stretch vibration) at 1149 cm−1.The spectrum of ES showed a broad band characteristic of carbonyl groups (C=O) at 1727 cm−1, characteristic bands of hydroxyl groups (C-H stretch vibration) at 2957 cm−1. Two other spectra at 1152 and 3087 cm-1 were also indicative of C-O and O-H stretch vibration, respectively. FTIR of formulation F1 exhibited the same spectra which in result there would be no significant shift in spectra and interactions between drug and polymer was not seen. Interaction between ionizable drugs and eudragits was investigated in some researchs.ForexampleHeun et al. foundinteractions between drugs and Eudragits RL/RS resins in aqueous environment.23 Also in the other study ionic interaction between propranolol hydrochloride and three different anionic polymers Eudragit S 100, Eudragit L 100-55 andsodium carboxymethylcellulose wasdemonstrated.24 However, in our study there was no any significant ionic or hydrogenic interaction between drug and polymers.
Figure 6.
FTIR spectra of (a) indomethacin, (b) Eudragit RS, (c) Eudragit S, and (d) formulation F1
Conclusion
Combination of Eudragit RS and Eudragit S for prepration of nanofibers containing indomethacin using electrospinning method was successfully tried.Accurate selection of solvent, viscosity, and ratios of ERS:ES and drug:polymer was important for successful preparation of electrospunnanofibers. In the entire composite nanofibers drug was present in an amorphous state. The optimized formulations were capable of drug loading up to 66% and could be useful for further studies on possible colonic delivery of indomethacin.
Acknowledgments
This work is the Pharm.D thesis of Mrs Z. Heshmati which is supported by a grant from research chancellor of Ahvaz Jundishapur University of Medical Sciences. The authors would like to thank Darupakhsh pharmaceutical co. for their collaboration and providing samples used in this paper.
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.Watts PJ, Illum L. Colonic drug delivery. Drug Dev Ind Pharm . 1997;23:893–913. [Google Scholar]
- 2.Goskonda SR, Upadrashta SM. Avicel RC-591/Chitosan beads by extrusion-spheronization technology. Drug Dev Ind Pharm . 1994;19:915–27. [Google Scholar]
- 3.Rodriguez M, Vila-Jato JL, Torres D. Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Control Release . 1998;55:67–77. doi: 10.1016/s0168-3659(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 4.Ahn YC, Park SK, Kim GT, Hwang YJ, Lee CG, Shin HS. et al. Development of high efficiency nanofilters made of nanofibers. Curr Appl Phys . 2006;6:1030–5. [Google Scholar]
- 5.Lannutti J, Reneker D, Ma T, Tomasko D, Farson D. Electrospinning for tissue engineering scaffolds. Mater Sci Eng C . 2007;27(3):504–9. [Google Scholar]
- 6.Hunley MT, Long TE. Electrospinning functional nanoscale fibers: a perspective for the future. Polym Int . 2008;57:385–9. [Google Scholar]
- 7.Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S. et al. Evaluation of electrospun PCL/gelatin nano fibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater . 2007;3:321–30. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Thomas S. Wound Management and Dressings. London: Pharmaceutical Press; 1990. [Google Scholar]
- 9.Kenawy el R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH. et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release . 2002;81(1-2):57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Mo X, Qing F. Electrospinning of collagen-chitosan complex. Mater Lett . 2007;61:3490–4. [Google Scholar]
- 11.Moghe AK, Gupta BS. Co-axial electrospinning for nanofiber structures: preparation and applications. Polym Rev . 2008; 48:353–77. [Google Scholar]
- 12.Qi H, Sui X, Yuan J, Wei Y, Zhang L. Electrospinning of cellulose-based fibers from NaOH/urea aqueous system. Macromol Mater Eng . 2010;295:695–700. [Google Scholar]
- 13.Liu Y, Ma G, Fang D, Xu J, Zhang H, Nie J. Effects of solution properties and electric field on the electrospinning of hyaluronic acid. Carbohyd Polym . 2011;83(2):1011–5. [Google Scholar]
- 14.ShenX ShenX, Yu D, Zhu L. Electrospundiclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. Int J Pharm . 2011;408:200–7. doi: 10.1016/j.ijpharm.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC. et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol . 2005;120:327–39. doi: 10.1016/j.jbiotec.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Nagapudi K, Apkarian RP, Chaikof EL. Engineered collagen-PEO nanofibers and fabrics. J Biomat Sci Polym E . 2001;12:979–93. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 17.Son WK, Youk JH, Lee TS, Park WH. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly (ethylene oxide) fibers. Polymer . 2004;45:2959–66. [Google Scholar]
- 18.Ding B, Kim HY, Lee SC, Shao CL, Lee DR, Park SJ. et al. Preparation and characterization of a nanoscale poly (vinyl alcohol) fiber aggregate produced by an electrospinning method. Polym Phys . 2002;40: Polym Phys 2002;40. [Google Scholar]
- 19.Koski A, Yim K, Shivkumar S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater Lett . 2004;58:493–7. [Google Scholar]
- 20.Chowdhury M, Stylios G. The effect of experimental parameters on the morphology of electrospun Nylon 6 fibres. Int J BasApplSci . 2010;10 (6):116–31. [Google Scholar]
- 21.Siepmann F, Le Brunsurname V, Siepmann J. Drug acting as plasticizers in polymeric systems: a quantitative treatment. J Control Release . 2006;115:298–306. doi: 10.1016/j.jconrel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Yu DG, Shen XX, Branford-White C, White K, Zhu LM, Bligh SW. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology . 2009;20(5):055104. doi: 10.1088/0957-4484/20/5/055104. [DOI] [PubMed] [Google Scholar]
- 23.Heun G, Lambov N, Groning R. Experimental and molecular modeling studies on interactions between drugs and Eudragit RL/RS resins in aqueous environment. Pharm Act Helv . 1998;73:57–62. [Google Scholar]
- 24.Takka S. Propranolol hydrochloride-anionic polymer binding interaction. Il Farmaco . 2003;58(10):1051–6. doi: 10.1016/S0014-827X(03)00181-2. [DOI] [PubMed] [Google Scholar]