Abstract
Purpose: The aim of this study was to determine the toxic effect of Nigella sativa powder on the liver function which was evaluated by measuring liver enzymes and through histopathological examination of liver tissue. Methods: Twenty four male Sprague Dawley rats were allotted randomly to four groups including: control (taking normal diet); low dose (supplemented with 0.01 g/kg/day Nigella sativa); normal dose (supplemented with 0.1 g/kg/day Nigella sativa) and high dose (supplemented with 1 g/kg/day Nigella sativa). All of supplements administered in powder form mixed with rats’ pellet for 28 days. To assess liver toxicity, liver enzymes measurement and histological study were done at the end of supplementation. Results: The finding revealed that there was no significant change in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) between treatment groups. Histopathological study showed very minimal and mild changes in fatty degeneration in normal and high doses of Nigella sativa treated group. Inflammation and necrosis were absent. Conclusion: The study showed that supplementation of Nigella sativa up to the dose of 1 g/kg supplemented for a period of 28 days resulted no changes in liver enzymes level and did not cause any toxicity effect on the liver function.
Keywords: Enzyme, Liver, Function, Nigella sativa, Toxicity, Rat
Introduction
Herbal medicines have been used by human for thousands years to treat medical illness or to improve physical performance. Plants have been always a major source of nutrition and health care for both humans and animals.1 In recent years, there has been growing interest in alternative therapies and the therapeutic use of natural products, especially those derived from plants.2 Despite the move toward synthetic medicine and use of sophisticated drugs, traditional plant-based remedies still play an important role in the world’s medicine.3 Extremely, 80% of the world’s population use plant-based remedies as their primary form of healthcare and the world market of herbal medicines based on traditional knowledge are estimated at USD 60 thousand million.4 Nigella sativa (Black seed) is an annual plant belongs to the botanical family of Ranunculaceae5 (Figure 1 A,B) and commonly grows in Europe, Middle East and Western Asia. Nigella sativa (NS) is usually used as a traditional medicine in Arabian countries,6 Indian sub-continent and Europe,7 for a wide range of therapeutic purpose including immunomodulative,8 antibacterial,9 anti-tumor,10 diuretic and hypotensive,11 genoprotective,12 hepato-protective and antidiabetic11 as well as bronchodilator activity8 and estrogenic activity.13 Since 1970 to 2001, about 530 studies have been conducted on the Nigella sativa and only 3.4% concern about its toxicity.14 Another study showed Nigella sativa fixed oil has a low toxicity with the evidence of high value of LD50 and no morphological changes on the histopathological examination on heart, liver, kidney, and pancreas tissue of treated rats.15 There are a wide range of studies which proved hepato-protective effect of Nigella sativa16,17 as well as its mild hepatotoxicity in animals.18 Even though it is now commercially found in supplement, but the consumption in raw form is still popular. People usually consume it at a low dose because of unknown effect at high dose. This study aimed to determine the toxic effect of Nigella sativa seed consumption at different doses on the liver function of rat. Liver contains enzymes which help the body detoxifying poisonous substances.19 Therefore, high dose of Nigella sativa might cause damage to the liver tissue and this can be evaluate by measuring several liver enzymes levels and microscopic examination of liver tissue after receiving Nigella sativa supplementation for 28 days. Therefore current study aimed to determine the toxic effect of Nigella sativa powder on the rats’ liver function which was evaluated by Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and through histopathological examination of liver tissue.
Figure 1.
(A) Nigella sativa flower (B) Nigella sativa seeds
Materials and Methods
Plant Materials
Nigella sativa seeds (imported from India) were purchased from a local herb store in Serdang, Malaysia. Voucher specimens of seeds were kept at the Cancer Research Laboratory of Institute of Biosciences and the seed was identified and authenticated by Professor Dr. Nordin Hj Lajis, Head of the Laboratory of Natural Products, Institute of Bioscience, University Putra Malaysia. After cleaning the seeds under running tap water for 10 min, they were rinsed twice with distilled water and air dried in an oven at 40 °C overnight until a constant weight was attained. The seeds were grounded to a powder shape using an electric grinder (National, Model MX-915, Kadoma, Osaka, Japan) for 6 minutes and were mixed with rat chow pellet powder and water into different doses including 0.01 (low dose), 0.1 (Normal dose) and 1 (High dose) g/kg body weight. Afterward, dough was baked in an oven at 40 °C until it received instant weight.
Animals
The protocol of the study was approved by Animal Care and Use Committee (ACUC), Faculty of Medicine and Health Sciences, University Putra Malaysia (UPM) with UPM/FPSK/PADS/BR/UUH/F01-00220 reference number for notice of approval. Twenty four male Sprague Dawley rats with 300-350 g body weight were supplied by Faculty of Medicine and Health Sciences, University Putra Malaysia and placed in the Animal House of the Faculty. They were housed individually in cages under standard laboratory conditions with a period of 12 h light/dark at 29 to 32°C and 70 to 80% relative humidity in the Animal House, Faculty of Medicine and Health Sciences, University Putra Malaysia. The animals were allowed to acclimatize for at least 10 days before the start of the experiments. The rats were fed with a standard rat chow pellet and allowed to drink water ad libitum. All animal received human care according to the criteria outlined in the “Guide for care and use of laboratory animals” prepared by the ACUC of Faculty of Medicine and Health Sciences, University Putra Malaysia and animal handling were conducted between 08.00 and 10.00 am to minimize the effects of environmental changes. The treatments were given to the rats for 4 weeks. The body weight was measured once a week.
Experimental Design
Animals were assigned into four treatment groups which are considered as control, low, normal, and high. Control groups were given normal pellet without Nigella sativa while the other treatment groups are given pellet containing different doses of Nigella sativa respectively. The dosages were chosen based on human Nigella sativa consumption which is equal to 2 g/day and considering conversion rate to rats, 0.1 g/kg was selected as normal dose. The low dose was ten times (10 X) less than the normal dose while the high dose was ten times (10 X) higher than the normal dose. The treatments were given to the rats for 4 weeks.
Blood Collection
The blood samples were collected at the end of study. The rats were fasted for 12 h before blood collection. Prior to blood sampling, the rats were anesthetized with diethyl ether to ease handling. The blood samples were collected by cardiac puncture using 25 G, 1" needle. Approximately 5 ml of blood volume were taken and dispensed into labelled plain tubes. The blood samples were then centrifuged at 3000 rpm for 10 min to separate the serum. The serum was stored at -40°C until enzyme assays were carried out.
Biochemical Analysis
Stocks and working solutions were maintained at 0°C in a refrigerator. Serum was obtained by high speed centrifugation. The hepatic enzymes were measured by an automatic analyzer (Hitachi 902) using Roche Liver Enzyme Kits based on the manufacturer’s instructions.
Histological Examination
All rats were sacrificed by cervical dislocation under and then midline laparotomy was performed. Resected liver specimens of each rat in all groups were fixed in 10% buffered formaldehyde for 24 hours and embedded into paraffin after 16 h of alcohol process. Five μm thick sections were obtained from the paraffin blocks and stained with hematoxylin and eosin. Each slide was examined under a light microscope by the same pathologist, who was blinded to the study group allocations. Central venous congestion, congestion and dilation of the hepatic sinusoids and inflammation of the portal tracts were noted and graded from 0 to 3, with “0” indicating no change, “1” slight change, “2” moderate change and “3” severe change. A sum of all grades was regarded as total score, which ranged between 0-9.
Statistical Analysis
Data were expressed as means ± standard deviation. The data were analyzed using SPSS windows program version 15 (SPSS Institute, Inc., Chicago, IL, USA). The One-way Analysis of Variance (ANOVA) and General linear Model (GLM) followed by Duncan Multiple Range Test (DMRT) were used for analysis of data. A p-value less than 0.05 (P<0.05) was considered to be significant.
Results
Body Weight
The means of rats’ body weight supplemented with Nigella sativa powder at various doses for 4 weeks period illustrated graphically in Figure 2. Measurement of the body weight was used to evaluate the health status of the rats during the treatment period. There was a slight weight reduction in treated rat groups compared to control, which was not statistically significant. This is indicating the healthy status of rats following Nigella sativa supplementation.
Figure 2 .
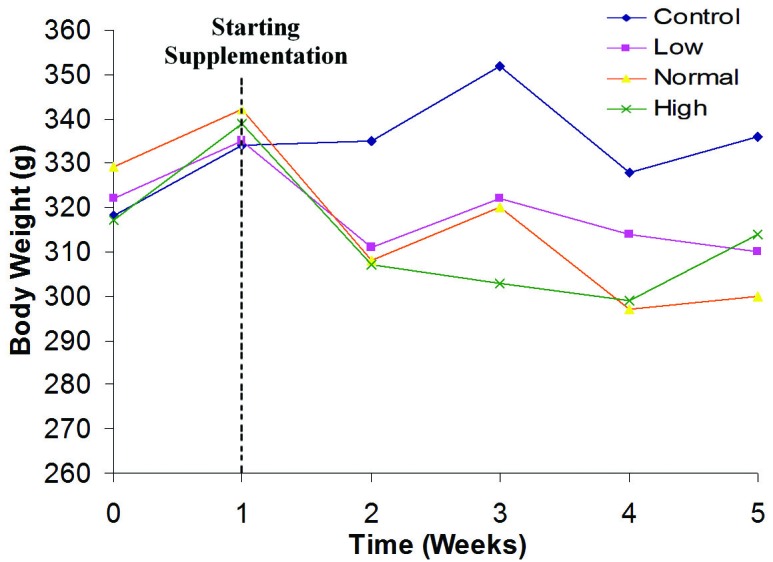
Changes in body weight of rats supplemented with various doses of Nigella sativa for 4 weeks.
Alanine Aminotransferase (ALT)
The results obtained in Figure 3 showed no significant decrease in serum ALT following supplementation with Nigella sativa. This small reduction of ALT was dose dependent. It means that consumption of high dose NS resulted in higher reduction of serum ALT level compared to other groups.
Figure 3 .
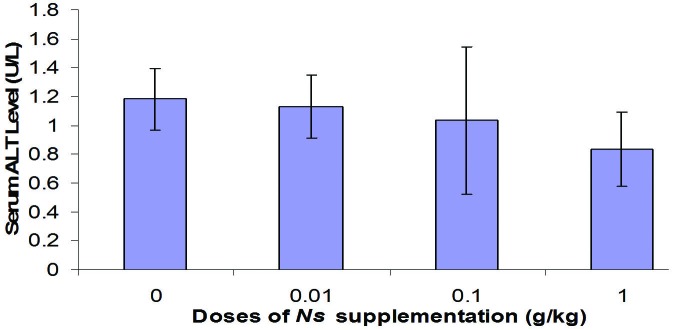
Changes in serum ALT level of rats supplemented with various doses of Nigella sativa for 4 weeks
Aspartate Aminotransferase (AST)
The results of liver function tests in this study revealed reduction in serum AST concentration in all NS group in comparison with control group, while the serum AST level was decreased in High dose NS administrated group more than other treatment group (Figure 4). It was indicated that AST level has not been affected by supplementation.
Figure 4 .
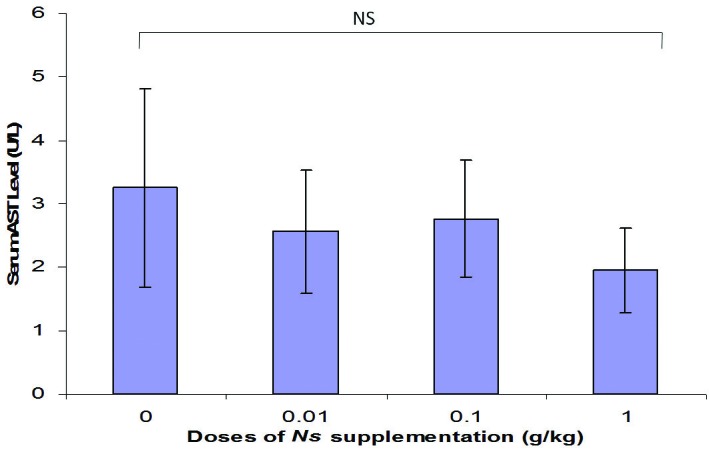
Changes in serum AST level of rats supplemented with various doses of Nigella sativa for 4 weeks
Histopatological Findin)
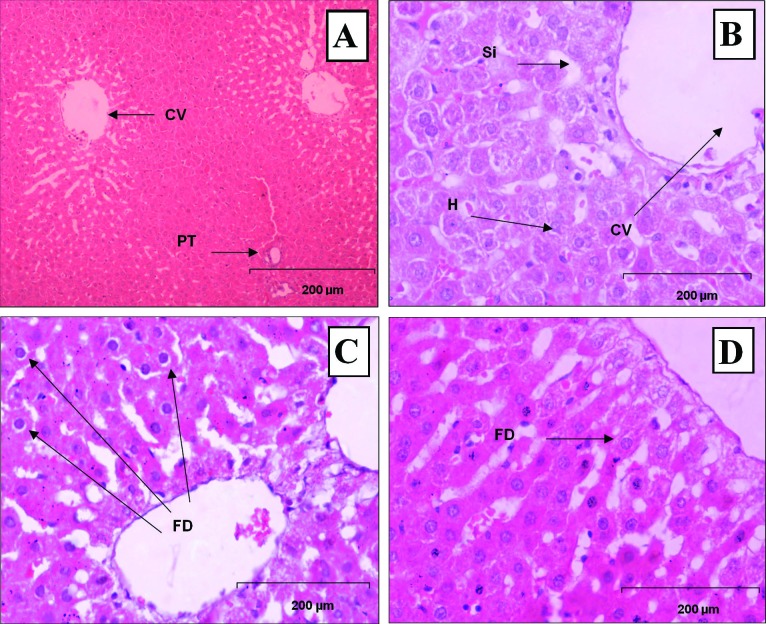
The results of liver histopathologic examination are shown in Table 1. The results of histopathologic examination of the liver showed no hepatic vacuolization, degeneration, inflammation and necrosis. Among treatment group, there were very minimal and mild fatty degeneration in the portal tracts of normal and high doses (0.1 g/kg and 1.0 g/kg body weight ) Nigella sativa treated rats as shown in Figure 5 (A, B, C, D).
Table 1. Histopathology scoring of liver tissue for rats supplemented with different doses of Nigella sativa supplementation for 28 days.
| Doses (g/kg) of Nigella sativa |
0.0 | 0.01 | 0.1 | 1 |
| Score | 0 1 2 3 4 | 0 1 2 3 4 | 0 1 2 3 4 | 0 1 2 3 4 |
| Hepatic vacuolization | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 |
| Hepatic degeneration | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 |
| Hepatic inflammation | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 |
| Necrosis | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 | 6 0 0 0 0 |
| Fatty degeneration | 0 0 0 0 0 | 0 5 1 0 0 | 0 5 1 0 0 | 0 2 4 0 0 |
Figure 5.
Histopathological section in the liver tissue of A) control group(0 g/kg NS), displaying central vein (CV) and portal triad (PT) B) Supplemented with Low dose NS(0.01 g/kg NS), displaying central vein (CV), sinusoid (Si), and hepatocyte (H), C) Supplemented with Normal Dose NS(0. 1 g/kg NS), displaying very minimal change, fatty degeneration (FD) was observed in normal doses treatment. Normal central vein (CV) and portal tract (PT) infiltrated with lymphocytes. D) Supplemented with High Dose NS (1 g/kg NS) displaying very minimal change, fatty degeneration (FD) was observed in high doses treatment. Normal central vein (CV) and portal tract (PT) infiltrated with lymphocytes. (H&E, X200).
Discussion
The present study was designed to investigate the toxicity effect of Nigella sativa on liver function evaluating Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and histopathological changes of liver. ALT is an enzyme normally present in liver and heart cells. When the liver or heart is damaged, ALT in blood will increased, and thus indicates liver or heart injury. During hepatocellular injury, enzymes which are normally located in the cytosol are released into the blood flow. Their qualification in plasma is useful biomarkers of the extent and type of hepatocellular damage.20 ALT and AST are used to assess the hepatocellular integrity of liver tissue. ALT is more predominantly found in liver while AST is normally found in equal amounts in the liver, heart, muscle, kidney and brain. Therefore, ALT is more liver-specific than AST. Normal range both for ALT and AST in human is 25 U/L to 50 U/L.21 From the biochemical study, it showed that the treatment doses (low dose 0.01 g/kg body weight, normal dose 0.1 g/kg body weight and high dose 1.0 g/kg body weight) of Nigella sativa supplementation reduced the liver enzymes (ALT and AST) level in treated rats compared to control group of rats. However, there was no significant different between dosage. The slowdown of body weight in Nigella sativa treated rats might be related to the liver enzyme (ALT and AST) level decrease, possibly effect of Nigella sativa treatment in rats with different doses for 28 days period.
The results of present study showed that the supplementation of Nigella sativa to the diets of rats for 28 days did not change the biochemical parameters of liver function as well as histopathological examinations which illustrated normal architecture of liver. It’s proved by no significant changes of serum ALT and AST level in treatment group compare to the control group. Absent of pathological condition of liver tissue in histological evaluation confirmed the result. This study also found that body weights of the rats in all groups are maintained during the experiment which indicating healthy status of animals.
In accordance, the oral administration of aqueous extract of Nigella sativa seeds showed no significant changes in liver function evaluating hepatic enzymes level as well as histopathological changes of liver tissue.22 Al Ammen and his colleagues16 reported no toxic effects of Nigella sativa on hepatic enzymes among asthmatic patients. Another studies also failed to show any toxicity for Nigella sativa fixed oil in mice.9,16
Our study showed that oral administration of Nigella sativa has no toxicity in the doses used. These results agree with previous data reporting that Nigella sativa has a wide margin of safety.2,2 Current study showed that Nigella sativa did not give any toxicity effect on liver to the parameters used, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The supplementations of Nigella sativa reduce the ALT level and AST level treated rats compared to the control doses of rats. This indicated that Nigella sativa dose up to 1.0 g/kg body weight showed no hepatocllular damage or hepatobiliary obstructive diseased and did not cause toxicity to the liver. Serum ALT and AST should increase as Itraconazole will induce liver damage.2 Histopathology examination showed very minimal and mild fatty degeneration change in the portal tracts in normal (0.1 g/kg) and high doses (1.0 g/kg body weight) Nigella sativa supplementation for 28 days. There are no hepatic vacuolization, degeneration, inflammation and necrosis in liver tissues. Therefore, histopathological changes can be due to environmental factors such as malnutrition or loss body weight. Almost of studies suggested a hypoprotective effects of Nigella sativa due to some components such as either thymoquinone and monoterpenes2 or tocopherols, phytosterols, and phenols.2 To the best of our knowledge two compounds of Nigella sativa probably exerted potent cytotoxic activity. These two compounds were found to be terpenoids, one of which showed presence of carbonyl functionality whereas the other showed the presence of both carbonyl and hydroxyl functionalities.
Conclusion
With the evidence of normal ALT and AST level in blood and normal liver tissue in histology examination for all treatment groups, it is suggested that there are no toxic effect on liver function of Nigella sativa at different doses for 4 weeks period. As a conclusion, popular consumption of Nigella sativa powder by human did not cause any toxicity effect on the liver function and safe to be consumed for many purposes.
Acknowledgments
The authors would like to thank the authorities of Faculty of Medicine and Health Sciences, University Putra Malaysia, for providing analytical facilities.
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.The World Medicine Situation 2011. 3xxsuprdxysup ed. Geneva: World Health Organization; 2011.
- 2.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR. et al. Anticancer drug discovery and development throughout the world. J Clin Oncol . 2002;20(18 Suppl):47S–59S. [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod . 2007;70(3):461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 4.WHO traditional medicine strategy 2002-2005. Geneva: World Health Organization; 2002.
- 5.Saad SI. Classification of flowering plants. 2xxsupndxysup ed. Alexandria: The general Egyptian Book Co; 1975. p.412–3.
- 6.Sayed MD. Traditional medicine in health care. J Ethnopharmacol . 1980;2(1):19–22. doi: 10.1016/0378-8741(80)90023-9. [DOI] [PubMed] [Google Scholar]
- 7.Nadkarni AK. Indian Materia Medica. Bombay: Popular Parkishan; 1976. p.854.
- 8.Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR. Potential immunomodulation effect of the extract of nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B . 2011;12(3):201–9. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaoui A, Cherrah Y, Lacaille-Dubois MA, Settaf A, Amarouch H, Hassar M. Diuretic and hypotensive effects of nigella sativa in the spontaneously hypertensive rat. Therapie . 2000;55(3):379–82. [PubMed] [Google Scholar]
- 10.Turkdogan MK, Agaoglu Z, Yener Z, Sekeroglu R, Akkan HA, Avci ME. The role of antioxidant vitamins (c and e), selenium and nigella sativa in the prevention of liver fibrosis and cirrhosis in rabbits: New hopes. Dtsch Tierarztl Wochenschr . 2001;108(2):71–3. [PubMed] [Google Scholar]
- 11.Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration/proliferation of the beta-cells in the islets of langerhans by nigella sativa lIn streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2003;201(4):213–9. doi: 10.1620/tjem.201.213. [DOI] [PubMed] [Google Scholar]
- 12.Babazadeh B, Sadeghnia HR, Safarpour Kapurchal E, Parsaee H, Nasri S, Tayarani-Najaran Z. Protective effect of Nigella sativa and thymoquinone on serum/glucose deprivation-induced DNA damage in PC12 cells. Avicenna Journal of Phytomedicine 2012:2(3):125-132. [PMC free article] [PubMed] [Google Scholar]
- 13.Parhizkar S, Latiff L, Rahman S, Dollah MA. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmacol . 2011;5(2):137–142. [Google Scholar]
- 14.Anwar MA. Nigella sativa: A bibliometric study of the literature on Habbatul-Barakah. Malays J Libr & Inf Sci . 2005;10(1):1–18. [Google Scholar]
- 15.Al Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Al-Yahya MA, Rafatullah S. et al. Gastroprotective effect of an aqueous suspension of black cumin nigella sativa on necrotizing agents-induced gastric injury in experimental animals. official journal of the Saudi J Gastroenterol . 2008;14(3): official journal of the Saudi J Gastroenterol 2008;14(3). doi: 10.4103/1319-3767.41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ghamdi MS. Protective effect of nigella sativa seeds against carbon tetrachloride-induced liver damage. Am J Chin Med . 2003;31(5):721–8. doi: 10.1142/S0192415X03001399. [DOI] [PubMed] [Google Scholar]
- 17.Ilhan N, Seçkin D. Protective effect of nigella sativa seeds on ccl4-induced hepatotoxicity. FÜ Sağlık Bil Dergisi . 2005;19(3):175–9. [Google Scholar]
- 18.Ezzat S, Daly EL. The effect of Nigella sativa seeds on certain aspects of carbohydrates and key hepatic enzymes in serum of rat. J Islamic Acad Sci . 1994;7(2):93–9. [Google Scholar]
- 19.Marieb EN. The digestive system. In Human anatomy and physiology, 6th ed. San Francisco: Pearson Education; 2004. P.912-5.
- 20.Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmacol Res . 2004;49(5):481–6. doi: 10.1016/j.phrs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Anderson SC, Cockayne S. Liver function. In: Clinical Chemistry: Concepts and Applications, New York: McGrow-Hill; 2003. P. 286-96.
- 22.Mohammed AK. Ameliorative effect of black seed (Nigella sativa L ) on the toxicity of aluminum in rabbits. Iraqi J Vet Med . 2010;34(2):110–6. [Google Scholar]
- 23.EL-Kholy WM, Hassan HA, Nour SE, Abe Elmageed ZE, Matrougui K. Hepatoprotective effects of Nigella sativa and bees’ honey on hepatotoxicity induced by administration of sodium nitrite and sunset yellow. FASEB J . 2009;23: 733. [Google Scholar]
- 24.Al Ameen NM, Altubaigy F, Jahangir T, Mahday IA, Mohammed EA, Musa OAA. Effect of Nigella sativa and bee honey on pulmonary, hepatic and renal function in Sudanese in Khartoum state. J Med Plants Res . 2011;5(31):6857–63. [Google Scholar]
- 25.Somchit N, Norshahida AR, Hasiah AH, Zuraini A, Sulaiman MR, Noordin MM. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: A comparative in vivo study. Hum Exp Toxicol . 2004;23(11):519–25. doi: 10.1191/0960327104ht479oa. [DOI] [PubMed] [Google Scholar]
- 26.El Tahir KE, Ashour MM, Al-Harbi MM. The respiratory effects of the volatile oil of the black seed (nigella sativa) in guinea-pigs: Elucidation of the mechanism(s) of action. Gen Pharmacol . 1993;24(5):1115–22. doi: 10.1016/0306-3623(93)90358-5. [DOI] [PubMed] [Google Scholar]
- 27.Ramadan MF, Kroh LW, Morsel JT. Radical scavenging activity of black cumin (nigella sativa l), coriander (coriandrum sativum l), and niger (guizotia abyssinica cass) crude seed oils and oil fractions. J Agric Food Chem. 2003;51(24):6961–9. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]