Abstract
Purpose: Fumaria parviflora Lam (Fumariaceae) has been used in traditional medicine in the treatment of several diseases such as diabetes. The present work was designed to evaluate the hypoglycaemic effects of methanolic extract (ME) of F. parviflora in normal and streptozotocin-induced diabetic rats. Methods: The rats used were allocated in six (I, II, III, IV, V and VI) experimental groups (n=5). Group I rats served as ‘normal control’ animals received distilled water and group II rats served as ‘diabetic control’ animals. Diabetes mellitus was induced in groups II, V and VI rats by intraperitoneal single injection of streptozotocin (STZ, 55 mg kg-1). Group V and VI rats were addi-tionally treated with ME (150 mg kg-1 day-1 and 250 mg kg-1 day-1, i.p. respectively) 24 hour post STZ injection, for seven consecutive days. Groups III and IV rats received only ME 150 mg kg-1 day-1 and 250 mg kg-1 day-1, i.p. respectively for seven days. The levels of blood glucose were determined using a Glucometer. Results: Administra-tion of F. parviflora extract showed a potent glucose lowering effect only on streptozo-tocin (STZ) induced diabetic rats below 100 mg/dl (P<0.001). However, no significant differences in the blood glucose levels were recorded between diabetic rats received 125 or 250 mg/kg of plant extracts. Conclusion: The findings of the study indicated that F. parviflora has significant hypoglycemic effect on STZ-induced diabetic rats with no effects on blood glucose levels of normal rats.
Keywords: Fumaria parviflora, Fumariaceae, Hypoglycemic, Streptozotocin, Anti diabetic
Introduction
Diabetes mellitusa metabolic disorder characterized by an inappropriate hyperglycemia caused by a relative or absolute deficiency of insulin or by a resistance to the action of insulin at the cellular level is found in all parts of the world.1 If diabetes is not being controlled effectively, the patient would have advanced risks of developing complications, like hypoglycemia, ketoaci-dosis, and nonketotic hyper-osmolar coma. The longer term complications might be cardiovascular disease, nerve damage, chronic kidney failure, retinal damage, and scanty healing of wounds, followed by gangrene on the feet leading to amputation.2-5 It has been well recognized that these complications bring about significant morbidity and mortality statistics world-wide, which is affecting negatively the quality of life in patients with diabetes.
From the patient’s perspective, the major objective is to design a regimen that will improve the progression of the disease complications. It has been considered very acceptable to include herbal or botanical extracts as a part of medical treatment. Although, herbs for diabetes treatment are not new and the plants and plant extracts since ancient times were used to combat diabetes, administration of herbal remedies in the case of diabetes diseases should certainly not be discounted.1,6-9, Accordingly, the present study was conducted to analyze one of the herbs, Fumaria parviflora, being employed traditionally by native people in the treatment of diabetes with an inadequate knowledge base.
Fumaria parviflora Lam. (Fumariaceae) an annual herbaceous plant that grows in wide variety parts of Iran, Pakistan and Turkey that has been reported to be used traditionally in dermatological diseases, in stimulation of liver function and gall bladder and also as antiscabies, antiscorbite, antibronchite, diuretic, expectorant, antipyretic, diaphoretic, appetizer and laxative.10 Studies dealing with the bioactivity and potential health benefits of F. parviflora in different aspects have been previously reported. The antinoiciceptive and histopathological effects of the percolated and soxhlet methanol extract of F. parviflora in formalin test was determined, especially at the late phase and hot-plate test in mice and rats.11 Various species of Fumaria have been studied by Orhan et al. which showed anticholinesterase activity for extracts of the aerial parts of F. parviflora.12 Moreover, phytochemical analyses of some plants of genus Fumaria, including F. parviflora has indicated presence of isoquinoline alkaloids.13-17 Elsewhere, the antipyretic activity of the hexane, chloroform and water-soluble extracts of F. parviflorain rabbits was verified.18 Akhtar et al. in 1984 published a paper in which they examined the blood glucose levels of the normal and alloxan-diabetic male albino rabbits after oral administration of various doses of the powdered F. parviflora and concluded that the plant contained some hypo-glycaemic properties.19 However, firm recommendations for general use of any herbal remedies needs detailed documentations of the glucose or insulin lowering effects of the plants in diabetes. Herein, we have evaluated the effect of the methanol extract of F. parvifloraon blood glucose level in normal and streptozotocin STZ-induced diabetic rats.
Materials and Methods
Plant Material
The aerial parts of Fumaria parviflora Lam were collected during June–July from Aharin East Azerbayjan province in Iran. A voucher specimen of the plant representing this collection has been retained in the herbarium of the Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran.
Preparation of Plant Extract
Aerial parts of plants (200 g) were air dried, powdered and extracted with maceration in chloroform and methanol (4×1L) consecutively and then evaporated in vacuo to afford chloroform and methanol extracts (ME). Since, in our preliminary study chloroform extract showed no effect on blood glucose level in normal and diabetic rats, the assessment of hypoglycemic activity of the plant set out on ME. Without any further purification, the crude methanol extract was refrigerated and subsequently used in this study. Aliquot portions of the crude ME residue was weighed and dissolved in distilled water for use on each day of our experiments.
Animals
Thirty male Wistar albino rats weighing 200-220 g were obtained from animal facility of pasture institute of Iran and were used in this survey. The animals were housed in stainless steel cages under controlled environmental conditions (temperature 25±5 ºC, with a light/dark cycle of 12 hours). The cage contained 5 rats and each rat had a tag number. Rats were allowed free access to concentrated animal food and water every day. Housing and caring conditions for the animals and the study protocols for the animal experiments were carried out in accordance with the internationally accepted principles and the national laws concerning the care and the use of laboratory animals.
Induction of Experimental Diabetes
Diabetes type 2 was induced with a single intra-peritoneal injection of a freshly prepared solution of streptozotocin (STZ) (55mg/kg), dissolved in cold citrate buffer (0.1M, pH 4.5), immediately before use due to the instability of STZ in aqueous media.
Measurement of Blood Glucose Levels
The blood was drawn from the tail and basal blood glucose levels were determined using an automated blood glucose analyzer (Glucometer One touch, Germany) prior to STZ injection and every 24h after STZ injection in groups II, V and VI and 1h after ME i.p. injection. Rats with blood glucose concentrations above 250 mg/dl were declared diabetic.
Experimental Designand Animal Grouping
The experimental animals were allowed 2-week acclimatization to laboratory environment and they were subsequently divided into 6 groups comprising of 5 animals in each group as follows: Group I, was considered as normal rats (distilled water-treated, Control), group II, diabetic control (STZ-treated), group III, extract-treated (normal + 125 mg/kg body weight/day of ME) rats, group IV extract-treated (normal + 250 mg/kg/day of ME) rats, group V, diabetic + 125 mg/kg/day of ME rats and group VI, diabetic + 250 mg/kg/day of ME rats. In groups V and VI administration of ME commenced 24h after injection of STZ and continued for the next 7 days.
Statistical Analysis
All the data are expressed as Mean ± S.E.M (standard error of means) and a probability level of P < 0.05 was taken to be statistically significant in the analyses.
Results
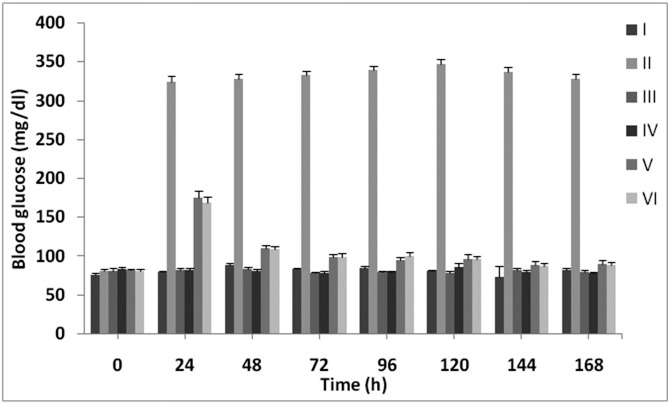
Blood glucose levels in different groups of control and experimental pre and after intra-peritoneal administration of STZ and plant extract were represented in Figure 1. In the control diabetic rats, blood glucose was reached maximum to its peak of about 346.88 mg/dl and remained high up to 192h during the experiment. Whereas, control group of normal rats, group I, did show no changes in mean±SEM blood glucose levels. Additionally, administration of ME in groups III and IV showed no effect on blood glucose levels when compared with normal rats. The findings of the study verified in diabetic treated rats with different concentrations of plant methanol extract (125 mg/kg and 250 mg/kg) levels of blood glucose were markedly controlled below 100 mg/dl at the end of the experiments. However, no significant differences in the blood glucose levels were recorded between diabetic rats received 125 or 250 mg/kg/day of plant extract and they both decreased the blood glucose levels, evidently.
Figure 1.
Effect of Methanol extract of F. parviflora on blood glucose levels in normal and diabetic rats. Data were given as mean ± SEM for five animals in each group.
* Groups [I: Normal controls, II: Diabetic controls, III: Normal + 125 mg/kg/day of extract, IV: Normal + 250 mg/kg/day of extract, V: Diabetic + 125 mg/kg/day of extract, VI: Diabetic + 250 mg/kg/day of extract].
Discussion
The use of medicinal plants in treatment of diseases have been an important part of medicinal therapy as observed for thousands of years contributing to scientific search of safer phytotherapeutic products, in this regard. Ever-increasing diabetes mellitus draw scores of researchers' attention to this phenomenon as a serious threat to mankind health in all parts of the world. In spite of the traditional use of botanicals in treatment of diabetes, paucity of definitive data on efficacy of these herbal remedies still deals a challenge in this field. In view of the fact that the diabetogenic effect of STZ is the direct consequence of irreversible damage to the pancreatic beta cells, resulting in degradation and loss of insulin secretion, STZ induced hyperglycemia has been recognized as a convenient experimental model to evaluate the activity of hypoglycemic agents.20,21 Intra-peritoneal administration of STZ (55 mg/kg) effectively induced diabetes in normal rats as reflected by hyperglycaemia when compared with normal rats.22-24 In our present study we have assessed the hypoglycemic activity of F. parviflora on normal and STZ induced diabetic rats.
The results revealed that F. parviflorahad no effect on the blood glucose level in normal rats (group III and IV). Therefore, it is possible to propose that in addition to insulin-like activity of the ME other mechanism such as alteration in the insulin-resistance pathway may be involved by ME. Hence, this pathway has no role in the normal animals, the extract was not able to change the blood glucose level in groups III and IV. Additionally, it can be suggested that insulin–resistance pathway in the peripheral tissues, possibly is superior to its insulin-like activity. However, this theory should be confirmed with further in vivo and in vitro studies.
Considering the results, administration of the plant extract exhibited a superb blood glucose lowering activity in diabetic rats providing an evidence for the merit of the traditional use of F. parviflora among the nations. In all probability, F. parviflora do appear to be helpful in diabetic patients as claimed and do have potential anti-diabetic effects in a rat model system of diabetes mellitus since the results are as striking in this study.
Conclusion
The present investigation is an evidence for the anti-diabetic activity of F. parviflora in STZ induced diabetic rats. The authors believe that F. parviflora could be considered as an excellent candidate for further studies on verifying the mechanisms of hypoglycemic activity, as well as for the isolation and identification of the foremost hypoglycemic phytochemical responsible for anti-diabetic activity of the plant. Besides, further comprehensive pharmacological surveys, involving experimental chronic studies, will be of value to assess the possible toxicological effects of this anti-diabetic plant.
Acknowledgments
Financial support of this study by the Research Vice-Chancellor of Tabriz University of Medical Sciences is faithfully appreciated.
Conflict of Interest
The authors report no conflicts of interest in this work.
References
- 1.Samad A, Shams MS, Ullah Z, Wais M, Nazish I, Sultana Y. et al. Status of herbal medicines in the treatment of diabetes: A review. Curr Diabetes Rev . 2009;5(2):102–11. doi: 10.2174/157339909788166837. [DOI] [PubMed] [Google Scholar]
- 2.Santiago JV. Overview of the complications of diabetes. Clin Chem . 1986;32(10 Suppl):B48–53. [PubMed] [Google Scholar]
- 3.Lundman B, Engstrom L. Diabetes and it's complications in a swedish county. Diabetes Res Clin Pract . 1998;39(2):157–64. doi: 10.1016/s0168-8227(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 4.Detournay B, Simon D, Guillausseau PJ, Joly D, Verges B, Attali C. et al. Chronic kidney disease in type 2 diabetes patients in france: Prevalence, influence of glycaemic control and implications for the pharmacological management of diabetes. Diabetes Metab . 2012;38(2):102–12. doi: 10.1016/j.diabet.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Losito A, Pittavini L, Ferri C, De Angelis L. Reduced kidney function and outcome in acute ischaemic stroke: Relationship to arterial hypertension and diabetes. Nephrol Dial Transplant . 2012;27(3):1054–8. doi: 10.1093/ndt/gfr378. [DOI] [PubMed] [Google Scholar]
- 6.Ceylan-Isik AF, Fliethman RM, Wold LE, Ren J. Herbal and traditional chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr Diabetes Rev . 2008;4(4):320–8. doi: 10.2174/157339908786241142. [DOI] [PubMed] [Google Scholar]
- 7.Wang E, Wylie-Rosett J. Review of selected chinese herbal medicines in the treatment of type 2 diabetes. Diabetes Educ . 2008;34(4):645–54. doi: 10.1177/0145721708320559. [DOI] [PubMed] [Google Scholar]
- 8.Suksomboon N, Poolsup N, Boonkaew S, Suthisisang CC. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. J Ethnopharmacol . 2011;137(3):1328–33. doi: 10.1016/j.jep.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev . 2004;3:CD003642. doi: 10.1002/14651858.CD003642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilani AH, Bashir S, Janbaz KH, Khan A. Pharmacological basis for the use of fumaria indica in constipation and diarrhea. J Ethnopharmacol . 2005;96(3):585–9. doi: 10.1016/j.jep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Heidari M, Mandgary A, Enayati M. Antinociceptive effects and toxicity of fumaria parviflora lam. In mice and rats. Daru . 2004;12(4):136–40. [Google Scholar]
- 12.Orhan I, Sener B, Choudhary MI, Khalid A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some turkish medicinal plants. J Ethnopharmacol . 2004;91(1):57–60. doi: 10.1016/j.jep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Suau R, Cabezudo B, Rico R, Najera F, Lopez-Romero JM. Direct determination of alkaloid contents in fumaria species by gc-ms. Phytochem Anal . 2002;13(6):363–7. doi: 10.1002/pca.669. [DOI] [PubMed] [Google Scholar]
- 14.Popova ME, Simanek V, Dolejs L, Smysl B, Preininger V. Alkaloids from fumaria parviflora and f. Kralikii. Planta Med . 1982;45(2):120–2. doi: 10.1055/s-2007-971259. [DOI] [PubMed] [Google Scholar]
- 15.Valka I, Walterova D, Popova ME, Preininger V, Simanek V. Separation and quantification of some alkaloids from fumaria parviflora by capillary isotachophoresis1. Planta Med . 1985;51(4):319–22. doi: 10.1055/s-2007-969501. [DOI] [PubMed] [Google Scholar]
- 16.Kirjakov H, Panov P. On the alkaloids of fumaria parviflora lam. Folia Med (Plovdiv) . 1974;16(2):101–3. [PubMed] [Google Scholar]
- 17.Valka I, Simanek V. Determination of alkaloids of fumaria parviflora and fumaria capreolata by high-performance liquid chromatography and capillary isotachophoresis. J Chromatogr . 1988;445(1):258–63. doi: 10.1016/s0021-9673(01)84530-1. [DOI] [PubMed] [Google Scholar]
- 18.Khattak SG, Gilani SN, Ikram M. Antipyretic studies on some indigenous pakistani medicinal plants. J Ethnopharmacol . 1985;14(1):45–51. doi: 10.1016/0378-8741(85)90027-3. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar MS, Khan QM, Khaliq T. Effects of euphorbia prostrata and fumaria parviflora in normoglycaemic and alloxan-treated hyperglycaemic rabbits. Planta Med . 1984;50(2):138–42. doi: 10.1055/s-2007-969653. [DOI] [PubMed] [Google Scholar]
- 20.Szkudelski T. The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas. Physiol Res . 2001;50(6):537–46. [PubMed] [Google Scholar]
- 21.Soon YY, Tan BK. Evaluation of the hypoglycemic and anti-oxidant activities of morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J . 2002;43(2):077–85. [PubMed] [Google Scholar]
- 22.Perez Gutierrez RM. Evaluation of hypoglycemic activity of the leaves of malva parviflora in streptozotocin-induced diabetic rats. Food Funct . 2012;3(4):420–7. doi: 10.1039/c2fo10153j. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesan T, Sorimuthu Pillai S. Antidiabetic activity of gossypin, a pentahydroxyflavone glucoside, in streptozotocin-induced experimental diabetes in rats. J Diabetes . 2012;4(1):41–6. doi: 10.1111/j.1753-0407.2011.00145.x. [DOI] [PubMed] [Google Scholar]
- 24.Jana K, Chatterjee K, Ali KM, De D, Bera TK, Ghosh D. Antihyperglycemic and antioxidative effects of the hydro-methanolic extract of the seeds of caesalpinia bonduc on streptozotocin-induced diabetes in male albino rats. Pharmacognosy Res . 2012;4(1):57–62. doi: 10.4103/0974-8490.91044. [DOI] [PMC free article] [PubMed] [Google Scholar]