Abstract
Purpose: As a typical Chinese herbal medicine, Citri reticulatae pericarpium (CRP) possesses various pharmacological effects involved in antioxidant ability. However, its antioxidant effects have not been reported yet. The objective of this work was to investigate its antioxidant ability, then further discuss the antioxidant mechanism. Methods: CRP was extracted by ethanol to obtain ethanol extract of Citri reticulatae pericarpium (ECRP). ECRP was then measured by various antioxidant methods, including DNA damage assay, DPPH assay, ABTS assay, Fe3+-reducing assay and Cu2+-reducing assay. Finally, the content of total flavonoids was analyzed by spectrophotometric method. Results: Our results revealed that ECRP could effectively protect against hydroxyl-induced DNA damage (IC50 944.47±147.74 μg/mL). In addition, it could also scavenge DPPH· radical (IC50349.67±1.91 μg/mL) and ABTS+• radical (IC5011.33±0.10 μg/mL), reduce Fe3+ (IC50 140.95±2.15 μg/mL) and Cu2+ (IC50 70.46±1.77 μg/mL). Chemical analysis demonstrated that the content of total flavonoids in ECRP was 198.29±12.24 mg quercetin/g. Conclusion: Citri reticulatae pericarpium can effectively protect against hydroxyl-induced DNA damage. One mechanism of protective effect may be radical-scavenging which is via donating hydrogen atom (H·), donating electron (e). Its antioxidant ability can be mainly attributed to the flavonoids, especially hesperidin and narirutin.
Keywords: Citri reticulatae pericarpium, Antioxidant activity, DNA oxidative damage, Chenpi, Hesperidin, Narirutin
Introduction
Reactive oxygen species (ROS) include hydroxyl radical (·OH), superoxide anion (·O2-), hydrogen peroxide (H2O2), and nitric oxide (NO), with the ·OH being the most harmful. All macromolecules are sensitive to free radical damage. For example, DNA can be easily damaged by ·OH then lead to severe biological consequences including mutation, cell death, carcinogenesis, and aging.1
Therefore, it is vital to search for potential therapeutic agents for DNA oxidative damage. Over the last two decades, much attention has been focused on the antioxidant of medicinal plants especially Chinese medicinal herbals.
As a typical Chinese herbal medicine, Citri reticulatae pericarpium (CRP, 陈皮 in Chinese, Figure 1A) has been used in traditional Chinese medicine (TCM) for about 2000 years2,3 CRP is the dried and ripe pericarpium of Citri reticulatae Blanco (Figure 1B). From the viewpoint of TCM, CRP can invigorate spleen, replenish qi, eliminate dampness and phlegm.3
Figure 1.

Dried Citri reticulatae pericarpium (A) and its plant Citri reticulatae Blanco (B)
Figure 1A was contributed by Weikang Chen, Figure 1B was contributed by www.plantphoto.cn
Modern medicine has demonstrated that CRP possessed various pharmacological effects. For example, the extract from CRP could induce the apoptosis on SNU-C4 (human colon cancer cells) via Bax-related caspase-3 activation.4 Ou reported that the extract from CRP could protect rats against myocardial ischemia.5 Fang pointed out that CRP had antibacterial action.6 Fan indicated an insecticidal effect of CRP.7 In addition, CRP was also proved to be of anti-ulcer and anti-inflammatory.8 However, according to free radical biology & medicine,9 all these pharmacological effects may be associated with antioxidant ability.
Until now, its antioxidant ability has not been reported. Therefore, the purpose of the study was to investigate the antioxidant ability, then further discuss the antioxidant mechanism.
Materials and Methods
Plant Material
Citri reticulatae pericarpium was purchased from Caizhilin pharmacy located in Guangzhou University of Chinese Medicine (Guangzhou, China), and authenticated by Professor Shuhui Tan. A voucher specimen was deposited in our laboratory.
Chemicals
DPPH• (1,1-diphenyl-2-picryl-hydrazl radical), ABTS [2,2′-azino-bis(3-ethylbenzo- thiazoline-6-sulfonic acid diammonium salt)], BHA (butylated hydroxyanisole), Trolox [(±)-6- hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid], DNA sodium salt (fish sperm), and neocuproine (2,9-dimethyl-1,10-phenanthroline) were purchased from Sigma Co. (Sigma-Aldrich Shanghai Trading Co., China). Other chemicals used in this study were of analytic grade.
Preparation of Extracts from Citri reticulatae Pericarpium
Citri reticulatae pericarpium was powdered then extracted by absolute ethanol using a Soxhlet extractor for 6 hr. The extract was filtered using a Buckner funnel and Whatman No 1 filter paper. The filtrate was then concentrated to dryness under reduced pressure to yield ECRP (ethanol extract of Citri reticulatae pericarpium). It was stored at 4°C for analysis.
Protective Effect against Hydroxyl-Induced DNA Damage
The experiment was conducted as described in previous report.10 However, deoxyribose was replaced by DNA sodium. Briefly, sample was dissolved in methanol at 8 mg/mL. Various amounts (20-100 μL) of sample methanolic solutions were then separately taken into mini tubes. After evaporating the sample solutions in tubes to dryness, 400 μL of phosphate buffer (0.2 mol/L, pH 7.4) was added to the sample residue. Subsequently, 50 μL DNA sodium (10.0 mg/mL), 50 μL H2O2(50 mmol/L), 50 μL FeCl3(3.2 mmol/L) and 50 μL Na2EDTA (1 mmol/L) were added. The reaction was initiated by adding 50 μL ascorbic acid (18 mmol/L) and the total volume of the reaction mixture was adjusted to 800 μL with buffer. After incubation in a water bath at 55 °C for 20 min, the reaction was terminated by adding 250 μL trichloroacetic acid (10g/100mL water). The color was then developed by addition of 150 μL of TBA (2-thiobarbituric acid)(0.4 mol/L, in 1.25% NaOH aqueous solution) and heating in an oven at 105 °C for 15 min. The mixture was cooled and absorbance was measured at 530 nm against the buffer (as blank). The percent of protection against DNA damage is expressed as follows:
Protective effect % = (1 - A/A0)×100%
Where A0 is the absorbance of the mixture without sample, and A is the absorbance of the mixture with sample.
DPPH• Radical-Scavenging Assay
DPPH• radical-scavenging activity was determined as previously described by Li.11 Briefly, 1 mL DPPH• ethanolic solution (0.1 mM) was mixed with 0.5 mL sample alcoholic solution (4.0 mg/mL). The mixture was kept at room temperature for 30 min, and then measured with a spectrophotometer (Unico 2100, Shanghai, China) at 519 nm. The DPPH• inhibition percentage was calculated as:
Inhibition % = (1 - A/A0)×100%,
where A is the absorbance with sample, while A0 is the absorbance without sample.
ABTS+• Radical-Scavenging Assay
The ABTS+• -scavenging activity was measured as described12 with some modifications. The ABTS+• was produced by mixing 0.35 mL ABTSdiammonium salt (7.4 mmol/L) with potassium 0.35 mL persulfate (2.6 mmol/L). The mixture was kept in the dark at room temperature for 12 h to allow completion of radical generation, then diluted with 95% ethanol (about 1:50) so that its absorbance at 734 nm was 0.70 ± 0.02. To determine the scavenging activity, 1.2 mL aliquot of diluted ABTS+• reagent was mixed with 0.3 mL of sample ethanolic solution (0.08-0.4 mg/mL). After incubation for 6 min, the absorbance at 734 nm was read on a spectrophotometer (Unico 2100, Shanghai, China). The percentage inhibition was calculated as:
Inhibition % = (1 - A/A0)×100%
Here, A0 is the absorbance of the mixture without sample, A is the absorbance of the mixture with sample.
Reducing Power (Fe3+) Assay
Ferric (Fe3+) reducing power was determined according to the method of Oyaizu.13 In brief, sample solution x μL (2 mg/mL, x = 30, 60, 90, 120, and 150) was mixed with (350-x) μL Na2HPO4/KH2PO4 buffer (0.2 mol/L, pH 6.6) and 250 μL K3Fe(CN)6 aqueous solution (1 g/100 mL). After incubation at 50 °C for 20 min, the mixture was added by 250 μL of trichloroacetic acid (10 g/100 mL), then centrifuged at 3500 r/min for 10 min. As soon as 400 μL supernatant was aliquoted into 400 μL FeCl3 (0.1 g/100 mL in distilled water), the timer was started. At 90 s, absorbance of the mixture was read at 700 nm (Unico 2100, Shanghai, China). Samples were analyzed in groups of three, and when the analysis of one group has finished, the next group of three samples was aliquoted into FeCl3 to avoid oxidization by air. The relative reducing ability of the sample was calculated by using the formula:
Relative reducing effect % = (A-Amin)/(Amax-Amin)×100%
Here, Amax is the maximum absorbance and Amin is the minimum absorbance in the test. A is the absorbance of sample.
Cu2+-Reducing Power Assay
The Cu2+-reducing capacity was determined by the method,14 with minor modifications. Briefly, 125 μL CuSO4 aqueous solution (0.01 mol/L), 125 μL neocuproine ethanolic solution (7.5 mmol/L) and (750-x) μL CH3COONH4 buffer solution (0.1 mol/L, pH 7.5) were brought to test tubes. Then, different volumes of samples (2mg/mL, x = 40-120 μL) were added to the tubes. Then, the total volume was adjusted to 1000 μL with the buffer and mixed vigorously. Absorbance against a buffer blank was measured at 450 nm after 30 min (Unico 2100, Shanghai, China). The relative reducing power of the sample as compared with the maximum absorbance, was calculated by the formula:
Relative reducing effect % = (A-Amin)/ (Amax-Amin) ×100%
where, Amax is the maximum absorbance at 450 nm and Amin is the minimum absorbance in the test. A is the absorbance of sample.
Determination of Total Flavonoids
The content of total flavonoids was measured using the NaNO2 -Al (NO3)3 method.15 In brief, 0.05mL sample methanolic solution (20 mg/mL) was mixed with 0.15 mL NaNO2 aqueous solution (5%, w/w). The mixture stood for 6 min, followed by the addition of 0.15 mL Al (NO3)3 aqueous solution(10%, w/w). After incubation at ambient temperature for 6 min, 2 mL NaOH aqueous solution (4%, w/w) was added to the mixture which was then adjusted to 5 mL with distilled water. The absorbance was read at 508 nm on a spectrophotometer (Unico 2100, Shanghai, China). The standard curve was prepared using different concentrations of quercetin and the results were also expressed as quercetin in milligrams per gram extract.
Statistical Analysis
Data are given as the mean ± SD of three measurements. The IC50 values were calculated by linear regression analysis. All linear regression in this paper was analyzed by Origin 6.0 professional software. Significant differences were performed using the T-test (p < 0.05). The analysis was performed using SPSS software (v.12, SPSS, USA).
Results and Discussion
It is well known that hydroxyl radical (•OH) is generated in human body via Fenton reaction. Since •OH radical has extreme reactivity, it can easily damage DNA to produce malondialdehyde (MDA) and various oxidative lesions.16,17 MDA combines TBA (2-thiobarbituric acid) to produce TBARS (thiobarbituric acid reactive substances) which present a maximum absorbance at 530 nm.18 On the other hand, as the oxidative lesions mentioned above have no conjugative system in the molecules (Figure 2), they cannot be detected by a spectrophotometer at 530 nm. It means that these oxidative lesions can bring about no interference with the determination of MDA.
Figure 2 .
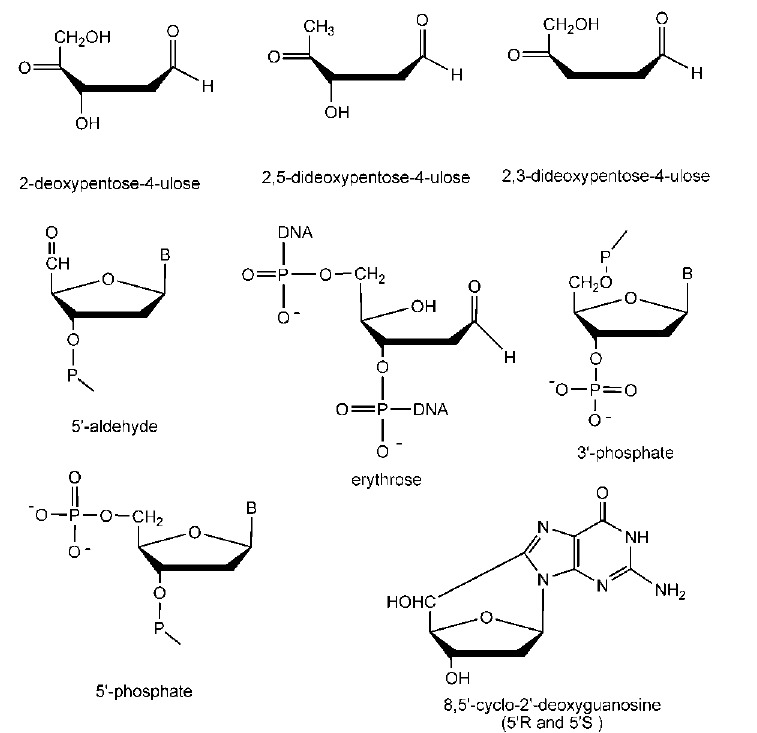
The structures of some oxidative lesions.
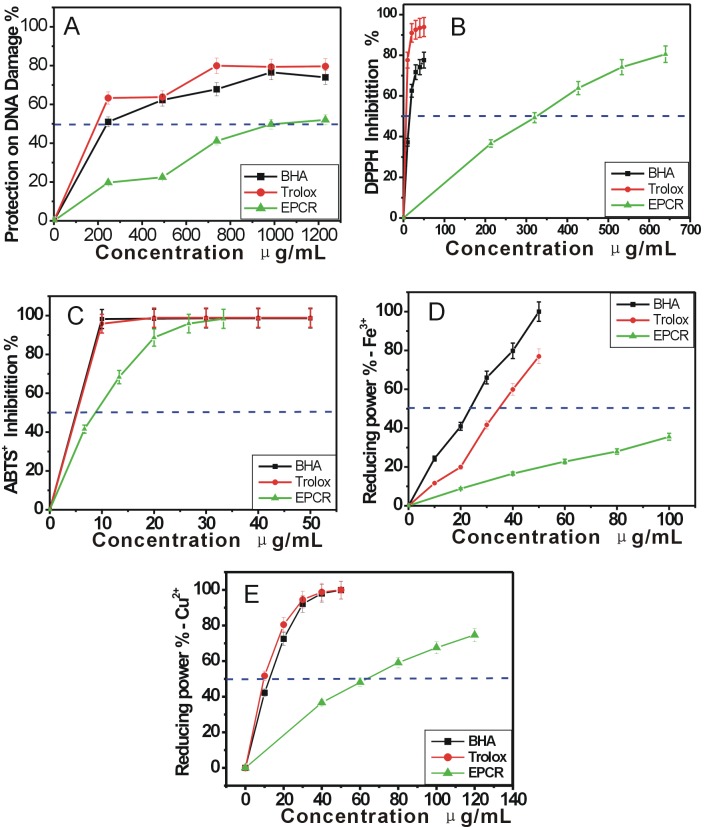
Hence, the value of A532nm can evaluate the amount of MDA, and ultimately reflect the extent of DNA damage. Based on the formula “protective effect % = (1 - A/A0) ×100%”, it can be deduced that the decrease of A530nmvalue indicates a protective effect against DNA damage. As seen in Figure 3A, ECRP dose-dependently increased the protective effect against DNA damage from 0-1240 μg/mL and its IC50 value was 944.47±147.74 μg/mL (Table 1).
Figure 3.
The dose response curves of ECRP in the antioxidant assays: (A) protective effect against DAN damage; (B)DPPH· scavenging; (C)ABTS+· scavenging (D) Fe-reducing; (E) Cu2+-reducing.
ECRP, absolute ethanol extract of Citri reticulatae pericarpium. Trolox and BHA (butylated hydroxyanisole) were used as the positive controls. Each value is expressed as Mean±SD (n=3).
Table 1. The IC50 values of ethanol extract from Citri reticulatae pericarpium (ECRP) (μg/mL).
| ECRP | Positive controls | ||
| Trolox | BHA | ||
| Protecting DNA damage | 944.47±147.74c | 306.13±26.11a | 344.89±30.28b |
| DPPH· scavenging | 349.67±1.91c | 9.75±0.06a | 22.35±0.58b |
| ABTS+· scavenging | 11.33±0.10b | 5.09±0.02a | 5.21±0.25a |
| Fe3+-reducing | 140.95±2.15c | 34.58±1.45b | 22.88±1.03a |
| Cu2+-reducing | 70.46±1.77c | 13.82±0.30a | 16.09±0.47b |
| IC50 value is defined as the concentration of 50% effect percentage and expressed as Mean±SD (n=3). Means values with different superscripts in the same row are significantly different (p<0.05), while with same superscripts are not signifiacntly different (p<0.05). BHA , butylated hydroxyanisole. | |||
Previous works have shown that there were two approaches for natural antioxidant to protect DNA oxidative damage: one was to scavenge the •OH radicals then to reduce its attack; one was to fast repair the deoxynucleotide radical cations which were damaged by •OH radicals.19 To further confirm whether the protective effect of ECRP was associated with its radical-scavenging ability, we determined the DPPH· and ABTS+· radical-scavenging abilities.
The DPPH and ABTS assays have been widely used to determine the free radical-scavenging activity of various plants and pure compounds. Both DPPH• and ABTS+· are stable free radicals which dissolve in methanol or ethanol, and their colors show characteristic absorptions at 519 nm or 734 nm, respectively. When an antioxidant scavenges the free radicals, the values of A519nm or A734nm will decrease. On this basis, the inhibition percentages were defined as: inhibition % = (1 - A/A0)×100%.
As can be seen in Figure 3B, ECRP can effectively inhibit DPPH• radical from 0-648 μg/mL and its IC50 was 349.67±1.91 μg/mL ( Table 1). The previous study suggested that DPPH· may be scavenged by an antioxidant through donation of hydrogen atom (H·) to form a stable DPPH-H molecule.20 For example, hesperidin which occurred in Citri reticulatae pericarpium,21 may scavenge DPPH• via the following proposed reaction22,23 (Figure 4).
Figure 4.
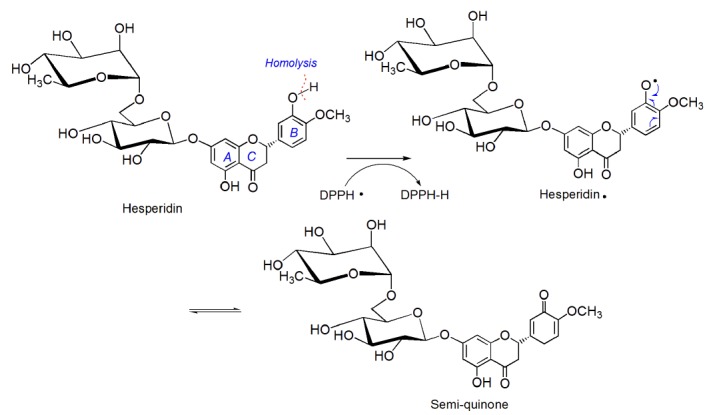
The proposed reaction of hesperidin with DPPH•.
The data in Figure 3C indicated that ECRP could also scavenge ABTS+· in a dose-dependent manner (0-35μg/mL) and its IC50 was 11.33±0.10μg/mL (Table 1). However, ABTS·+ scavenging is regarded as an electron (e) transfer reaction.24
The fact that ECRP can effectively scavenge both DPPH· and ABTS+·radicals, suggests that: (1) the protective effect of ECRP against DNA oxidative damage was associated with its radical-scavenging ability; (2) ECRP exerted its radical-scavenging action by donating hydrogen atom (H·) and electron (e).
Although a reductant is not necessarily an antioxidant, an antioxidant is commonly a reductant.25 The reducing power of an antioxidant may therefore serve as a significant indicator of its potential antioxidant activity.26 Figure 3D&3E showed that ECRP exhibited its reducing powers on Fe3+ and Cu2+in a concentration dependent manner. The IC50 values were 140.95±2.15 µg/mL & 70.46±1.77 µg/mL, respectively for Fe3+-reducing and Cu2+-reducing) (table 1). Obviously, these data further support the findings mentioned above.
Previous studies have shown that flavonoids can be responsible for the antioxidant ability in plants, we then determined the content of total flavonoids in ECRP. Our results indicated a high amount of total flavonoids (198.29±12.24 mg quercetin/g) in ECRP. In fact, at least 9 flavonoids have been isolated from Citri reticulatae pericarpium until now, including hesperidin,27 narirutin,27 nobiletin,27 tangeretin,27 natsudaidain,21 3,5,6,7,8,3', 4'-heptamethoxylflavones,27 5-hydroxyl-6,7,8,3',4'–pentamethoxylflavone,28 5,6,7,8, 4'–pentamethoxylflavone,28 and 5,6,7,8,3',4'–hexamethoxylflavone (Figure 5).28 Among them, hesperidin and narirutin presented much higher content that the others in CRP.27 Therefore, hesperidin and narirutin were regarded as two main active components of antioxidant in CRP.
Figure 5.
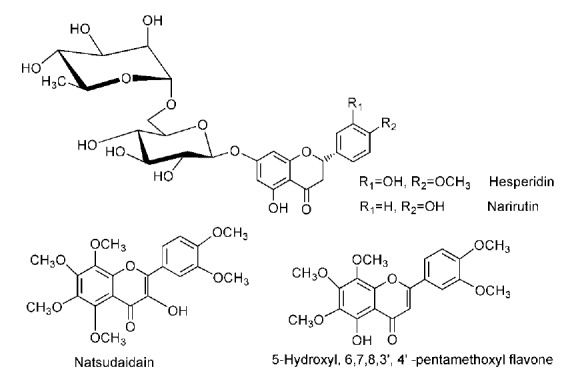
The structures of some flavonoids in Citri reticulatae pericarpium.
Conclusion
As a typical Chinese herbal medicine, Citri reticulatae pericarpium can effectively protect against hydroxyl-induced DNA damage. One mechanism of protective effect may be radical-scavenging which is via donating hydrogen atom (H·), donating electron (e). Its antioxidant ability can be mainly attributed to flavonoids (especially hesperidin and narirutin).
Conflict of Interest
The authors declare there is no Conflict of interest in the content of this study.
References
- 1.Bhattacharjee S, Deterding LJ, Chatterjee S, Jiang J, Ehrenshaft M, Lardinois O. et al. Site-specific radical formation in DNA induced by cu(ii)-h2o2 oxidizing system, using esr, immuno-spin trapping, lc-ms, and ms/ms. Free Radic Biol Med . 2011;50(11):1536–45. doi: 10.1016/j.freeradbiomed.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luan YJ, Hou WS. The Divine Farmer’s Materia Medica. China: People's Medical Press; 2010. [Google Scholar]
- 3.China pharmacopoeia committee. Pharmacopoeia of the people's republic of China. China: Chemical Industry Press; 2005. [Google Scholar]
- 4.Kang SA, Park HJ, Kim MJ, Lee SY, Han SW, Leem KH. Citri reticulatae Viride Pericarpium extract induced apoptosis in SNU-C4, human colon cancer cells. J Ethnopharmacol . 2005;97(2):231–5. doi: 10.1016/j.jep.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Ou LJ, Sun XP, Liu QD, Mi SQ, Wang NS. Effects of rhizoma zingiberis and pericarpium citri reticulatae extracts on myocardial ischemia in rats. Zhong Yao Cai . 2009;32(11):1723–6. [PubMed] [Google Scholar]
- 6.Fang YF, Wei YP, Ding XA. Tangerine peel on the the superficial fungal test tube the antibacterial experiments and clinical efficacy observed. Chin J Dermatol Venereol . 1997;11(5):275. [Google Scholar]
- 7.Fan J, Ding ZM. The tangerine peel several citrus bark extract on aphids, mites, worms insecticidal activity preliminary study. Tradit Chin Med . 1995;20(7):397–8. [PubMed] [Google Scholar]
- 8.Yu H, Li CX, Gan QX. Pharmacological effects of Citrus. Biomagnetism . 2005;5:44–5. [Google Scholar]
- 9.Zheng RL, Huang ZY. Free radical biology. 3rd Ed. China: Higher Education Press; 2007. [Google Scholar]
- 10.Wang X, Li X, Chen D. Evaluation of antioxdiant activity of isoferulic acid in vitro. Nat Prod Commun . 2011;6:1285–8. [PubMed] [Google Scholar]
- 11.Li X, Chen C. Systematic Evaluation on Antioxidant of Magnolol in vitro. Int Res J Pure Appl Chem . 2012;2(1):68–76. [Google Scholar]
- 12.Gao Y, Hu Q, Li X. Chemical composition and antioxidant activity of essential oil from Syzygium samarangense (BL.) Merr.et Perry flower-bud. Spatula DD . 2012;2(1):23–33. [Google Scholar]
- 13.Oyaizu M. Studies on product of browning reaction prepared from glucoseamine. Jpn J Nutr . 1986;44:307–15. [Google Scholar]
- 14.Li X, Wang X, Chen D, Chen S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct Foods Health Dis . 2011;7:232–44. [Google Scholar]
- 15.Li XC, Chen D, Mai Y, Wen B, Wang X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi) Nat prod Res . 2012;26(11):1050–3. doi: 10.1080/14786419.2010.551771. [DOI] [PubMed] [Google Scholar]
- 16.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic Biol Med . 2002;32(11):1102–15. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 17.Jaruga P, Rozalski R, Jawien A, Migdalski A, Olinski R, Dizdaroglu M. DNA damage products (5'r)- and (5's)-8,5'-cyclo-2'-deoxyadenosines as potential biomarkers in human urine for atherosclerosis. Biochemistry . 2012;51(9):1822–4. doi: 10.1021/bi201912c. [DOI] [PubMed] [Google Scholar]
- 18.Cheeseman KH, Beavis A, Esterbauer H. Hydroxyl-radical-induced iron-catalysed degradation of 2-deoxyribose. Quantitative determination of malondialdehyde. Biochem J . 1988;252(3):649–53. doi: 10.1042/bj2520649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang RL, Zheng Y, Huang R. Theory and application of free radical biology. China: Science Press; 2002. [Google Scholar]
- 20.Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT-Food Sci Technol . 1997;30(6):609–15. [Google Scholar]
- 21.Qian SH, Brand-Williams W, Chen L. Tangerine peel flavonoids in the study. Chin Herb Med. 1998;6:57–9. [Google Scholar]
- 22.Tsimogiannis DI, Oreopoulou V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′,4′-hydroxy substituted members. Innov Food Sci Emerg Technol . 2006;7(1-2):140–6. [Google Scholar]
- 23.Khanduja KL, Bhardwaj A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J Biochem Biophys . 2003;40(6):416–22. [PubMed] [Google Scholar]
- 24.Aliaga C, Lissi EA. Reaction of 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radicals with hydroperoxides: Kinetics and mechanism. Int J Chem Kinet . 1998;30:565–70. [Google Scholar]
- 25.Prior RL, Cao G. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radic Biol Med . 1999;27(11-12):1173–81. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 26.Jung MJ, Heo SI, Wang MH. Free radical scavenging and total phenolic contents from methanolic extracts of Ulmus davidiana. Food Chem . 2008;108(2):482–7. doi: 10.1016/j.foodchem.2007.10.081. [DOI] [PubMed] [Google Scholar]
- 27.Feng YF, Zhang HW, Zou ZM, Sun CH. HPLC simultaneous determination of contents of five flavonoids in Pericarpium Citri Reticulatae. Pharm Anal mag . 2009;20:47–8. [Google Scholar]
- 28.Zhang ZH, Wang CY, Yang TM, Zhou JB, Huang YB. The tangerine peel chemical composition and pharmacological research. Northwest Pharm J . 2005;29:10–5. [Google Scholar]