Abstract
Purpose: In the present study in vivo analgesic activity of some previously synthesized 1,2,4-triazole derivatives containing pyrazole, tetrazole, isoxazole and pyrimidine ring have been evaluated. Methods: Acetic acid induced writhing method and Hot plate method has been described to study analgesic activity of some 1,2,4-triazole derivatives containing pyrazole, tetrazole, isoxazole and pyrimidine as a pharmacological active lead. Results: Thirty six different derivatives containing 1,2,4-triazole ring were subjected to study their in vivo analgesic activity. Chloro, nitro and methoxy, hydroxy and bromo substituted derivatives showed excellent analgesic activity and dimethylamino, furan and phenyl substituted derivatives showed moderate analgesic activity in both of the methods. Compounds IIIa, IIId, IIIf, IIIi, IIIj, IVa, IVb, IVd, IVf, IVh, IVj IV3a and IIj were found to be superior analgesic agents after screening by Acetic acid induced writhing method. Compounds IIIb, IIId, IIIf, IIIh, IIIj, IVa, IVb, IVd, IVf, IVh, IVi, IV3c, IV3e and IIj were showed analgesic potential after screening of Hot plate method. Conclusion: All tested compounds containing 1,2,4-triazole were found to be promising analgesic agents, for this activity pyrazole, tetrazole, isoxazole and pyrimidine leads might be supported.
Keywords: Triazole, Analgesic Activity, Pyrazole, Tetrazole, Isoxazole, Pyrimidine
Introduction
Analgesic and anti-inflammatory drugs are one of the most valuable medicaments that used in many of disease for relief of pain and inflammation. Most analgesic and anti-inflammatory drugs available in the market, still present a wide range of many problems such as efficacy and undesired effects including GIT disorders and other unwanted effects,1 that limit their clinical usefulness and remain to be solved and leaving an open door for new and better compounds.2 This situation highlights the need for advent of safe, novel and effective analgesic and anti-inflammatory compounds.3 1,2,4-triazole received sheer attention of medicinal chemists because of their many therapeutic applications like anticancer,4,5 antimicrobial,6-9 anticonvulsant,10 anti-inflammatory, analgesic,11 antidepressant,12 antitubercular,13 antimalarial14 and hypoglycemic15 activities.
We have reported that 5-methyl-2-[(5-substituted aryl-4H-1,2,4-triazol-3-yl)methyl]-2,4-dihydro-3H-pyrazol-3-one and 5-phenyl-1-[(5-substituted aryl-4H-1,2,4-triazol-3-yl)methyl]-1H-tetrazole had significant anticancer activity specially on renal cancer cell lines (UO-31) as well in vitro antibacterial activity against gram positive bacterial S. aureus NCIM 2079, B. subtillis NCIM 2063 and gram negative bacterial E. coli NCIM 2065, P. aeruginosa NCIM 2863 strains.16 More recently we have reported antimicrobial, antitubercular and anticancer activity of 1-[5-(substituted aryl)-1,2-oxazol-3-yl]-3,5-diphenyl-1H-1,2,4-triazole (4a-g).17 In continuation of our previous work18-20in this article the attempts have been made to explore the analgesic potential of some formerly synthesized 1,2,4-triazoles clubbed with pyrazole, tetrazole, isoxazole and pyrimidine heterocycles.
Materials and Methods
The standard analgesic drugs Ibuprofen and Pentazocine, solvents used for the experimental work were commercially procured from E. Merck India and Qualigens India. Swiss strain albino mice for study were procured from National Toxicology Center, Pune.
Evaluation of analgesic activity
Study protocol was approved by the Institutional Animal Ethics Committee for the purpose of control and supervision of experiments on animals (IAEC, Approval No.1211/ac/08/CPCSEA) before experiment. Swiss strain albino mice of either sex weighing 25–30 g were used for this study. The test compounds were administered intraperitoneally in 10% v/v Tween 80 suspension.
Acute toxicity study
The acute toxicity for the test compounds was determined by the Miller and Tainter method administering the compounds intraperitoneally. LD50 of the test compounds calculated by Miller and Tainter (1944) method,21 initially least tolerated (smallest) dose (100% mortality) and most tolerated (highest) dose (0% mortality) were determined by hit and trial method. After determination of two doses we have selected five doses in between the least tolerated and most tolerated doses were given intraperitoneally to 5 groups of mice, 10 animals in each group. The animals were observed for first 2 hours and then at 6th and 24th hour for any toxic symptoms. After 24 hours, the number of deceased animals was counted in each group and percentage of mortality calculated. From the obtained data determined the LD50of the test compounds by using probit value transformations.
Acetic acid induced writhing method (Abdominal Constriction Test)
The animals were divided into 38 groups of six mice each. The control group of animals was administered with 10% v/v Tween 80 (0.5 ml) suspension. The animals of another group were injected intraperitoneally with standard drug Ibuprofen (10 mg/kg). After 20 min of the administration the test compounds, all the groups of mice were given with the writhing agent 3% v/v aqueous acetic acid in a dose of 2 ml/kg intraperitoneally. The writhing produced in these animals was counted visually for 15 min and the numbers of writhings produced in treated groups were compared with control group. The results of analgesic activity are recorded in Table 1. Analgesic activity in percent was calculated by using following formula.
Table 1. Evaluation of analgesic activity by acetic acid induced writhing method.
| Sr. No. | Treatment | Dose mg/kg | Writhing episodes in 15 min (Mean ± S.E.M.) | Percent protection |
| 1 | Control | -- | 38.70±0.5547 | - |
| 2 | Ibuprofen | 10 | 13.19±0.6921** | 66 |
| 3 | IIIa | 50 | 17.28±0.6647** | 55 |
| 4 | IIIb | 50 | 18.24±0.6425** | 53 |
| 5 | IIIc | 50 | 24.89±0.5354** | 36 |
| 6 | IIId | 50 | 15.65±0.7845** | 60 |
| 7 | IIIe | 50 | 26.45±0.8121** | 32 |
| 8 | IIIf | 50 | 16.46±0.5744** | 57 |
| 9 | IIIg | 50 | 27.65±0.5478** | 29 |
| 10 | IIIh | 50 | 18.57±0.4545** | 52 |
| 11 | IIIi | 50 | 16.49±0.6545** | 57 |
| 12 | IIIj | 50 | 16.47±0.2254** | 57 |
| 13 | IVa | 50 | 16.45±0.5456** | 57 |
| 14 | IVb | 50 | 17.49±0.5641** | 55 |
| 15 | IVc | 50 | 21.73±0.5974** | 44 |
| 16 | IVd | 50 | 15.44±0.6133** | 60 |
| 17 | IVe | 50 | 22.45±0.5525** | 42 |
| 18 | IVf | 50 | 15.36±0.6455** | 60 |
| 19 | IVg | 50 | 19.65±0.7322** | 49 |
| 20 | IVh | 50 | 17.22±0.4855** | 56 |
| 21 | IVi | 50 | 19.47±0.6513** | 50 |
| 22 | IVj | 50 | 14.24±0.4745** | 63 |
| 23 | IV3a | 100 | 15.44±0.4454** | 60 |
| 24 | IV3b | 100 | 22.65±0.5546** | 42 |
| 25 | IV3c | 100 | 19.47±0.5941** | 50 |
| 26 | IV3d | 100 | 21.77±0.4423** | 44 |
| 27 | IV3e | 100 | 25.49±0.4473** | 34 |
| 28 | IV3f | 100 | 19.42±0.6651** | 50 |
| 29 | IV3g | 100 | 26.56±0.4329** | 31 |
| 30 | IV3h | 100 | 28.56±0.7325** | 26 |
| 31 | IV3i | 100 | 23.58±0.6423** | 39 |
| 32 | IV3j | 100 | 19.26±0.4523** | 50 |
| 33 | Ih | 25 | 20.54±0.6425** | 47 |
| 34 | Ii | 25 | 22.89±0.5214** | 41 |
| 35 | Ij | 25 | 18.34±0.4527** | 53 |
| 36 | IIh | 25 | 20.57±0.8592** | 47 |
| 37 | IIi | 25 | 22.44±0.6854** | 42 |
| 38 | IIj | 25 | 15.47±0.8957** | 60 |
| ** P < 0.01 represent significant difference when compared with control groups. | ||||
Protection = 100-[{(No. of writhes in treated mice)/(No. of writhes in untreated mice)}×100]
Hot plate method
The method of Eddy and Leimbach was adopted for the study. The temperature of a metal surface in the hot plate test was set at 55±1.0ºC. The time taken by the animals to lick the fore or hind paw or jump out of the place was taken as the reaction time. Latency to the licking paws or jumping from plate was determined before and after treatment. The latency was recorded at the time of 0 (just before any treatment) and 15, 30 and 60 min after intraperitoneal administration of test compounds. A latency period of 15 sec was defined as complete analgesia as cut off time to prevent damage to mice. The reference compound Pentazocine was administered in a dose of 5 mg/kg. The time course of hot plate latency was expressed as the percentage of the maximum possible effect (%MPE) according to the following formula:
%MPE=[(post drug latency) - (pre-drug latency) /(cut-off time) - (pre-drug latency)]×100
After the treatment of test and reference compounds, the pain thresholds of the animals were observed and presented in Table 2.
Table 2. Evaluation of analgesic activity by Hot plate method.
| Treatment | Average Reaction Time in seconds before treatment (Mean ± S.E.M.) | Reaction time in seconds after treatment (Mean ± S.E.M.) | %MPE | ||
| 15 min | 30 min | 60min | |||
| Control | 4.75±0.1547 | 4.75±0.1583 | 4.75±0.2563 | 4.75±0.4941 | - |
| Pentazocine | 4.70±0.4012** | 7.70±0.5211** | 9.67±0.4302** | 11.81±0.3254** | 69.02 |
| IIIa | 4.56±0.5221** | 7.23±0.6326** | 9.23±0.5745** | 10.76±0.4369** | 59.38 |
| IIIb | 4.63±0.4785** | 7.49±0.2234** | 9.87±0.3865** | 11.15±0.5356** | 62.87 |
| IIIc | 4.67±0.4523** | 6.52±0.6542** | 9.34±0.5413** | 9.53±0.4356** | 47.04 |
| IIId | 4.63±0.6374** | 7.46±0.7585** | 9.52±0.2658** | 10.47±0.7541** | 56.31 |
| IIIe | 4.69±0.4474** | 6.45±0.5428** | 8.36±0.6756** | 9.27 ±0.5854** | 44.42 |
| IIIf | 4.65±0.4469** | 7.42±0.3769** | 9.49±0.5369** | 10.71±0.5775** | 58.55 |
| IIIg | 4.56±0.5854** | 6.70±0.4459** | 8.49±0.7474** | 9.27±0.6525** | 45.11 |
| IIIh | 4.43±0.3544** | 7.50±0.7585** | 9.57±0.6456** | 10.52 ±0.6785** | 57.61 |
| IIIi | 4.71±0.4675** | 6.39±0.3646** | 9.03±0.5236** | 9.46±0.5359** | 46.16 |
| IIIj | 4.68±0.7548** | 7.56±0.5636** | 9.52±0.6552** | 11.37±0.6426** | 64.82 |
| IVa | 4.72±0.5957** | 7.68±0.4523** | 9.56±0.3356** | 11.09±0.5517** | 61.96 |
| IVb | 4.67±0.4358** | 7.45±0.6984** | 9.16±0.3548** | 10.59±0.5478** | 57.30 |
| IVc | 4.55±0.4785** | 6.59±0.4578** | 9.44±0.5349** | 9.69±0.8689** | 49.18 |
| IVd | 4.58±0.5878** | 7.66±0.6689** | 9.50±0.4245** | 11.56±0.6741** | 66.98 |
| IVe | 4.39±0.4589** | 6.89±0.7481** | 8.59±0.6478** | 9.75 ±0.4589** | 50.51 |
| IVf | 4.66±0.5958** | 7.53±0.3549** | 9.80±0.3358** | 11.61±0.6358** | 67.21 |
| IVg | 4.45±0.5869** | 7.72±0.5269** | 9.58±0.5895** | 10.46±0.5692** | 56.96 |
| IVh | 4.41±0.5321** | 7.80±0.4781** | 9.57±0.5696** | 10.82 ±0.3324** | 60.52 |
| IVi | 4.64±0.2966** | 7.56±0.3826** | 9.45±0.7851** | 10.89±0.5369** | 60.32 |
| IVj | 4.52±0.2853** | 7.70±0.5239** | 9.43±0.4665** | 10.44±0.6745 ** | 56.48 |
| IV3a | 4.64±0.4789** | 7.34±0.3545** | 9.12±0.3456** | 10.12±0.2985** | 52.89 |
| IV3b | 4.56±0.5884** | 7.11±0.5789** | 8.36±0.6398** | 9.47±0.6489** | 47.03 |
| IV3c | 4.67±0.3956** | 7.49±0.5212** | 9.27±0.3369** | 10.76±0.6245** | 58.95 |
| IV3d | 4.73±0.4525** | 7.13±0.2845** | 9.10±0.3235** | 9.46±0.5469** | 46.05 |
| IV3e | 4.59±0.5823** | 6.93±0.5861** | 9.14±0.6478** | 10.74 ±0.4369** | 59.07 |
| IV3f | 4.54±0.4969** | 7.23±0.5326** | 9.50±0.3756** | 10.32±0.6542** | 55.25 |
| IV3g | 4.49±0.5478** | 7.02±0.4689** | 8.48±0.5225** | 8.93±0.5692** | 42.24 |
| IV3h | 4.61±0.4526** | 6.80±0.4369** | 8.55±0.5236** | 9.51 ±0.5236** | 47.16 |
| IV3i | 4.67±0.6325** | 6.56±0.3989** | 8.47±0.5364** | 9.37±0.6346** | 45.49 |
| IV3j | 4.71±0.5963** | 7.31±0.2845** | 9.46±0.3623** | 10.20±0.4126 ** | 53.35 |
| Ih | 4.46±0.6522** | 7.25±0.4545** | 8.55±0.2625** | 9.88±0.4121** | 51.18 |
| Ii | 4.29±0.3532** | 7.45±0.4666** | 9.12±0.3644** | 9.83±0.5481** | 51.72 |
| Ij | 4.34±0.5418** | 7.75±0.6641** | 9.85±0.4236** | 10.24±0.3678** | 55.34 |
| IIh | 4.54±0.6645** | 6.96±0.4425** | 8.86±0.3356** | 9.87±0.4356** | 50.95 |
| Iii | 4.59±0.5414** | 6.45±0.3784** | 8.22±0.3541** | 9.45±0.4678** | 46.68 |
| IIj | 4.74±0.4775** | 7.35±0.4125** | 9.45±0.5925** | 10.96±0.6486** | 60.62 |
| Dose: 25 mg/kg for compounds Ih-Ij, 50mg/kg for IIIa-j and IVa-j, 100mg/kg for IV3a-j and 5mg/Kg for Pentazocine. ** p<0.01 represent the significant difference when compared control group. | |||||
Statistical analysis
Data were presented as arithmetic mean±SEM. Statistical analysis was performed by one way variance (ANOVA) followed by Dunnett’s test. ‘‘p’’ value of less than 0.05 was considered as statistically significant.
Results and Discussion
Acetic acid induced writhing method
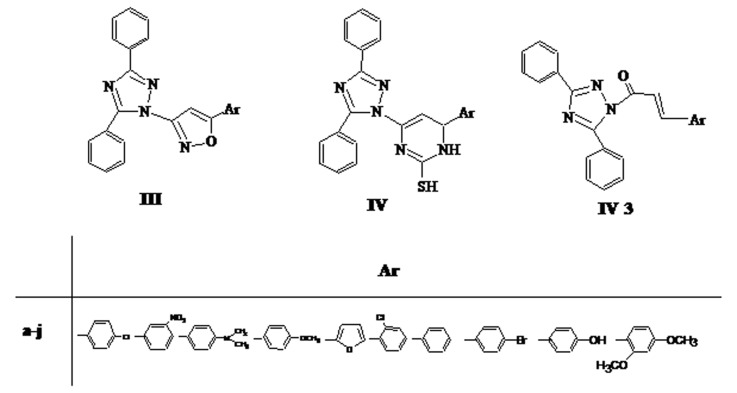
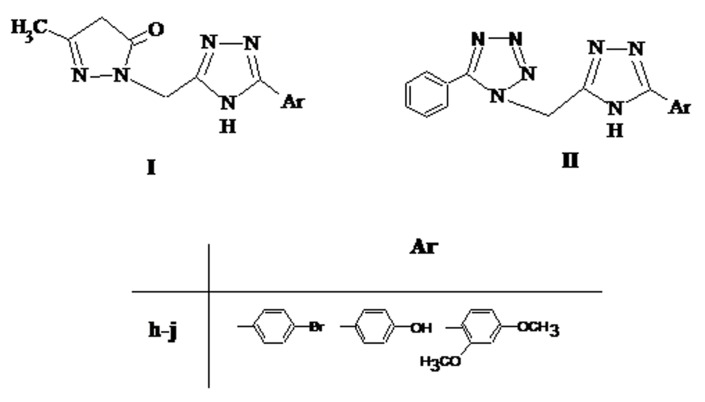
Abdominal construction responses induced by acetic acid is a sensitive procedure to establish efficacy of peripherally acting analgesics, it may causes increase in the level of PGE2 and PGF2a by intraperitoneally administration of acetic acid. The analgesic activity was expressed as percentage of protection. All tested compounds exhibited activity in a dose range of 25-100 mg/kg. Writhing episodes and percent protection of tested compounds for analgesic activity are summarized in Table 1. Compound IIIa, IIId, IIIf, IIIi, IIIj, IVa, IVb, IVd, IVf, IVh, IVj IV3a and IIj (Figure 1 and 2) were found to be superior analgesic agents with 55, 60, 57, 57, 57, 57, 55, 60, 60, 56, 63, 60 and 60% analgesic activity respectively as compared to other tested compounds. Chloro, nitro, methoxy, hydroxy and bromo substituted derivatives exhibited excellent analgesic activity where as dimethylamino, furan and phenyl substituted derivatives showed moderate analgesic activity. Substituted phenyl ring present on isoxazole, pyrimidine and 1,2,4-triazole nucleus might have attributed crucial role to show analgesic activity. Compounds with substitution of chloro, methoxy, bromo on 4th position of phenyl ring present on isoxazole, pyrimidine and triazole nucleus showed maximum analgesic activity. Compounds IIId, IVd, IVf, IVj, IV3a and IIj were exhibited comparative analgesic property (up to 60% protection) with standard drug Ibuprofen (66%) as illustrated in Table 1. because of 4-methoxy, 4-chloro, 2-chloro, 2,4-dimethoxy groups present on phenyl ring of pharmacologically active isoxazole, pyrimidine, and triazole chalcone heterocycles.
Figure 1.
Structures of compounds of scheme III, IV and IV3.
Figure 2.
Structures of compounds of scheme I and II.
Hot plate method
All compounds tested by Eddy’s hot plate method exhibited activity in a dose range of 25-100 mg/kg. The analgesic activity measured by central analgesia. Pentazocine 5 mg/kg significantly increased the hot plate latency producing a highest %MPE at 69.02. Compounds IIIb, IIIj, IVa, IVd, IVf, IVh, IVi, and IIj significantly increased the hot plate latency when compared to the control group. The highest antinociception induced by compounds IIIj, IVd and IVf at dose of 50 mg/kg were observed with 64.82, 66.98 and 67.21 %MPE respectively. The analgesic activity of compounds IIIb, IIId, IIIf, IIIh, IIIj, IVa, IVb, IVd, IVf, IVh, IVi, IV3c, IV3e and IIj were comparable to Pentazocine after 15, 30 and 60 min. 2-chloro, 3-nitro, 4-chloro, 4-methoxy, 4-bromo, 4-hydroxy and 2,4-dimethoxy substituted analogs exhibited dynamic analgesic activity. 4-dimethyl amino and 2-furyl substituted compound of tested series of 1-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-3-(substituted aryl) prop-2-en-1-one (Chalcones) were also acquired higher hot plate latency with 10.76 and 10.74 sec respectively after 60 min. Compounds from series of 1-[5-(substituted aryl)-1,2-oxazol-3-yl]- 3,5-diphenyl-1H-1,2,4-triazoles and 6-(substituted aryl)-4-(3,5-diphenyl-1H-1,2,4-triazol-1-yl)-1,6-dihydropyrimidine-2-thiol obtained excellent analgesic potential.
Conclusion
The results of the present investigation reveals that the increase in analgesic activity is attributed to the presence of 2-chloro, 3-nitro, 4-chloro, 4-methoxy, 4-bromo, 4-hydroxy and 2,4-dimethoxy groups on phenyl ring of isoxazole, pyrimidine, pyrazole, tetrazole and triazole. All tested heterocycles posses central and peripheral analgesic property. Perceptibly the comparative evaluation of active compounds will required in the further studies, the data reported in this article may be helpful guide for the medicinal chemist who is working in this area.
Acknowledgments
Authors are highly thankful to National Toxicology Center, Pune, for providing animals to caring out analgesic activity and acute toxicity study. We are also grateful of Principal M.E.S. College Pharmacy, Sonai and Prashant Patil Gadakh, Secreatary, Mula Education Society for providing excellent research facilities for this work.
Conflict of interest
All the authors report no conflicts of interest.
References
- 1.Girard P, Verniers D, Coppe MC, Pansart Y, Gillardin JM. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur J Pharmacol . 2008; 584:263–71. doi: 10.1016/j.ejphar.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Tao YM, Li QL, Zhang CF, Xu XJ, Chen J, Ju YW. et al. LPK-26, a novel κ- opioid receptor agonist with potent antinociceptive effects and low dependence potential. Eur J Pharmacol . 2008; 584:306–11. doi: 10.1016/j.ejphar.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Kolesnikov Y, Soritsa D. Analgesic synergy between topical opioids and topical non-steroidal anti-inflammatory drugs in the mouse model of thermal pain. Eur J Pharmacol . 2008; 579:126–33. doi: 10.1016/j.ejphar.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Bhat KS, Poojary B, Prasad DJ, Naik P, Holla BS. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur J Med Chem . 2009; 44:5066–70. doi: 10.1016/j.ejmech.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Al-Soud YA, Al-Masoudi NA, Ferwanah AE. Synthesis and properties of new substituted 1,2,4-triazoles: Potential antitumor agents. Bioorg Med Chem . 2003; 11:1701–8. doi: 10.1016/s0968-0896(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 6.Lingappa B, Girisha KS, Kalluraya BN, Rai S, Kumari NS. Regioselective reaction: Novel Mannich bases derived from 3-(4,6-disubstituted-2-thiomethyl)3-amino-5-mercapto-1,2,4-triazoles and their antimicrobial properties. Ind J Chem . 2008; 47B:1858–64. [Google Scholar]
- 7.Rao G, Rajasekran S, Attimarad M. Synthesis and Antimicrobial activity of Some 5-phenyl-4-substituted amino-3-mercapto (4H) 1,2,4-triazoles. Ind J Pharm Sci . 2000; 6:475–7. [Google Scholar]
- 8.Jalilian AR, Sattari S, Bineshmarvasti M, Shafiee A, Daneshtalab M. Synthesis and in vitro antifungal and cytotoxicity evaluation of thiazolo-4H-1,2,4-triazoles and 1,2-thiadiazolo-4H-1,2,4-triazoles-thiazoles-1,2,3-thiadiazoles. Arch Der Pharm . 2000; 333:347–54. doi: 10.1002/1521-4184(200010)333:10<347::aid-ardp347>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic M, Dimova V, Molnar GD, Kakurinov V, Colanceska RK. Synthesis of some N1-aryl/heteroarylaminomethyl/ethyl-1,2,4-triazoles and their antibacterial and antifungal activities. Hetero Comm . 2001; 7:577–82. [Google Scholar]
- 10.Chimirri A, Bevacqua F, Gitto R, Quartarone S, Zappala MD, Sarro A. et al. Synthesis and anticonvulsant activity of new 1-H-triazolo[4,5-c][2,3]benzodiaze-pines. Med Chem Res . 1999; 9:203–12. [Google Scholar]
- 11.Hunashal RD, Ronad PM, Maddi VS, Satyanarayana D, Kamadod MA. Synthesis, anti-inflammatory and analgesic activity of 2-[4-(substituted benzylideneamino)-5-(substitutedphenoxymethyl)-4H-1,2,4-triazol-3-yl-thio] acetic acid derivatives. Arab J Chem 2011; 1-9. [Google Scholar]
- 12.Kane MJ, Dudley MW, Sorensen MS, Miller FP. Synthesis of 1,2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents. J Med Chem . 1988; 31:1253–8. doi: 10.1021/jm00401a031. [DOI] [PubMed] [Google Scholar]
- 13.Husain MI, Amir M, Singh E. Synthesis and antitubercular activities of [5-(2furyl)-1,2,4-triazoles-3yl thio] acehydrazide derivatives. Ind J Chem . 1987; 26B:2512–54. [Google Scholar]
- 14.Xiao Z, Waters NC, Woodard CL, Li PK. Design and synthesis of pfmrk inhibitors as potential antimalarial agents. Bioorg Med Chem Lett . 2001; 11:2875–8. doi: 10.1016/s0960-894x(01)00578-9. [DOI] [PubMed] [Google Scholar]
- 15.Deliwala CV, Mhasalkar MY, Shah MH, Pilankar PD, Nikam ST, Anantanarayan KG. Synthesis and hypoglycaemic activity of 3-aryl(or pyridyl)-5-alkyl amino-1,3,4, Thiadiazole and some sulfonyl ureas derivatives of 4H-1,2,4 triazoles. J Med Chem . 1971; 14:1000–3. doi: 10.1021/jm00292a035. [DOI] [PubMed] [Google Scholar]
- 16.Khanage SG, Mohite PB, Raju SA. Synthesis, Anticancer and Antibacterial Activity of Some Novel 1,2,4-Triazole Derivatives Containing Pyrazole and Tetrazole Rings. Asian J Res Chem . 2011; 4(4):567–73. [Google Scholar]
- 17.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Synthesis and pharmacological evaluation of isoxazole derivatives containing 1,2,4-triazole Moiety. Mar Pharm J . 2012; 16(2):134–40. doi: 10.5681/apb.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Investigation of pyrazole and tetrazole derivatives containing 3,5 disubstituted-4H 1,2,4-triazole as a potential antitubercular and antifungal agent. Bioint Res App Chem . 2012; 2(2):277–83. [Google Scholar]
- 19.Khanage SG, Mohite PB, Pandhare RB, Raju SA. Study of analgesic activity of novel 1,2,4-triazole derivatives bearing pyrazole and tetrazole moiety. J Pharm Res . 2011; 4(10):3609–11. [Google Scholar]
- 20.Khanage SG, Raju SA, Mohite PB, Pandhare RB. Synthesis and Pharmacological Evaluation of Some New Pyrimidine Derivatives containing 1, 2, 4-triazole. Adv Pharm Bull . 2012; 2(2):213–22. doi: 10.5681/apb.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller LC, Tainter ML. Estimation of LD50 and its error by means of log-probit graph paper. Proc Soc Exp Bio Med . 1944; 57: 261. [Google Scholar]