Abstract
Purpose: Progressive loss of dopaminergic neurons of the substantia nigra pars compacta (SNc) in Parkinson’s disease (PD) leads to impairment of motor skills. Several evidences show that the role of serotonergic system in regulation of normal movement is pivotal and mediates via 5-HT1A receptors. Our previous study has shown that fluoxetine in acute injections able to attenuate catalepsy in 6-hydroxydopamine (6-OHDA)-lesioned rats. Since drugs are used chronically in clinic, in this study we attempted to evaluate effect of chronic administration of fluoxetine on 6-OHDA-induced catalepsy. Methods: Catalepsy was induced by unilateral infusion of 6-OHDA (8 µg/2 µl/rat) into the central region of SNc and assayed by using bar-test. Fluoxetine (1, 2.5, 5 and 10 mg/kg) was injected intraperitonealy (ip) for 10 days and its anti-cataleptic effect was assessed at the 10th day. Results: Fluoxetine in high doses (5 and 10 mg/kg) worsened 6-OHDA-induced catalepsy while it had anti-cataleptic effect at the dose of 1mg/kg. The anti-cataleptic effect of fluoxetine (1mg/kg) was reversed by co-administration with NAN-190 (0.5 mg/kg, ip), as a5-HT1Areceptor antagonist. Conclusion: According to the results it can be concluded that fluoxetine has anti-cataleptic effect in parkinsonian rats only at low doses, whereas at higher doses it worsens catalepsy. It’s anti-cataleptic effect is exerted through affecting on 5-HT1Areceptors. However, at high doses other mechanisms may be involved. Further clinical studies are needed to prove it’s possible clinical application as an adjuvant therapy in reducing catalepsy of PD.
Keywords: Fluoxetine, 6-Hydroxydopamine, Catalepsy, Rat
Introduction
Parkinson's Disease (PD) is the second most common neurodegenerative disorder that is characterized by a marked loss of dopaminergic neurons of mainly the substantia nigra pars compacta (SNc) leading to a reduction of dopamine (DA) in the striatum. Dopamine deficiency results in motor disabilities, such as muscle rigidity, akinesia, tremor and postural abnormalities as well as cognitive disturbances.1-3 Investigation of PD in animals is based on modeling of the disease by injection of some neurotoxins such as 6-OHDA that selectively destroys catecholaminergic neurons. Intracerebral injection of 6-OHDA into the rat nigrostriatal pathway degenerate virtually all dopaminergic neurons in the SNc and leading to stable motor deficits.3,4
In 6-OHDA-treated rats hyperinnervation of serotonergic (5-HT) fibers within the affected area takes place which compensate some activities of lost dopaminergic neurons.5-7 All components of basal ganglia receive serotonergic neurons from dorsal raphe nucleus.8 It seems that serotonergic system has a pivotal role in regulation of voluntary movements and disturbances in serotonine transmission might contribute to the neural mechanisms underlying disorders of basal ganglia such as PD, Tourette's syndrome and obsessive compulsive disorder.5,9 Thus 5-HT transmission may be critical in treating the symptoms of PD and other motor disorders.8 Several studies have shown anti-cataleptic effect of 5HT1A agonists in rodent model of PD but the effect of selective serotonin reuptake inhibitors (SSRIs) in improving of catalepsy is controversial.9-11 Our previous study indicated that single-dose administration of flouxetine could abolish catalepsy in 6-OHDA lesioned rats.5 Sinces the long-term effect of drugs can vary with their acute administration, therefore in this study we attempted to investigate the chronic effect of flouxetine in catalepsy induced by 6-OHDA.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma Chemical Co. (USA). Solutions were prepared freshly on the days of experimentation. Fluoxetine and 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)buthyl]piperazine hydrobromide (NAN-190) were dissolved in 0.9% saline and 6-OHDA was dissolved in 0.9% saline containing 0.2% (w/v) ascorbic acid. The drugs were injected intraperitoneally (ip) except for 6-hydroxydopamine (6-OHDA) which was injected into right subestentia nigra pars compacta (SNc) in a total volum of 8 µl /rat with a constant injection rate of 0.2 µl/min.
Animals
The experiments were carried out on male Wistar rats weighing between 270-300 g. Before and during of study these animals were kept in polypropylene cages, four per cage, under standard conditions (12:12h) light/dark cycle at an ambient temperature about 25±2°C and had access to standard pellet and water ad labium. Animals were acclimated to the testing conditions 2 days before the behavioral assessments were done. All of the procedures were carried out under the ethical guidelines of Tabriz University of Medical Sciences.
Surgical procedures
The animals were anesthetized intraperitoenally by ketamine (50 mg/kg) and xylazine (5 mg/kg). After deep anaesthetization, rats were fixed in a stereotaxic frame in the flat skin positions. Scalp hairs of the rats were completely shaved with a standard electric shaving machine, swabbed with povidone iodine and a central incision made to reveal skull. A 0.7 millimeter bar hole was drilled and 23 gauge sterile stainless steel cannula, as a guide cannula inserted for subsequent injection of 6-OHDA in to the SNc. The coordinates for this site were based on the rat brain atlas12: anteroposterior (AP): -5.0 mm from the bregma; mediolateral (ML): ±2.1 mm from the midline and dorsoventral (DV):-7.7 from the skull. Desipramine (25 mg/kg) was injected intraperitoneally 30 min before intra-nigral injection of 6-OHDA to avoid degeneration of noradrenergic neurons. Then 6-OHDA (8 μg/ per rat in 2 μl saline with 0.02 % ascorbic acid) was infused by infusion pump at the flow rate of 0.2 μl/min into the right subestentia nigra pars compacta. At the end of injection, guide cannula was kept for an additional 2 min and then slowly was withdrawed. All of these procedures were repeated in Sham-operated animals but they were received only 2 μl vehicle of 6-OHDA (0.9% saline containing 0.2% (w/v) ascorbic acid). For approving the accuracy of the site of injection, we provided a histological slice of striatum region that showed the site of inserted guide cannula in accordance with rat brain atlas. After three weeks as a recovery period, only the rats that showed marked immobilization in bare test were subjected to further experimentation. Then parkinsonian rats were divided randomly into equal groups and received once daily (9 a.m.) injections of fluoxetine (1, 2.5, 5 and 10 mg/kg, ip) for 10 days. NAN-190 (0.5 mg/kg, ip), as a 5-HT1A receptor antagonist, was injected concomitantly with the effective dose of fluoxetine.
Catalepsy test
Catalepsy was assessed by using of standard wooden bar test mean. Anterior limbs of rat gently extended on 9 cm high bar (0.9 cm in diameter). Elapsed time for each rat in this imposed posture was considered as a bar test time. The endpoint of catalepsy was designated to occur when both front pows were removed from the bar or if the animal moved its head in an exploratoty manner. The cut-off time of the test was 720 seconds. This test was carried out 5, 60, 120 and 180 minutes after drugs administration in the 10th day. All observations were made between 9 AM and 4 PM.
Statistical analysis
Statistical analysis of each data set was calculated by use of SPSS software (version 16.0). Data were expressed as the mean+SEM, and were analyzed by one-way ANOVA in each experiment. In the case of significant variation (p<0.05), the values were compared by Tukey test.
Results
Effect of Flouxetine on 6-OHDA induced catalepsy
Three groups of rats were shedoulded as normal (control), sham-operated (receiving 2 μl vehicle) and 6-OHDA (8 μg/2μl/rat, intra-SNc)-lesioned rats. As it has been shown in Figure 1, 6-OHDA could induce significant (p<0.001) catalepsy when compared with normal (control) and sham-operated animals. In other experiment five groups of 6-OHDA-lesioned rats received saline or one of four different doses of flouxetine (1, 2.5, 5 and 10 mg/kg, ip), respectively for 10 days. The results showed that flouxetine decreased the severity of 6-OHDA-induced catalepsy dose dependently (p < 0.001) (Figure 2).
Figure 1.
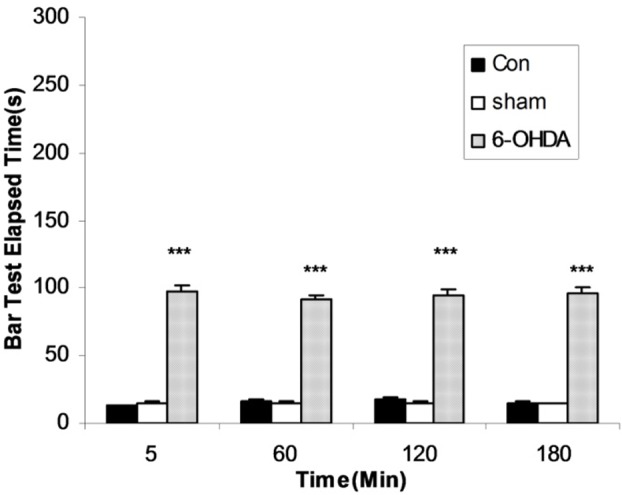
The results of bar test in control, Sham-operated and 6-OHDA (8μg/2μl/rat)-lesioned rats. Each bar represents the mean±SEM of elapsed time (s), n = 8 rats for each group; *** p<0.001 when compared with normal (Con) and sham operated groups.
Figure 2.
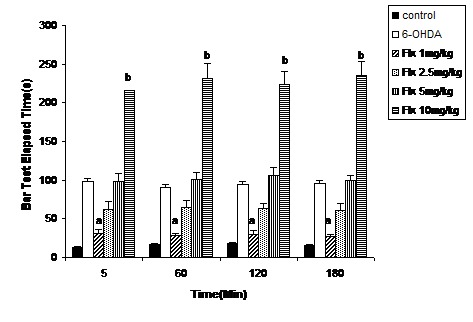
The bar test results of 6-OHDA (8 μg/2 μl/rat)-lesioned rats treated with Flouxetine (1, 2.5, 5 and 10 mg/kg, ip for 10 days). Each bar represents the mean ± SEM of elapsed time (s); n = 8 rats for each group; a p< 0.001 when compared with 6-OHDA-lesioned rats and b p< 0.001 when compared with control group.(Flx=Flouxetine)
Effect of NAN-190 co-treatment with flouxetine on 6-OHDA-induced catalepsy
Three groups of 6-OHDA-lesioned animals received saline, flouxetine (1 mg/kg, ip) or flouxetine (1mg/kg ip) with NAN-190 (1mg/kg ip) respectively. The results showed that catalepsy-ameliorating effect of flouxetine was reversed (p <0.001) by NAN-190 (Figure 3).
Figure 3.
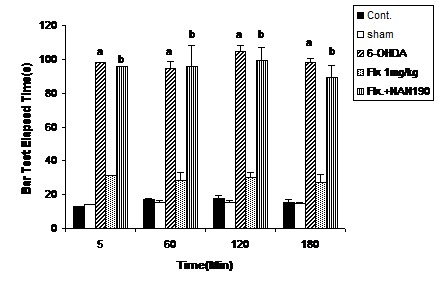
The bar test results from the co-administration of NAN-190 (0.5 mg/kg,ip) with flouxetine (1mg/kg, ip for 10 days) in 6-OHDA-lesioned rats. Each bar represents the mean ± SEM of elapsed time (s); n = 8 rats for each group; a p<0.001 when compared with 6-OHDA-lesioned rats; b p< 0.001 when compared with 6-OHDA-lesioned rats co-treated with NAN- 190 and flouxetine.
Discussion
Striatal DA deficiency or direct striatal damage for any reason may lead to PD which is characterized by tremor at rest, muscle rigidity and slowness of voluntary movement.4 6-OHDA is a neurotoxin which is used generally to produce experimental model of PD.5 In this study intra-SNc injection of 6-OHDA caused noticeable catalepsy when was assessed by bar test. This is standard test frequently used for evaluating catalepsy induced by 6-OHDA and neuroleptic drugs in rodents.13 Our results showed that chronic injections of flouxetine for ten days attenuated 6-OHDA-induced catalepsy only at low doses. The most anticatleptic effect was observed at the dose of 1mg/kg, whereas flouxetine at the high doses (5 and 10 mg/kg) trebeled catalepsy. This may be due to increase of flouextine dose and involvement of some other neuronal effects.
5-HT1A receptors are widely distributed through the basal ganglia. They are located on dorsal raphe neurons with efferents to the striatum, and are also localized on cortical neurons sending glutamatergic projections to the basal ganglia.14,15 Release of dopamine following stimulation of 5-HT1A receptors in these regions is via the inhibition of adenyl cyclase and the opening of potassium channels. These findings show that modulation of 5-HT transmission by 5-HT1A receptor agonists can be a potential therapeutic approach in PD.16,17 On the other hand it is supposed that the striatum is a central neuroanatomical site for both antidyskinetic and anti-parkinsonian actions of 5-HT1A receptor agonists.18 In particular, the striatum and the output regions of the basal ganglia, the substantia nigra pars reticulata (SNr), and medial globus pallidus (GPm) receive a dense serotonergic input, thus suggesting a potential role for serotonin in PD.19 Specific serotonin reuptake inhibitors (SSRIs) which increase serotonin levels in synaptic cleft may have similar effect through affecting on 5-HT1A receptors. In this study, NAN-190 (5-HT1A receptor antagonist) reversed the catalepsy-improving effect of fluoxetine in 6-OHDA lesioned rats. Thus, it seems that low doses of fluoxetine improves anti-cataleptic effect of 6-OHDA by affecting on 5-HT1Areceptors. While high dose of fluoxetine worsened catalepsy. It has been shown that serotonin modulates dopamine in basal ganglia by inhibiting its production and release.8 Furthermore, SSRIs may worsen the symptoms of pre-existing PD or depressive symptoms of anhedonia and social isolation.11 Worsening of PD by SSRIs can be explained by two mechanisms:.First, over activation of serotonergic projections of dorsal raphe which project directly to the substantia nigra and subsequent inhibition of the dopaminergic neurons;20 second, extrapyramidal side effects of SSRI.19,21-23
According to the results, we conclude that flouxetine improves catalepsy of parkinsonian rats in a dose-dependent manner. This effect is mediated by the stimulation of 5-HT1A receptors. We suggest a possible clinical application for fluoxetine in attenuating catalepsy of PD. To prove this hypothesis further clinical investigations should be carried out.
Acknowledgments
We wish to thank the Director of Drug Applied Research Center of the Tabriz University of Medical Sciences for supporting this study.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol . 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Di Filippoa M, Chiasserini D, Tozzi A, Picconi B, Calabresi P. Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimers Dis . 2010;20 (Suppl 2):S369–79. doi: 10.3233/JAD-2010-100543. [DOI] [PubMed] [Google Scholar]
- 3.Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res . 2005;162(1):1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson's Disease: Mechanisms and Models. Neuron . 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi J, Nayebi AM, Reyhani-Rad S, Samini M. Fluoxetine improves the Effect of levodopa on 6-hydroxy dopamine-induced motor impairments in rats. Adv Pharm Bull . 2012;2(2):149–55. doi: 10.5681/apb.2012.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda T, Kannari K, Huo SH, Arai A, Tomiyama M, Matsunaga M. et al. Increase of the striatal serotonergic fibers after nigrostriatal dopaminergic denervation in adult rats. Int Congr Ser . 2003; 1251:211–5. [Google Scholar]
- 7.Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 Map Kinase pathway. J Comp Neurol. 2006;498(3):415–30. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: Therapeutic implication for parkinson's disease and other motor disorders. Prog in Brain Res . 2008;172:423–63. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- 9.Caley CF, Friedman JH. Does fluoxetine exacerbate Parkinson's disease? J Clin. Psychiatry . 1992;53:278–82. [PubMed] [Google Scholar]
- 10.Steur EN. Increase of Parkinson disability after fluoxetine medication. Neurology . 1993;43(1):211–3. doi: 10.1212/wnl.43.1_part_1.211. [DOI] [PubMed] [Google Scholar]
- 11.Gönül AS, Aksu M. SSRI-induced Parkinsonism may be an early sign of future Parkinson's Disease. J Clin Psychiatry . 1999;60(6):410. doi: 10.4088/jcp.v60n0611d. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1982.
- 13.Pires JG, Bonikovski V, Futuro-Neto HA. Acute effects of selective serotonin reuptake inhibitors on neuroleptic-induced catalepsy in mice. Braz J Med Biol Res . 2005;38(12):1867–72. doi: 10.1590/s0100-879x2005001200015. [DOI] [PubMed] [Google Scholar]
- 14.Mohajjel Nayebi AA, Sheidaei H. Buspirone improves haloperidol-induced Parkinson disease in mice through 5-HT(1A) recaptors. Daru . 2010;18(1):41–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Navailles S, De Deurwaerdere P. Imbalanced dopaminergic transmission mediated by serotonergic Neurons in L-DOPA-induced dyskinesia. Parkinsons Dis . 2012;2012:323686. doi: 10.1155/2012/323686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nayebi AM, Rad SR, Saberian M, Azimzadeh S, Samini M. Buspirone improves 6-hydroxydopamine-induced catalepsy through stimulation of nigral 5-HT(1A) receptors in rats. Pharmacol Rep . 2010;62(2):258–64. doi: 10.1016/s1734-1140(10)70264-4. [DOI] [PubMed] [Google Scholar]
- 17.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther . 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupre KB, Eskow KL, Barnum CJ, Bishop C. Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology . 2008;55(8):1321–8. doi: 10.1016/j.neuropharm.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox SH, Chuang R, Brotchie JM. Serotonin and parkinson's disease: On movement, mood, and madness. Mov Disord . 2009;24(9):1255–66. doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- 20.Eltayb A, Svensson TH, Ahlenius S. Catalepsy induced by the 5-HT(1A) receptor antagonist WAY 100635 in rats pretreated with the selective serotonin reuptake inhibitor citalopram. Eur J Pharmacol . 2001;411(3):275–7. doi: 10.1016/s0014-2999(00)00924-9. [DOI] [PubMed] [Google Scholar]
- 21.van de Vijver, Roos RA, Jansen PA, Porsius AJ, de Boer A. Start of a selective serotonin reuptake inhibitor (SSRI) and increase of antiparkinsonian drug treatment in patients on levodopa. Br J Clin Pharmacol . 2002;54(2):168–70. doi: 10.1046/j.1365-2125.2001.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schapira AH. Present and future drug treatment for parkinson's disease. J Neurol Neurosurg Psychiatry . 2005;76(11):1472–8. doi: 10.1136/jnnp.2004.035980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya DK. Extrapyramidal symptoms with selective serotonin reuptake inhibitors. Br J Psychiatry . 1994;165(6):728–33. doi: 10.1192/bjp.165.6.728. [DOI] [PubMed] [Google Scholar]


