Abstract
Purpose: This research was aimed to formulate and characterize a microemolsion systems as a topical delivery system of naproxen for relief of symptoms of rheumatoid arthritis, osteoarthritis and treatment of dysmenorrheal. Methods: ME formulations prepared by mixing of appropriate amount of surfactant including Tween 80 and Span 80, co-surfactant such as propylene glycol (PG) and oil phase including Labrafac PG – transcutol P (10:1 ratio). The prepared microemolsions were evaluated regarding their particle size, zeta potential, conductivity, stability, viscosity, differential scanning calorimetry (DSC), scanning electron microscopy (SEM), refractory index (RI) and pH. Results: The mean droplets size of microemulsion formulation were in the range of 7.03 to 79.8 nm, and its refractory index (RI) and pH were 1.45 and 6.75, respectively. Viscosity range was 253.73- 802.63cps. Drug release profile showed that 26.15% of the drug released in the first 24 hours of experiment. Also, Hexagonal and bicontinuous structures were seen in the SEM photograph of the microemulsions. Conclusion: characterization, physicochemical properties and in vitro release were dependent upon the contents of S/C ratio, water and, oil phase percentage in formulations. Also, ME-6 may be preferable for topical naproxen formulation.
Keywords: Naproxen, Microemulsion, Phase Diagram, Characterization
Introduction
Microemulsions are macroscopically isotropic mixtures of at least a hydrophilic, a hydrophobic and an amphiphilic component. Their thermodynamic stability and their nanostructure are two important characteristics that distinguish them from ordinary emulsions which are thermodynamically unstable. Microemulsions were first observed by Schulman1 and Winsor2 in the 1950s. Then, the term “microemulsions” has been used to describe multi-component systems comprising non-polar, aqueous, surfactant, and cosurfactant components. Conventional microemulsions can be classified oil-in-water, (o/w), water-in-oil (w/o) and bicontinuous phase microemulsions.3 Some advantages offered by microemulsions include improvement in poorly drug solubility, enhancement of bioavailability, protection of the unstable drugs against environmental conditions and a long shelf life.
Naproxen (NAP, Figure 1) is a non-steroidal anti-inflammatory drug derived of propionic acid used widely as analgesic, antipyretic and for symptoms relief of dysmenorrheal pain.1 The most widely reported side effect of NSAIDs includes, gastrointestinal ulcer, accompanied by anaemia due to the bleeding, which is also true for naproxen. In order to avoid the gastric irritation, minimize the systemic toxicity and achieve a better therapeutic effect, one promising method is to administer the drug via skin.2 Transdermal drug delivery systems provide the most important route to achieve these goals.3 The transdermal delivery system also enable controlled or sustained release of the active ingredients and an enhanced patient compliance.4
Figure 1.
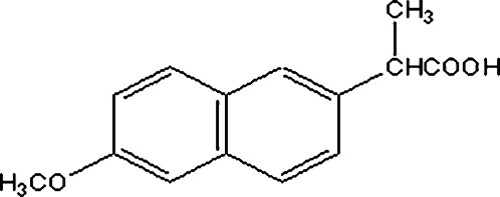
Chemical structure of naproxen.
Naproxen is poorly water solubility and it is possible to increase its solubility by utilizing microemulsion systems.4 In this study, The main aim of our investigations was to design and evaluate a microemulsion based naproxen (1%, w/w) for topical delivery.
Materials and Methods
Naproxen was purchased from Pars Darou company (Iran), Propylene glycol dicaprylocapraye (LabrafacTMPG), Median chain triglycerides (LabrafacTM Lipophile WL 1349), Diethylene glycol monoethyl ether (Transcutol P) was donated as gift by GATTEFOSSE Company (France). Span 80, Tween 80 and PG were obtained from Merck (Germany). All chemicals and solvents were of analytical grade. Freshly double distilled water was used in the experiments. Minitab15 software was used for experimental design and the evaluation of the effect of variables on responses. Sigma plot11 software was applied for providing tertiary phase diagrams.
Naproxen assay
The quantitative determination of naproxen was performed by UV spectrophotometry (BioWaveII,WPA) at λmax= 271 nm.
Solubility of naproxen
Solubility of naproxen was determined in different oil (Isopropyl myristate, Labrafac PG, LabrafacTM Lipophile WL 1349, Labrafac PG+Transcutol P(10:1)), surfactants (Span 80, Tween 80) and co-surfactant (Propylen glycol) by dissolving an excess amount of naproxen in 3ml of oil, and other components using a stirrer at 37 ºC+0.5 for 72 h. The equilibrated samples were then centrifuged at 10000 rpm for 30 min to remove undissolved drug, then the clear supernatant liquid was decanted. The solubility of naproxen was measured by analyzing the filtrate spectrophotometrically using nanospecterophotometer (Biochrom WPA Bioware) at 271 nm.
Pseudo-ternary phase diagram construction
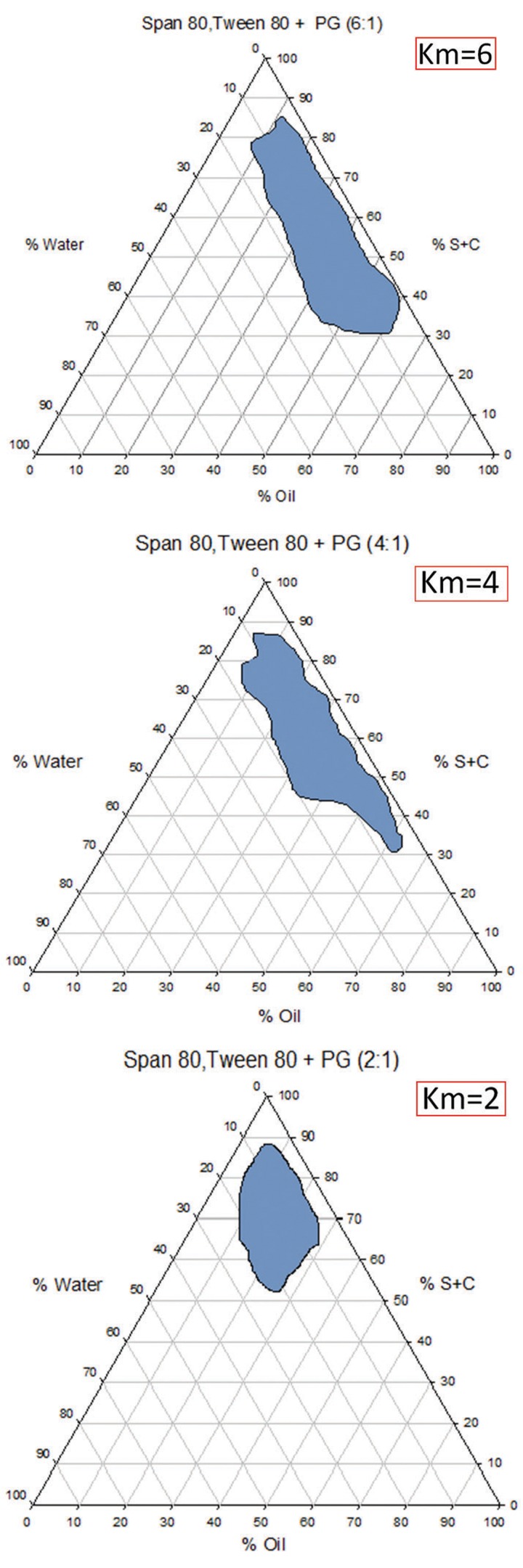
To investigate concentration range of components for the existing boundary of MEs, pseudo-ternary phase diagrams were constructed using the water titration method. Three phase diagrams were prepared with the 2:1, 4:1, and 6:1 weight ratios of (Span 80 /Tween 80) Propylen glycol respectively. Oil phase(Labrafac PG +Transcutol-P)(10:1) and the surfactant mixture were then mixed at the weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1.6 These mixtures were diluted dropwise with double distilled water, under moderate agitation. The samples were classified as microemulsions when they appeared as clear liquids.7
Several parameters influence on final properties of microemulsions. Full factorial design was used concerning with 3 variables at 2 levels for formulations. Major variables take part in determination of microemulsion’s properties includes surfactant/cosurfactant ratio (S/C), percentage of oil (% oil) and water percentage (%w). Eight different formulations with low and high values of oil (20% and 40%), water (5%, 10%), and S/Co mixing ratio (6:1, 4:1) were prepared for preparing of microemulsion formulation.
Preparation of naproxen Microemulsions
Various MEs were chosen from the pseudoternary phase diagram with 4:1, and 6:1 weight ratio of Span 80 /Tween 80/Propylen glycol. Naproxen (1%) was added to oil phase, then adding S/ CoS mixture and an appropriate amount of double distilled water was added to the mixture drop by drop and the MEs containing naproxen were obtained by stirring the mixtures at ambient temperature.8,9
Differential scanning calorimetry (DSC)
DSC measurements were carried out by means of a Metller Toldo DSC1 starR system equipped with refrigerated cooling system (Hubert Tc45). Approximately 5-10mg of microemulsion samples were weighted into hermetic aluminium pans and quickly sealed to prevent water evaporation from microemulsion samples. Simultaneously an empty hermetically sealed pan was used as a reference. Microemulsion samples were exposed in a temperature ranging from +30 ºC to - 50 ºC (scan rate:10 ºC/min). All measurements were preferred at least in triplicate.
In order to ensure accuracy and repeatability of data, DSC instrument was calibrated and checked under the conditions of use by indium standard. Changes of Enthalpy quantities (∆H) were calculated from endothermic and exothermic transitions of thermograms by Equation 1:10
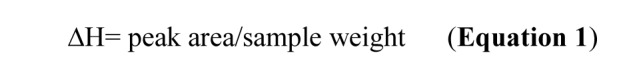
Zeta potential determination
Zeta potential of samples were measured by Zetasizer (Malvern instrument 1td ZEN3600, UK). Samples were placed in clear disposable zeta cells and results were recorded.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to characterize microstructure of micemulsions. SEM of samples were measured by LED 1455VP, Germany.
Particle size measurements
The average droplet size of samples was measured at 25 ºC by SCATTER SCOPE 1 QUIDIX (South Korea) and their refractory indices (RI) were also calculated.
Viscosity measurements
Viscosity of samples was measured at 25 ºC with a Brookfield viscometer (DV-II+Pro Brookfield., USA) using spindle no. 34. With shear rate 50 rpm. Each measurement was performed in triplicate.11-13
Conductivity measurements
Electrical conductivity of MEs was measured with a conductivity meter (Metrohm Model 712) using conductivity cells with a cell constant of 1.0 and consisting of two platinum plates separated by desired distance and having liquid between the platinum plate acting as a conductor.
Surface tension measurement
The surface tension of microemulsion was measured at 25 ºC with a Torsion balance (WHITE ELEC Model NO. 83944E).
Determination of pH
The pH values for microemolsion was determined at 25 ºC by pH meter (Mettler Toledo seven easy, Switzerland). All measurements were carried out in triplicate.12
Physical stability study
The physical stability of microemulsions was studied regarding the temperature stability and centrifugation. Microemulsions were kept in various temperatures (4 ºC, 25 ºC and 37 ºC) and observed for phase separation, flocculation or precipitation. Also, Microemulsions were centrifuged by HIGH SPEED BRUSHLESS CENTERIFUGE (Vs-35sMTi, vision) 10000 rpm for 30 minute at 25 ºC and inspected for any change in their homogeneity.14
Release study
Franz diffusion cells (area 3.4618 cm2) with a cellulose membrane were used to determine the release rate of naproxen from different microemulsion formulations. The cellulose membrane was first hydrated in distilled water at 25 ºC for 24 hours. The membrane was then clamped between the donor and receptor compartments of the cells. Each Diffusion cell was filled with 25 ml of phosphate buffer (pH =7.4). The receptor fluid was constantly stirred by externally driven magnetic bars at 300 rpm throughout the experiment. Naproxen microemulsion (5g) was accurately weighted and placed in donor compartment. At 0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 24h time intervals, 2ml sample was removed from receptor for spectrophotometric determination and replaced immediately with an equal volume of fresh receptor medium. Samples were analyzed by UV visible spectrophotometer (BioWaveII,WPA) at 271nm. The results were plotted as cumulative released drug percentage versus time.15
Statistical methods
All the experiments were repeated three times and data were expressed as the mean value±SD. Statistical data were analyzed by one-way analysis of variance (ANOVA) and P<0.05 was considered to be significant with 95% confidence intervals.
Results and Discussion
The results of solubility of naproxen are tabulated in Table 1. The maximum solubility of naproxen was found in Labrafac PG:Transcotol P (10:1) (14.067±0.023) as compared to other oils. In addition, the highest drug solubility of naproxen in surfactants were found in Span 80 (15.033±0.208), and Tween 80 (0.576±0.012). Based on the solubility studies of naproxen in oil, surfactant and co-surfactant and the preformulation studies it was found that Labrafac PG-Transcutol P, span 80, Tween 80 and propylene glycol could be the most appropriate combination for preparation of microemulsion.
Table 1. Solubility of Naproxen in different oils, surfactants and co-surfactants (mean±SD, n=3) .
| Phase type | Excipient | Solubility (mg/ml) |
| oil | Labrafac PG | 13.697 ± 0.536 |
| Isopropyl Myristat | 12.820 ± 0.044 | |
| LabrafacxxsupTMxysup Lipophile WL 1349 | 10.930 ± 0.100 | |
| Labrafac PG+Transcutol P(10:1) | 14.067 ± 0.023 | |
| surfactant | Tween 80 | 0.576 ± 0.012 |
| Span 80 | 15.033 ± 0.208 | |
| Co-surfactant | Propylene glycol | 14.120 ± 0.368 |
Pseudo-ternary phase diagrams of the investigated quaternary system water/ Labrafac PG, Transcotol P (10:1)/ Span 80-Tween 80/ PG are showed in Figure 2. Microemulsions were formed at ambient temperature. The phase diagrams clearly indicated that microemulsion existence region increased with increase in the weight ratio of surfactant/cosurfactant (Km=2-6).
Figure 2.
The pseudo-ternary phase diagrams of the oil-surfactant/cosurfactant mixture–water system at the 2:1, 4:1, and 6:1 weight ratio of Span 80 /Tween 80/ Propylene glycol at ambient temperature, dark area show microemulsions zone.
The mean particle size of formulations was from 7 to 79 nm (Table 2). Particle size of free drug MEs and drug loaded MEs were determined and there was no significant difference observed in average particle size after loading the drug. The ME 1 formulation had the lowest average particle size 7.03 ± 0.2nm with polydispersity index (PI) of 0.362±0.032 (Table 2). PI is a measure of particle homogeneity and it varies from 0.0 to 1.0. The closer to zero the PI value the more homogenous are the particles.
Table 2. Compositions of Selected Microemulsions (% w/w) and Particle Size (mean±SD, n=3).
| Formulation | Factorial | S/C | % Oil | % (S+C) | % Water | Particle size (nm) | Polydispersity |
| ME-1 | + + + | 6:1 | 40 | 50 | 10 | 7.03±0.2nm | 0.348±0.012 |
| ME-2 | + + - | 6:1 | 40 | 55 | 5 | 79.8±0.7 nm | 0.347±0.025 |
| ME-3 | + - + | 6:1 | 20 | 70 | 10 | 53.6±0.5 nm | 0.346±0.016 |
| ME-4 | + - - | 6:1 | 20 | 74 | 5 | 10.3±1.2 nm | 0.341±0.021 |
| ME-5 | - - - | 4:1 | 20 | 75 | 5 | 21.9±0.6 nm | 0.347±0.018 |
| ME-6 | - - + | 4:1 | 20 | 70 | 10 | 34.5±1.3 nm | 0.348±0.023 |
| ME-7 | - + - | 4:1 | 40 | 55 | 5 | 10.3±0.5 nm | 0.348±0.021 |
| ME-8 | - + + | 4:1 | 40 | 50 | 10 | 11.6±0.3 nm | 0.347±0.014 |
| +: high level; - : low level |
The PI indicated that ME formulations had narrow size distribution. Analysis of variance showed that correlation between mean particle size, PI and independent variables are not significant (p>0.05).
The refractive index (RI) of the ME formulation was found 1.45 that is near to oil phase which indicates ME formulations have water-in-oil structures. Analysis of variance is showed significant correlation between RI and independent variables (%w) and (% oil) (p<0.05). Equation 2 showed the effect of independent variables on RI.
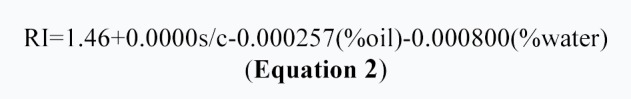
There was a strong correlation between the specific structure of the microemulsion systems and their electrical conductive behavior.16 The phase systems (o/w or w/o) of the microemulsions were determined by measuring the conductivity of the microemulsions.17 The ME formulations had the average conductivity in the range of 0.046-0.136ms/cm that is showed w/o structure of MEs. Also, w/o structure of MEs is confirmed with colour tests.
The ME formulations had appropriate observed pH value (6.750±0.09) that is best for topical application. Incorporation of naproxen did not significantly affect the observed pH value of the ME formulations (Table 3).
Table 3. pH, Refractive index,Conductivity and Zeta potential of selected Naproxen microemulsions (mean±SD, n=3).
| Formulation | PH | Refractive index | Zeta potential (mV) | Conductivity (mS/cm) |
| ME-1 | 6.60 ± 0.05 | 1.4460 ± 0.18 | 0.84 | 0.136±0.008 |
| ME-2 | 6.72 ± 0.08 | 1.4508 ± 0.19 | -0.66 | 0.134±0.002 |
| ME-3 | 6.85 ± 0.12 | 1.4531 ± 0.15 | -5.34 | 0.105±0.012 |
| ME-4 | 6.76 ± 0.09 | 1.4535 ± 0.20 | -10.50 | 0.077±0.009 |
| ME-5 | 6.70 ±0.06 | 1.4561 ± 0.17 | -11.50 | 0.067±0.004 |
| ME-6 | 6.70 ±0.09 | 1.4514 ± 0.16 | -18.00 | 0.046±0.018 |
| ME-7 | 6.89 ± 0.07 | 1.4505 ± 0.14 | -11.80 | 0.125±0.006 |
| ME-8 | 6.78 ± 0.10 | 1.4449 ± 0.19 | -15.70 | 0.067±0.013 |
The mean viscosity of formulations were from 253.73±1.88cps to 802.63±1.66cps. The highest viscosity belongs to ME-3 formulation with bicontinueous structure. Multivariate regression was applied for the analysis of correlation between independent variables and MEs viscosity. Linear Equation 3 shows all the main effects for viscosity is:

The percent of oil had more negative effect on viscosity. There was no significant difference found between the viscosities of free drug and drug loaded MEs (p>0.05).
Figure 3 shows the effect of main factors (S/C,%oil, %water) and their interactions on viscosity of microemulsions. The amount of %water and s/c had more effect on viscosity. Higher amount of %water and s/c ratio performed higher viscosity.
Figure 3.
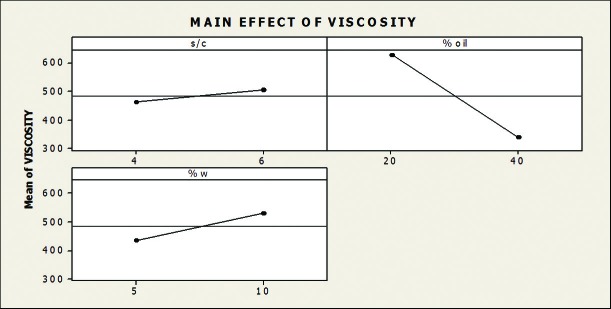
The effect of main effect of independent variables on viscosity
The ME formulations had the zeta potential average (-0.66 to+18.00mv) (Table 3). The highest zeta potential belongs to ME-6 formulation which seems to suggest micellar and bicontinuous structures and the lowest belong to ME-1 which seems to suggest reverse hexagonal and micellar structures. Multivariate regression was used for the analysis of correlation between independent variables and MEs zeta potential. The S/C ratio had more positive effect on zeta potential. There was found significant difference between the zeta potential and S/C ratio. (p<0.05). Linear Equation 4 shows all the main effects for zeta potential is:

The mean surface tension of formulations was from 44.17±1.46 to 52.17±1.25dynes/cm (Table 4). The surface tension data implies water-in-oil microemulsions because surface tension amounts of MEs is nearby to oil phase surface tension.
Table 4. Surface tension and viscosity of selected microemulsions(mean±SD, n=3).
| Formulation | Surface tension (dynes/cm) | Viscosity (cp) |
| ME-1 | 44.17 ± 1.46 | 282.90 ± 1.91 |
| ME-2 | 47.67 ± 0.85 | 253.73 ± 1.88 |
| ME-3 | 46.00 ±1.06 | 802.63 ± 1.66 |
| ME-4 | 47.67 ± 1.44 | 681.13 ± 1.98 |
| ME-5 | 47.33 ± 0.76 | 445.60 ± 1.15 |
| ME-6 | 49.33 ± 1.36 | 580.83 ± 1.92 |
| ME-7 | 52.17 ± 1.25 | 364.17 ± 1.60 |
| ME-8 | 48.50 ± 1.42 | 457.90 ± 1.83 |
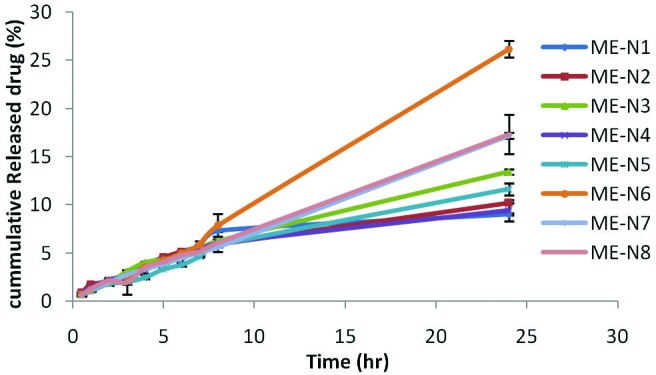
Figure 4 shows the release profile of naproxen ME formulations. The cumulative amount of naproxen that had permeated through the cellulose membrane (%) was plotted as a function of time (hours). In this study, ME-6 and ME-1 have the highest and lowest accumulative release percent, respectively. Table 5 shows release percent and kinetic of release in naproxen ME formulations. Multivariate regression was used for the analysis of correlation between independent variables and MEs release. Analysis of variance is showed no significant correlation between release percentage value of naproxen and independent variables (p>0.05). The percent of water and S/C ratio had more negative effect on accumulative release percent. Linear Equation 5 shows effect of independent variables on release percent:
Figure 4.
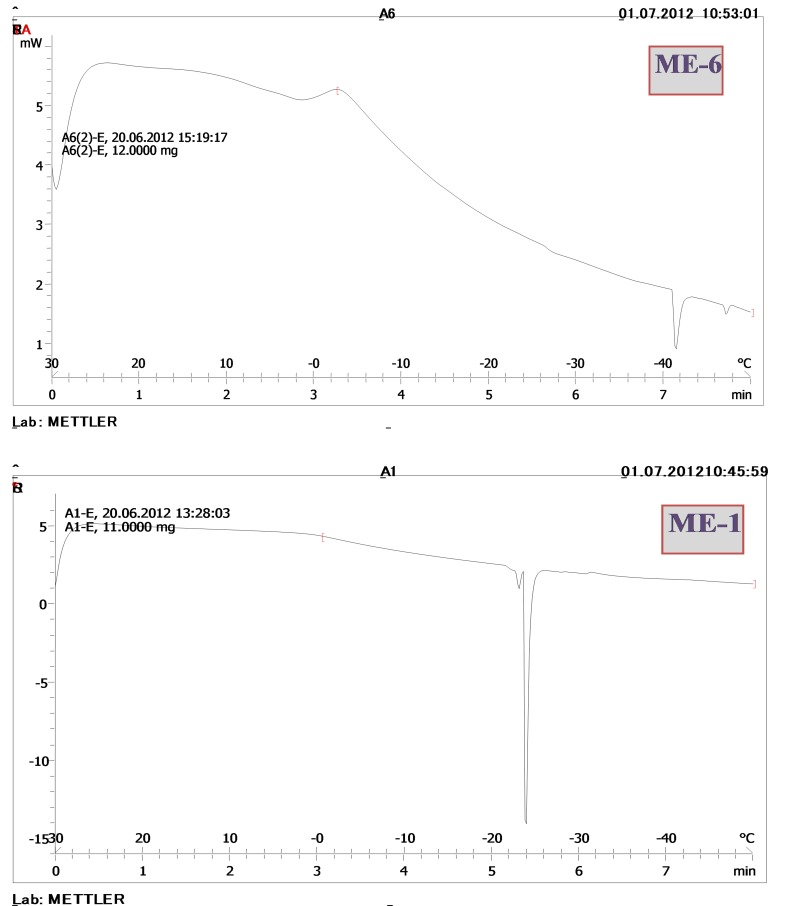
DSC cooling thermograms of ME-1 and ME-6 formulations.
Table 5. Percent release and kinetic release of selected microemulsions(mean±SD, n=3) .
| Formulation | % release | kinetic | Rxxref02 | Intercept |
| 1 | 9.0133 | Log Wagner | 0.9679 | -2.1891 |
| 2 | 10.2128 | Higuchi | 0.9929 | -0.0069 |
| 3 | 13.4262 | Weibul | 0.9954 | -4.3820 |
| 4 | 9.4373 | Log Wagner | 0.9899 | -2.2074 |
| 5 | 11.6378 | Pepas | 0.9832 | -4.5453 |
| 6 | 26.1512 | Zero | 0.9916 | -0.0068 |
| 7 | 17.1690 | Zero | 0.9983 | 0.004 |
| 8 | 17.3421 | 3/2 root of mass | 0.9970 | 0.0023 |

On the basis of Equation 5, it seems that release percent enhanced with decrease in the oil percent and s/c ratio.
In vitro release studies with an artificial hydrophobic membrane can provide information about the diffusion of a drug, which depends on the physico-chemical properties of components, vehicle internal structure, and interaction between drug and vehicle.18,19
The release profile of MEs were calculated by fitting the experimental data to equations describing different kinetic models. Linear regression analyses were made for zero-order (Mt/M0 = kt), first-order (ln (M0–Mt) = kt), Higuchi (Mt/M0 = (kt)1/2), Log Wagner, Linear wagner, Weibul, Second root of mass, Three-Seconds root of mass, and Pepas kinetics.
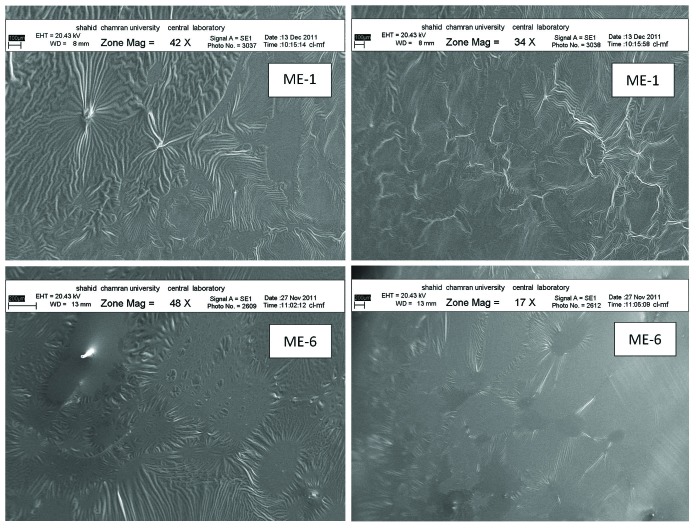
The amount of naproxen release differ between microemulsion carriers with different internal microstructure. Comparing the amounts of released naproxen after 24 hours as well as the release rate (Figure 3) the slowest release was observed for ME -1 with reverse hexagonal structure and the highest release was observed for ME-6 with bicontinuous and micellar structures.
Table 5 shows kinetic of release in naproxen ME formulations. Multivariate regression was applied for the analysis of correlation between independent variables and MEs kinetic release. Analysis of variance is showed no significant correlation between release kinetic of naproxen microemulsions and independent variables (p>0.05).Linear Equation 6 shows effect of independent variables on kinetic release:

The percent of water and S/C ratio had more positive and more negative effect on kinetic release, respectively, and percent of oil is not effect on the release kinetic of MEs. Three-seconds root of mass kinetic was obtained with increase in percent of water and decrease in s/c ratio whereas zero-order kinetic conversely.
Figure 5 shows the SEM images of ME-1 (with reverse hexagonal structure) and ME-6 (with micellar and bicontinuous structurs), respectively.
Figure 5.
SEM photographs of of ME-2 and ME-6.
The thermal behaviour of water can be a useful and rapid means with which to understand the microstructure of microemulsions.20 In this context, a small broadened peak at very low temperatures (below -30 ºC) has been suggested to be either internal water or water that is interacting strongly with the surfactants.21,22 When water is mixed in to a microemulsion system it can be either bound (interfacial) or free (bulk) water depending of its state in the system. In cooling curves of the samples (ME1-ME2-ME4), DSC thermogram showed one exothermic peak at around 0 to -3 ºC that indicate the freezing of bulk water in these formulations. The other endothermic peak at around -23 to -27 ºC belong to bound water freezing. In cooling curves of ME-3, DSC thermogram showed one exothermic peak at -2 ºC (bulk water) and one endothermic peak at -34 ºC that indicates bound water that interacts with surfactants strongly. DSC thermograms of ME-7 and ME-8 showed one exothermic peak at 0 to -1ºC (bulk water), also two endothermic at -19 to -21ºC (bound water) and -36.5 to -43 ºC (the water must be strongly bound or interacts with surfactants). In cooling curves of ME-5 and ME-6, DSC thermogrms showed one exothermic peak of around 0 ºC (ME-5) and -4 ºC (ME-6), which indicates bulk water. Also, in ME-6 showed one endothermic peak at -41 ºC which indicates the water must be strongly bound or interacts with surfactants. Figure 6 shows DSC cooling thermograms of ME-1and ME-6 formulations.
Figure 6.
DSC cooling thermograms of ME-1 and ME-6 formulations
The visual inspection experiment was carried out for 3 months by drawing ME sample at weekly interval for the first month and monthly interval for the subsequent months. The visual observation showed no evidence of phase separation or any precipitation or flocculation.
These samples also revealed no sign of phase separation under stress when subjected to centrifugation at 10000rpm for 30 min. The centrifugtion tests showed that microemulsions were remained homogenous without any phase separation throughout the test indicates good physical stability of both preparations.
Conclusion
This study estabilished that physicochemical properties and in vitro release were dependent upon the contents of S/C ratio, oil and water in formulations. Pseudo-ternary Phase diagrams indicated more width microemulsion region with a rise in S/C ratio. With decrease in S/C ratio and oil percent and increase in water percent could be obtained higher in vitro percentage release. The amount of naproxen release differ between microemulsion carriers with various internal microstructures. ME-6 may be preferable for topical naproxen formulation despite that the serious work still needs to be carried out to reveal the mechanisms of drug delivery into the skin.
Acknowledgments
This paper is extracted from pharm.D.thesis (Eftekhari,S) and financial support was provided by Ahvaz Jundishapur University of Medical Sciences. The authors are very thankful to Faratin company executive manager (Taheri,M,Iran) for providing gratis sample of Transcutol P, Labrafac PG and LabrafacTM Lipophile WL 1349 from GATTEFOSSE( France) and also GATTEFOSSE company (France).
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.Schulman JH, Hoar TP. Transparent water-in-oil dispersions: The oleopathic Hydromicelle. Nature . 1943; 152: 102. [Google Scholar]
- 2.Winsor PA. Solvent Properties of Amphiphilic Compounds. London: Butherworth; 1954.
- 3.Attwood D. Microemulsions. In: Kreuter J, editor. Colloidal Drug Delivery Systems. New York: Marcel Dekker; 1994.P.31-71.
- 4.Raffa RB. The Science and Practice of Pharmacy. In: Gennaro AR, editor. Remington. Philadelphia: Lippincott Williams & Wilkins; 2005.P. 1539.
- 5.Moghimipour E, Salimi A, Leis F. Preparation and Evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv Pharm Bull . 2012; 2(2):141–7. doi: 10.5681/apb.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilk KA, Zielinska K, Hamerska-Dudra A, Jezierski A. Biocompatible microemulsions of dicephalic aldonamide-type surfactants: Formulation, structure and temperature influence. J Colloid Interface Sci . 2009;334(1):87–95. doi: 10.1016/j.jcis.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 7.Sharif Makhmal zadeh, Torabi SH, Azarpanah A. Optimization of Ibuprofen Delivery through Rat Skin from Traditional and Novel Nanoemulsion Formulations. Iran J Pharm Res . 2012; 11(1):47–58. [PMC free article] [PubMed] [Google Scholar]
- 8.Langevin D. Microemulsions and liquid crystals. Mol Cryst Liq Cryst . 1986; 138:259–305. [Google Scholar]
- 9.Eicke HF. The microemulsion concept in nonpolar surfactant solutions. In: Robb ID, editor. Mikroemulsions. New York: Plenum; 1982.P. 17-32.
- 10.Sharif Makhmal zadeh, Yazdani M, Rezaai S, Salimi A. The effect of chemical enhancers on Tacrolimus permeation through rat skin. J Pharm Res . 2010; 5(3):1309–12. [Google Scholar]
- 11.Lapasin R, Grassi M, Coceani N. Effects of polymer addition on the rheology of o/w microemulsions. Rheol Acta . 2001; 40:185–92. [Google Scholar]
- 12.Ramesh Shah R, Shripal Magdum Ch, Shivagonda Patil Sh, Shanawaj Niakwade N. Preparation and Evaluation of Aceclofenac Topical Microemulsion. Iran J Pharm Res . 2010; 9(1):5–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Djordjevic L, Primorac M, Stupar M, Krajisnik D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm . 2004;271(1-2):11–9. doi: 10.1016/j.ijpharm.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Lv Q, Yu A, Xi Y, Li H, Song Z, Cui J, et al. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm . 2009;372(1-2):191–8. doi: 10.1016/j.ijpharm.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Araujo LM, Thomazine JA, Lopez RF. Development of microemulsions to topically deliver 5-aminolevulinic acid in photodynamic therapy. Eur J Pharm Biopharm . 2010;75(1):48–55. doi: 10.1016/j.ejpb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Yue Y, San-ming L, Pan D, Da-fang Z. Physicochemical properties and evaluation of microemulsion systems for transdermal delivery of meloxicam. Chem Res Chin Univ . 2007; 23(1):81–86. [Google Scholar]
- 17.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemlsions for topical delivery of estradiol. Int J Pharm . 2003;254:99–107. doi: 10.1016/s0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 18.Zvonar A, Rozman B, Bester Rogac M, Gasperlin M. The Influence of Microstructure on Celecoxib Releasefrom a Pharmaceutically Applicable System: Mygliol 812®/Labrasol®/Plurol Oleique®/Water Mixtures. Acta Chim Slov . 2009; 56:131–8. [Google Scholar]
- 19.Gradzielski M, Hoffmann H. Handbook of Microemulsion Science and Technology. New York: Marcel Dekker; 1999. P. 375.
- 20.Liu H, Wang Y, Lang Y, Yao H, Dong Y, Li S. Bicontinuous cyclosporin a loaded water-AOT/Tween 85-isopropylmyristate microemulsion: Structural characterization and dermal pharmacokinetics in vivo. J Pharm Sci . 2009;98(3):1167–76. doi: 10.1002/jps.21485. [DOI] [PubMed] [Google Scholar]
- 21.Podlogar F, Gasperlin M, Tomsic M, Jamnik A, Rogac MB. Structural characterisation of water-tween 40/imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int J Pharm . 2004;276(1-2):115–28. doi: 10.1016/j.ijpharm.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Podlogar F, Bester Rogac M, Gasperlin M. The effect of internal structure of selected water-tween 40-imwitor 308-ipm microemulsions on ketoprofene release. Int J Pharm . 2005;302(1-2):68–77. doi: 10.1016/j.ijpharm.2005.06.023. [DOI] [PubMed] [Google Scholar]