Abstract
Purpose: Recombinant tumor necrosis factor-alpha (TNF-α) has been utilized as an antineoplastic agent for the treatment of patients with melanoma and sarcoma. It targets tumor cell antigens by impressing tumor-associated vessels. Protein purification with affinity chromatography has been widely used in the downstream processing of pharmaceutical-grade proteins. Methods:In this study, we examined the potential of our produced anti-TNF-α scFv fragments for purification of TNF-α produced by Raji cells. The Raji cells were induced by lipopolysaccharides (LPS) to express TNF-α. Western blotting and Fluorescence-activated cell sorting (FACS) flow cytometry analyses were used to evaluate the TNF-α expression. The anti-TNF-α scFv selected from antibody phage display library was coupled to CNBr-activated sepharose 4B beads used for affinity purification of expressed TNF-α and the purity of the protein was assessed by SDS-PAGE. Results: Western blot and FACS flow cytometry analyses showed the successful expression of TNF-α with Raji cells. SDS-PAGE analysis showed the performance of scFv for purification of TNF-α protein with purity over 95%. Conclusion: These findings confirm not only the potential of the produced scFv antibody fragments but also this highly pure recombinant TNF-α protein can be applied for various in vitro and in vivo applications.
Keywords: TNF-α expression, Affinity Purification, Monoclonal antibody, LPS
Introduction
Cytokines, as low molecular-weight signaling molecules, are biologically functional in markedly low amounts. They play central roles upon the activity immune system, inflammation and cell growth. Of the cytokines, tumor necrosis factor alpha (TNF-α) possesses pleomorphic resulting in pivotal impacts on biological functions including inflammation, cell propagation, differentiation, immune regulation in addition to its ability to induce apoptosis within the tumor-associated endothelial cells.1,2 It is mainly expressed by monocytes/macrophages,3 even though other cells (T-lymphocytes, natural killer (NK) cells, astrocytes, fibroblasts, Kupffer cells, keratinocytes, smooth-muscle cells) as well as tumor cells can express TNF-α.4 The mature human TNF-α is a 157 amino acid (AA) protein (17 kDa) with an isoelectric point of 5.8, which contains one disulfide-bond (Cys69-Cys101). It is normally processed from a precursor form called transmembrane (a type II transmembrane protein with 26 kDa, 233 AA) revealing no glycosylation.5
Recombinant TNF-α has been harnessed as an antineoplastic agent alone or in combination with a conventional chemotherapy agent for the treatment of patients with melanoma and sarcoma.6-8 It is able to induce apoptosis within the tumor-associated endothelial cells, resulting in complete eradication of the tumor vasculature.8,9 Nevertheless, because of vasoplegia induction (also known as systemic inflammatory response), the therapeutic use of TNF in clinic was limited,7 while most of clinical phases revealed that TNF alone cannot effectively suppress the growth of tumor. Besides, TNF has several in vitro applications such as Enzyme-linked immunosorbent assay (ELISA), biopanning and Western blotting.
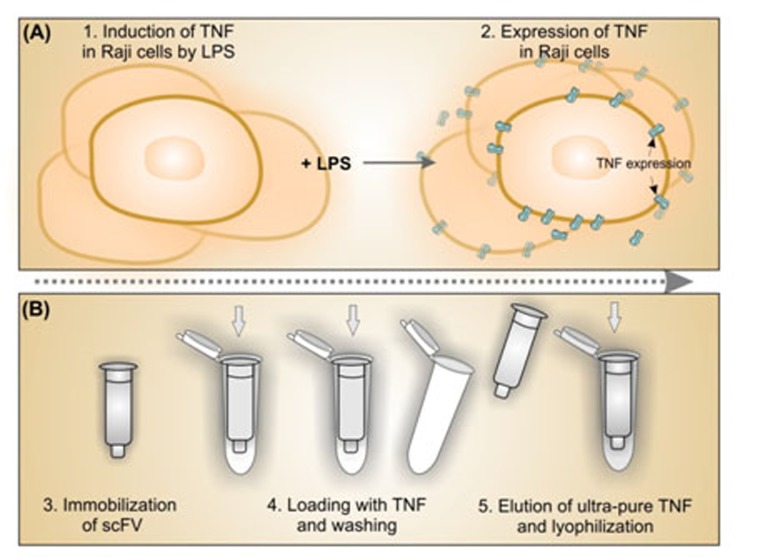
We have previously produced anti-TNF-α scFv antibody fragments using phage display technology.10 To examine the potential of these scFvs as ligate for affinity purification of expressed TNF-α, in the current study we stimulated Raji cells with lipopolysaccharide (LPS) that can elicit macrophages to produce TNF-α,11,12 and exploit the scFvs for purification of the induced TNF-α. Figure 1 represents schematic illustration for upstream production of TNF in Raji cells and downstream affinity purification process for the expressed TNF molecules.
Figure 1.
Schematic representation for upstream (A) and downstream (B) processing of TNF-α.
Materials and Methods
Culture of Raji Cell for induction of TNF expression
Human B-lymphoblastoid cells (Raji cell line)were cultured in T75 flasks and grown overnight in 18 ml RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) in a tissue culture incubator humidified with a 5% CO2 at 37oC. Then, cells were induced to produce TNF-α by addition of 10 µg/mL LPS (Sigma Chem. Co., St. Louis, MO, USA).
Cells were washed twice with cold PBS (pH 7.4) and incubated with the cell lysis buffer (PBS that contained 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, and 0.01% protease inhibitor cocktail) at 4ºC for 1 h. After centrifugation, the supernatant was collected and TNF-α concentration was determined using Western blotting and Fluorescence-activated cell sorting (FACS) methods.
Western blotting analysis; assessment of TNF-α expression
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis was conducted to determine the TNF-α expression in Raji cells. Cell lysates were mixed with sample buffer and separated by electrophoresis on reduced condition onto 12% gels. The protein profiles were electrically transferred on Polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA) using transfer buffer (25 mM Tris, 193 mM glycine, and 20% methanol). The membrane was then blocked with the blocking solution and incubated with anti-TNF-α scFv monoclonal antibody (mAb), followed by incubation with anti-myc and HRP-conjugated anti-mouse IgG antibodies. After extensive washing, the protein bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, Freiburg, Germany) substrate.
Flow cytometry analysis using FITC conjugated scFvn
For analysis of induced Raji cells for TNF-α expression, we harnessed the flow cytometry analysis using FITC conjugated scFv antibody fragments. To this end, we first purified scFv antibody fragments as described previously through antibody phage display technique10 and then conjugated these scFvs with fluorescein isothiocyanate (FITC) (Sigma Chemical Co., St. Louis, MO, USA). Briefly, anti-TNF-α scFv was dialysed against 0.1 M sodium carbonate buffer, pH 9 overnight at 4°C.The FITC was dissolved in anhydrous DMSO (Sigma Chemical Co., St. Louis, MO, USA)at 1 mg/mL and added to1 mg/mL protein while gently and continuously stirring the protein solution and incubated in the dark for 8 h at 4°C. After addition of NH4Cl to a final concentration of 50 mM, the reaction incubated for 2 h at 4°C. Gel filtration was used to separate the unbound FITC from the FITC-conjugated scFvs.13,14
For FACS flow cytometry analysis, the LPS induced Raji cells were incubated with 1 mL of FITC labeled scFv at 1:1000 dilution for 1 h. After washing with PBS containing 10% FBS, to assess the fluorescence intensity, 10000 events were measured for each sample using FACSCalibur and analyzed with CellQuest Pro software (Becton Dickinson Biosciences, San Jose, CA, USA).
Preparation of scFv-coupled sepharose column
Affinity purified scFv was coupled to CNBr-activated sepharose 4B beads(Sigma Chemical Co., St. Louis, MO, USA) for preparation ofthe affinity column. Purified scFv antibodyfragments were dialyzed against coupling buffer (0.2 M NaHCO3, 0.5 M NaCl, pH 9.0) overnight at 4°C. Sepharose beads were then introduced to 1 mM HCl and washed with coupling buffer. ThescFv antibody fragments were added to resin, incubated overnight at 4oC and blocked withblocking buffer (200 mM Glycine, pH 8.0). Antibody-coupled sepharose was transferred to column and washed (3×) with PBS.
Purification of TNF-α
TNF-αexpressed Raji cell lysates were dialyzed against PBS buffer (pH 7.4) and loaded on column. The bound proteins were eluted using elution Buffer (100 mM Glycine, pH 2.5) and neutralized with 2 M Tris, pH 8.0. The purity was assessed by SDS-PAGE.
SDS-PAGE analysis of purified protein
SDS-PAGE analysis was performed for the purity assessment of the eluted protein. The collected protein fractions were subjected to electrophoresis in 12% SDS-PAGE on reduced condition.
Protein bands were visualized by Coomassie blue staining technique.
Results
Western blotting analysis for functional assessment of TNF-α expression
The Raji cell lysates were resolved by 12% SDS-PAGE on reducing condition and electrically transferred onto the PVDF membrane. The membrane was probed with anti-TNF-α scFv, anti-myc and HRP conjugated anti-mouse IgG antibodies. The ECL substrate was used for the visualization of protein bands. Figure 2 represents the Western blot analysis of the expressed TNF-α in Raji cells, showing the presence of a TNF-α protein band with a molecular weight of 17 KDa.
Figure 2.
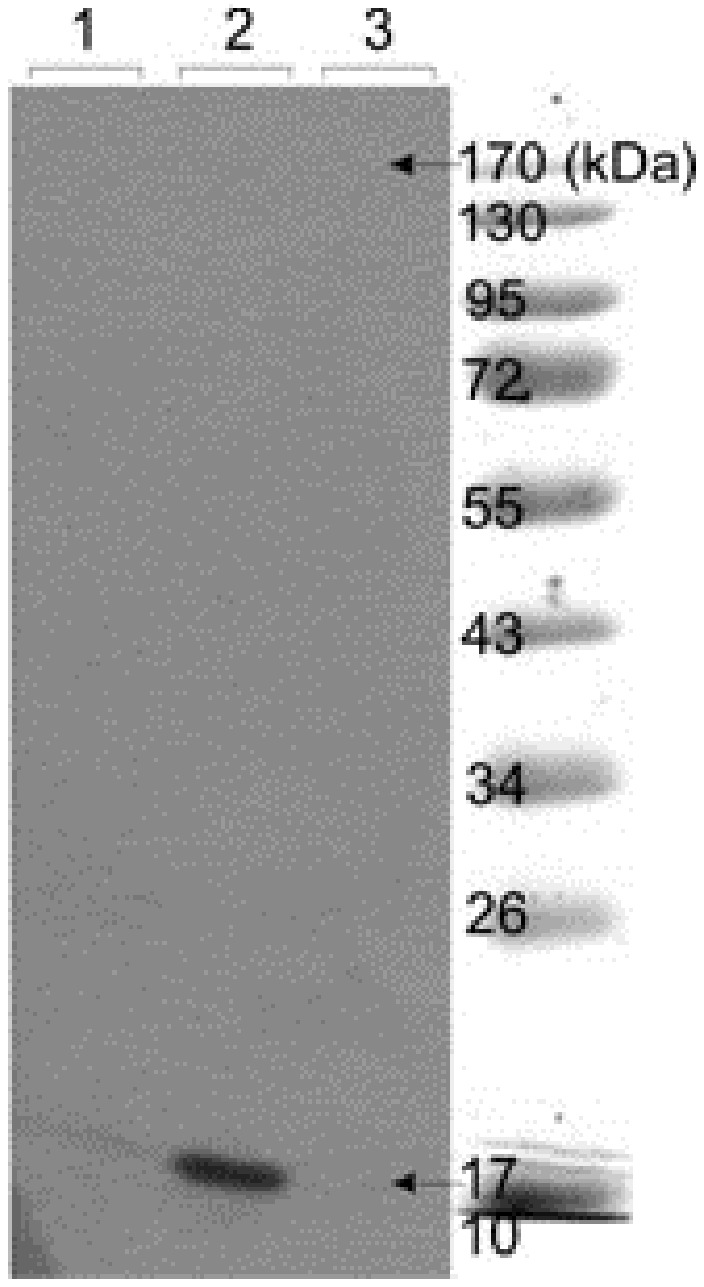
Western blot analysis of TNF-α in Raji cell lysates. Cultured human Raji cells were stimulated with 10 mg/mL LPS. SDS-PAGE was subjected onto cell lysates and blotted onto PVDF membrane. Lanes 1 and 3: Control. Lane 2: The detected band showing induction of the TNF-α.
FACS flow cytometry analysis for assessment of TNF-α expression
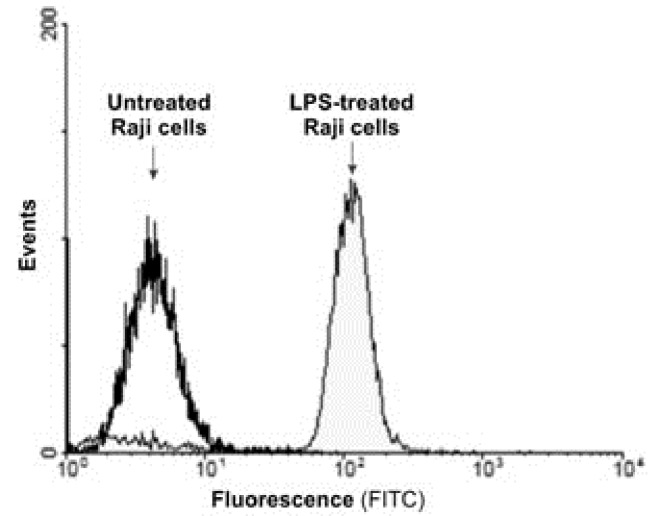
After induction of Raji cells with LPS for TNF-α expression, the cells were incubated with 1 mL of FITC labeled scFv at 1:1000 dilution for 1 h and washed with PBS containing 10% FBS. Figure 3 represents the fluorescence intensity of the TNF-α expressed by Raji cells and captured by FITC labeled scFvs.
Figure 3.
The fluorescence intensity of the TNF-α expressed by Raji cells and captured by FITC labeled scFvs.
The fluorescence intensity of the TNF-α expressed by Raji cells and captured by FITC labeled scFvs.
Purification of TNF-α from crude protein extract
Purified scFv was coupled to CNBr-activated sepharose 4B beads and used for purification of expressed TNF-α from Raji cell lysates. After dialysis of cell lysates against PBS buffer, pH 7.4, the protein was loaded on column. The purity of eluted protein was analyzed by SDS-PAGE. The purity of protein was up to 95%. Figure 4 represents the electrophoretic pattern of purified TNF-α with CNBr-activated sepharose 4B beads, showing the major band at a molecular weight of approximately 17kDa.
Figure 4.
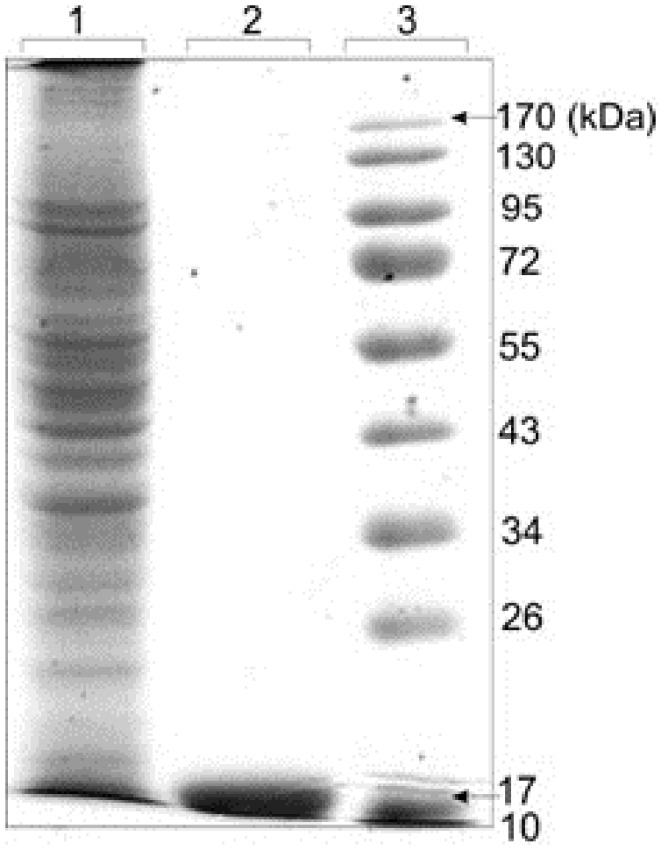
Electrophoretic pattern of purified TNF-α with CNBr-activated sepharose 4B beads. Expressed Raji cells lysates was dialyzed against PBS buffer, pH 7.4 and loaded on column containing coupled purified scFv-CNBr-activated sepharose 4B beads. The purity of eluted protein was analyzed by SDS-PAGE which was up to 95%. The major band at a molecular weight of approximately 17 kDa related to TNF-α protein was observed. Lane 1: Crude protein extract before chromatography, Lane 2: Pure elution fraction. Lane 3: Protein size marker. The gel was stained with Coomassie Blue R-25.
Discussion
Recombinant TNF-α has various applications in clinic as well as research studies. In clinic, it has been utilized as an antineoplastic agent either alone or in combination with chemotherapy for the treatment of patients with melanoma and sarcoma.6-8 It targets tumor cells and suppress the tumor-associated microvasculature.8,9 Nevertheless, it can induce vasoplegia that has some implications. Thus, its therapeutic use in clinic confined to administration through the limb perfusion.7 In research, however it has been widely used for designing several tests such as ELISA, biopanning and Western blotting.
To produce ultra-pure TNF-α, in the current study, we have induced Raji cells with LPS to express the TNF-α. LPS simulate TNF expression through LPS dependent pathway.16 The performance of such upstream bioprocessing (Figure 1) was further determined by Western blotting and FACS flow cytometry analysis. Western blot analysis of the TNF-α expression identified the prominent 17 kDa band as human TNF-α, representing the successful expression of TNF-α protein (Figure 2).
Having capitalized the phage display technology, we have previously produced a high affinity scFv antibody fragments against TNF-α.10 We utilized these scFvs for detection and purification of TNF-α (Figure 1). The expression of the TNF-α in the Raji cells induced by LPS was confirmed by the FACS flow cytometry analysis through conjugation of the anti-TNF-α scFvs with FITC (Figure 3).Induction of TNF-α by LPS have been reported in different cells.11,12,17,18
Chromatography technology is the fundamental technique in all biopharmaceutical downstream processing. In fact, affinity chromatography has great impacts upon downstream processing of recombinant proteins that are used as effective modalities for different diseases particularly for the immunotherapy.19-24 We harnessed the affinity chromatography using our previously produced scFvs for purification of expressed TNF-α in Raji cells. The produced scFvs through antibody phage display technology10 was immobilized onto the CNBr-activated sepharose 4B and used for purification of expressed TNF-α. The immobilized scFvs onto the CNBr-Sepharose, as the immuno-adsorbent, can be used continuously for rapid purification of TNF-α from any biological sources. The SDS-PAGE analysis showed a high purity (over 95%) for the extracted protein in elution fraction (Figure 4).
In fact, for capturing proteins with high purity, the affinity chromatography method appears to be a simple, one-step and time-efficient approach in comparison with the other chromatography methods such as ion exchange chromatography and gel filtration.25 We utilized this technique to produce highly pure recombinant TNF-α protein that can be applied successfully in clinic and different immunostaining methods. Thus, we propose this approach as basic technology for production of proteins therapeutics.
Conclusion
In conclusion, our results indicated that recombinant TNF-α protein successfully expressed in Raji cells and purified with the previously produced scFv, indicating that this scFv can be used effectively in affinity chromatography for production of ultra-pure recombinant TNF-α.
Acknowledgments
This study was supported by the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences. These data are the result of Ph.D thesis registered No: 89007 in Tabriz University of Medical Sciences.
Conflict of Interest
No conflict of interests to be declared.
References
- 1.Smyth MJ, Johnstone RW. Role of tnf in lymphocyte-mediated cytotoxicity. Microsc Res Tech . 2000;50(3):196–208. doi: 10.1002/1097-0029(20000801)50:3<196::AID-JEMT3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Ware CF. Network communications: Lymphotoxins, light, and tnf. Annu Rev Immunol . 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 3.Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA. et al. Human tumour necrosis factor: Precursor structure, expression and homology to lymphotoxin. Nature . 1984;312(5996):724–9. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 4.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev . 2005;16(1):35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Rink L. Tumor Necrosis Factor-α. In: Delves PJ, Roitt IM, editors. Encyclopedia of Immunology. 2nd ed. Oxford: Elsevier; 1998. p. 2435-40. [Google Scholar]
- 6.Grunhagen DJ, de Wilt JH, ten Hagen TL, Eggermont AM. Technology insight: Utility of tnf-alpha-based isolated limb perfusion to avoid amputation of irresectable tumors of the extremities. Nat Clin Pract Oncol . 2006;3(2):94–103. doi: 10.1038/ncponc0426. [DOI] [PubMed] [Google Scholar]
- 7.Lejeune F, Ruegg C. Recombinant human tumor necrosis factor: An efficient agent for cancer treatment. Bull Cancer . 2006;93(8):E90–100. [PubMed] [Google Scholar]
- 8.Lejeune FJ, Liénard D, Matter M, Ruegg C. Efficiency of recombinant human tnf in human cancer therapy. Cancer Immun . 2006;6:6. [PubMed] [Google Scholar]
- 9.Corti A. Strategies for improving the anti-neoplastic activity of tnf by tumor targeting. Methods Mol Med . 2004;98:247–64. doi: 10.1385/1-59259-771-8:247. [DOI] [PubMed] [Google Scholar]
- 10.Abdolalizadeh J, Nouri M, Zolbanin JM, Baradaran B, Barzegari A, Omidi Y. Downstream characterization of anti-tnf-α single chain variable fragment antibodies. Hum Antibodies . 2012;21(1):41–8. doi: 10.3233/HAB-2012-0260. [DOI] [PubMed] [Google Scholar]
- 11.Diya Z, Lili C, Shenglai L, Zhiyuan G, Jie Y. Lipopolysaccharide (lps) of porphyromonas gingivalis induces il-1beta, tnf-alpha and il-6 production by thp-1 cells in a way different from that of escherichia coli lps. Innate Immun . 2008;14(2):99–107. doi: 10.1177/1753425907088244. [DOI] [PubMed] [Google Scholar]
- 12.He X, Shu J, Xu L, Lu C, Lu A. Inhibitory effect of astragalus polysaccharides on lipopolysaccharide-induced tnf-a and il-1β production in thp-1 cells. Molecules . 2012;17(3):3155–64. doi: 10.3390/molecules17033155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Labeling antibodies with fluorochromes. CSH protocols 2006;2006(2). [DOI] [PubMed] [Google Scholar]
- 14.Goding JW. Conjugation of antibodies with fluorochromes: Modifications to the standard methods. J Immunol Methods . 1976;13(3-4):215. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 15.Affinity Chromatography, Principles and Methods. Uppsala, Sweden: GE Healthcare Bio-Sciences AB; 2002. [Google Scholar]
- 16.Jiang C, Ting AT, Seed B. Ppar-γ agonists inhibit production of monocyte inflammatory cytokines. Nature . 1998;391(6662):82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 17.Peng T, Shen E, Fan J, Zhang Y, Arnold JMO, Feng Q. Disruption of phospholipase cγ1 signalling attenuates cardiac tumor necrosis factor-α expression and improves myocardial function during endotoxemia. Cardiovasc Res . 2008;78(1):90–7. doi: 10.1093/cvr/cvm100. [DOI] [PubMed] [Google Scholar]
- 18.Chou YC, Sheu JR, Chung CL, Hsiao CJ, Hsueh PJ, Hsiao G. Hypertonicity-enhanced TNF-alpha release from activated human monocytic THP-1 cells requires ERK activation. Biochim Biophys Acta . 2011;1810(4):475–84. doi: 10.1016/j.bbagen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Cai R, Ye X. Preparation, purification and identification of the polyclonal antibody of phd finger protein 8. Sheng Wu Gong Cheng Xue Bao;26(3):393-7. [PubMed] [Google Scholar]
- 20.Loughran SaT, Loughran NB, Ryan BJ, D'Souza BN, Walls D. Modified his-tag fusion vector for enhanced protein purification by immobilized metal affinity chromatography. Anal Biochem . 2006;355(1):148–50. doi: 10.1016/j.ab.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Steen J, Uhlen M, Hober S, Ottosson J. High-throughput protein purification using an automated set-up for high-yield affinity chromatography. Protein Expression Purif . 2006;46(2):173–8. doi: 10.1016/j.pep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Cass B, Pham PL, Kamen A, Durocher Y. Purification of recombinant proteins from mammalian cell culture using a generic double-affinity chromatography scheme. Protein Expression Purif . 2005;40(1):77–85. doi: 10.1016/j.pep.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Wang GR, Shi Q, Zhang BY, Mei GY, Li Y. et al. Preparations of the specific antibodies against exon 2 and exon 3 of human tau protein. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi . 2009;23(2):146–8. [PubMed] [Google Scholar]
- 24.Bandehpour M, Khodabandeh M, Mosaffa N, Sharifnia Z, Ghazanfari T, Kazemi B. An efficient procedure for purification of recombinant human beta heat shock protein 90. Daru . 2010;18(1):64–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Arnau J, Lauritzen C, Petersen GE, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expression Purif . 2006;48(1):1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]